Abstract
The LATERAL ORGAN BOUNDARIES DOMAIN/ASYMMETRIC LEAVES2-LIKE (LBD/ASL) genes encode proteins harboring a conserved amino acid domain, referred to as the LOB (for lateral organ boundaries) domain. While recent studies have revealed developmental functions of some LBD genes in Arabidopsis (Arabidopsis thaliana) and in crop plants, the biological functions of many other LBD genes remain to be determined. In this study, we have demonstrated that the lbd18 mutant evidenced a reduced number of lateral roots and that lbd16 lbd18 double mutants exhibited a dramatic reduction in the number of lateral roots compared with lbd16 or lbd18. Consistent with this observation, significant β-glucuronidase (GUS) expression in ProLBD18:GUS seedlings was detected in lateral root primordia as well as in the emerged lateral roots. Whereas the numbers of primordia of lbd16, lbd18, and lbd16 lbd18 mutants were similar to those observed in the wild type, the numbers of emerged lateral roots of lbd16 and lbd18 single mutants were reduced significantly. lbd16 lbd18 double mutants exhibited additively reduced numbers of emerged lateral roots compared with single mutants. This finding indicates that LBD16 and LBD18 may function in the initiation and emergence of lateral root formation via a different pathway. LBD18 was shown to be localized into the nucleus. We determined whether LBD18 functions in the nucleus using a steroid regulator-inducible system in which the nuclear translocation of LBD18 can be regulated by dexamethasone in the wild-type, lbd18, and lbd16 lbd18 backgrounds. Whereas LBD18 overexpression in the wild-type background induced lateral root formation to some degree, other lines manifested the growth-inhibition phenotype. However, LBD18 overexpression rescued lateral root formation in lbd18 and lbd16 lbd18 mutants without inducing any other phenotypes. Furthermore, we demonstrated that LBD18 overexpression can stimulate lateral root formation in auxin response factor7/19 (arf7 arf19) mutants with blocked lateral root formation. Taken together, our results suggest that LBD18 functions in the initiation and emergence of lateral roots, in conjunction with LBD16, downstream of ARF7 and ARF19.
The LATERAL ORGAN BOUNDARIES DOMAIN/ASYMMETRIC LEAVES2-LIKE (LBD/ASL) genes (hereafter referred to as LBD) encode proteins harboring a LOB (for lateral organ boundaries) domain, which is a conserved amino acid domain that is detected only in plants, indicative of its function in plant-specific processes (Iwakawa et al., 2002; Shuai et al., 2002). There are 42 Arabidopsis (Arabidopsis thaliana) LBD genes, which have been assigned to two classes. Class I comprises 36 genes and class II comprises six genes (Iwakawa et al., 2002; Shuai et al., 2002). The class I proteins harbor LOB domains similar to those observed in the LOB protein, whereas the class II proteins are less similar to the class I proteins, which include the LOB domain as well as regions outside of the LOB domain. The LOB domain is approximately 100 amino acids in length and harbors a conserved 4-Cys motif with CX2CX6CX3C spacing, a Gly-Ala-Ser block, and a predicted coiled-coil motif with LX6LX3LX6L spacing, reminiscent of the Leu zipper found in the majority of class I proteins (Shuai et al., 2002). None of the class II proteins were predicted to form coiled-coil structures.
Although we currently understand very little about the biological roles of the LBD genes, there have been some reports describing the developmental functions of LBD genes in Arabidopsis on the basis of gain-of-function studies. The gain-of-function mutants of LBD36/ASL1, designated downwards siliques1, showed shorter internodes and downward lateral organs such as flowers (Chalfun-Junior et al., 2005). Although the lbd36 loss-of-function mutants did not show morphological phenotypes, the analysis of lbd36 as2 double mutants showed that these two members act redundantly to control cell fate determination in the petals. Another Arabidopsis gain-of-function mutant, jagged lateral organs-D (jlo-D), generates strongly lobed leaves and the shoot apical meristem prematurely arrests organ initiation, terminating in a pin-like structure (Borghi et al., 2007). During embryogenesis, JLO (=LBD30/ASL19) is necessary for the initiation of cotyledons and development beyond the globular stage. The results of misexpression experiments indicate that during postembryonic development, JLO function is required for the initiation of plant lateral organs. A recent study showed that the LOB domain of AS2 cannot be functionally replaced by those of other members of the LOB family, indicating that dissimilar amino acid residues in the LOB domains are important for characteristic functions of the family members (Matsumura et al., 2009).
Thirty-five LBD genes in rice (Oryza sativa) have been identified from the genome sequences of the two rice subspecies, a japonica rice (Nippobare) and an indica rice (9311; Yang et al., 2006). Analyses of rice mutants have provided evidence of the involvement of a variety of rice LBD genes in lateral organ development. CROWN ROOTLESS1 (CRL1), encoding a LBD protein, is crucial for crown root formation in rice (Inukai et al., 2005). The crl1 mutant showed auxin-related phenotypes, such as decreased lateral root number, auxin insensitivity in lateral root formation, and impaired root gravitropism. A rice AUXIN RESPONSE FACTOR (ARF) appears to directly regulate CRL1 expression in the auxin signaling pathway (Inukai et al., 2005). ADVENTITIOUS ROOTLESS1 encodes an auxin-responsive protein with a LOB domain that controls the initiation of adventitious root primordia in rice and turned out to be the same gene as CRL1 (Liu et al., 2005).
Lateral roots of Arabidopsis are derived from a subset of the pericycle cells (pericycle founder cells), which are positioned at the xylem poles within the parent root tissues (Casimiro et al., 2003). The mature pericycle cells dedifferentiate to form lateral root primordium (LRP), which undergoes consistent anticlinal and periclinal cell divisions to generate a highly organized LRP (Malamy and Benfey, 1997). The LRP emerges from the parent root via cell expansion, and the activation of the lateral root meristem results in continued growth of the organized lateral root. A growing body of physiological and genetic evidence has been collected to suggest that auxin plays a profound role in lateral root formation. For example, many auxin-related mutants have been shown to affect lateral root formation (Casimiro et al., 2003). Lateral root formation in Arabidopsis was shown to be regulated by ARF7 and ARF19 via the direct activation of LBD16 and LBD29/ASL16 (Okushima et al., 2007). Overexpression of LBD16 and LBD29 induced lateral root formation in the absence of ARF7 and ARF19, and the dominant repression of LBD16 inhibited lateral root formation, thus suggesting that these LBDs function downstream of ARF7- and ARF19-mediated auxin signaling during lateral root formation. The results of selection and binding assays demonstrated that a truncated LOB protein harboring only the conserved LOB domain can preferentially bind to unique DNA sequences, which is indicative of a DNA-binding protein (Husbands et al., 2007). Recently, LBD18 was shown to regulate tracheary element differentiation (Soyano et al., 2008).
In this study, we demonstrated that LBD18 is involved in the regulation of lateral root formation, based on the analysis of loss-of-function mutants and the complementation of lbd18 and lbd16 lbd18 mutants by dexamethasone (DEX)-inducible LBD18 expression. Double mutations in LBD16 and LBD18 resulted in a synergistic reduction in the number of lateral roots, particularly in initiation and emergence, compared with either the lbd16 or lbd18 single mutant. This finding is suggestive of a combinatorial interaction of LBD16 and LBD18 in the process of lateral root formation. LBD18 expression in arf7 and arf19 mutants by the DEX-inducible system increased the number of lateral roots, thus demonstrating that LBD18 functions downstream of ARF7 and ARF19 in lateral root formation.
RESULTS
Phenotype Analysis of lbd16, lbd18, and lbd16 lbd18 Mutants
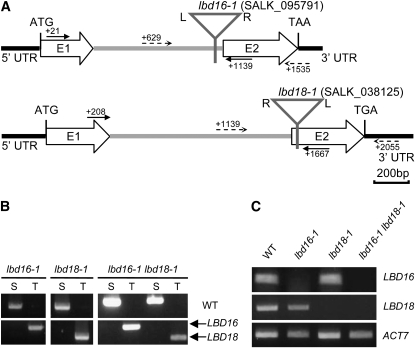
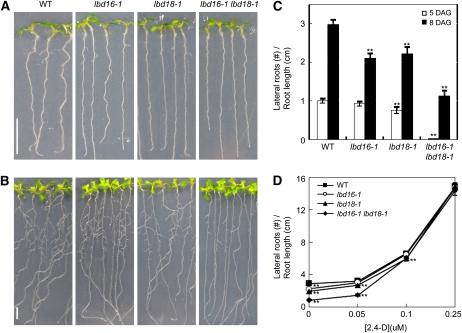
In an earlier study, we constructed a transgenic Arabidopsis that expressed the glucocorticoid receptor (GR)-fused stabilized indoleacetic acid1 (iaa1) protein harboring an amino acid change in domain II (Park et al., 2002). DEX treatment of this transgenic Arabidopsis evoked dramatic auxin-related phenotypes and repressed the auxin induction of a variety of Aux/IAA genes (Park et al., 2002; Ku et al., 2009). We previously assessed the effects of DEX-inducible iaa1 on auxin-regulated gene expression, focusing on early genes, with the Affymetrix full genome array and subsequently identified a transcriptome downstream of iaa1 during the auxin response (Lee et al., 2009). In order to characterize the biological functions of auxin response genes downstream of iaa1, we analyzed a variety of Arabidopsis T-DNA insertion mutants, but none of them evidenced notable morphological phenotypes. Then, we constructed double mutants of similarly classified single mutants and examined auxin-related phenotypes (data not shown). We found that among them, lbd16-1 lbd18-1 double mutants exhibited significantly reduced numbers of lateral roots. Figure 1, A and B, show the schematic view and the results of PCR analysis of the homozygous T-DNA insertion mutants of lbd16-1 and lbd18-1 single mutants. Figure 1C shows the results of reverse transcription (RT)-PCR analysis of homozygous T-DNA insertion mutants of lbd16-1 and lbd18-1 as well as lbd16-1 lbd18-1. We examined the auxin-related phenotypes and morphological changes of these single mutants but detected no significant phenotypes except for the change in lateral root formation. One example demonstrates the insignificant difference in root growth inhibition between these mutants and the wild-type plants, with varying concentrations of indole-3-acetic acid (IAA; Supplemental Fig. S1). However, we did note a significant reduction in lateral root number for lbd18-1 as well as lbd16-1 when compared with the wild-type plants at 5 and 8 d after germination (Fig. 2, A–C). The lbd16-1 lbd18-1 double mutants showed substantially reduced numbers of lateral roots compared with lbd16-1 and lbd18-1 by dissecting microscopy. The addition of the auxin 2,4-dichlorophenoxyacetic acid (2,4-D) to the lbd16-1, lbd18-1, and lbd16-1 lbd18-1 mutants rescued the reduced lateral root number to wild-type levels (Fig. 2D). These results indicate that LBD18, in combination with LBD16, is involved in lateral root formation during the auxin response.
Figure 1.
PCR analysis of the lbd16, lbd18, and lbd16 lbd18 mutants. A, Location of T-DNA insertions of LBD16 and LBD18 in Arabidopsis. T-DNA insertions are indicated by triangles. LBD16 and LBD18 are composed of two exons indicated by E1 and E2 and one intron indicated by the gray lines. Solid lines and dotted lines indicate the locations of the primers for the RT-PCR and the primers for the PCR of genomic DNA, respectively. Numbers indicate the position of the first nucleotide of each primer relative to the AUG initiation codon. L and R, Left and right borders of T-DNA; UTR, untranslated region. B, PCR analysis of T-DNA insertion mutants. Genomic DNA isolated from each mutant was subjected to PCR with primers specific for LBD16 or LBD18 and the primers for T-DNA. S and T, PCR products amplified by specific primers (S) or specific primers and T-DNA primers (T); WT, wild-type Columbia. C, RT-PCR analysis of T-DNA insertion mutants. Total RNA isolated from 7-d-old light-grown seedlings was subjected to RT-PCR. The ACTIN7 mRNA was utilized as a loading control.
Figure 2.
Lateral root phenotypes of lbd16, lbd18, and lbd16 lbd18 mutants. A and B, Lateral roots of T-DNA insertion mutants and the wild type (WT). Photographs were taken 5 d (A) or 8 d (B) after germination. Bars = 1 cm. C, Number of lateral roots at different days after germination (DAG). The white columns and black columns indicate the lateral root numbers of plants grown vertically 5 and 8 d after germination, respectively. n > 29. Lateral roots were counted with a dissecting microscope. Lateral root numbers per unit root length (cm) measured were plotted. D, Number of lateral roots with varying concentrations of 2,4-D. Lateral root numbers of 5-d-old seedlings grown vertically were counted 4 d after transfer to medium containing the indicated concentrations of 2,4-D. n > 18. Lateral root numbers per unit root length (cm) measured were plotted. Asterisks denote statistical significance at P < 0.01.
GUS Expression Patterns of ProLBD16:GUS and ProLBD18:GUS Transgenics
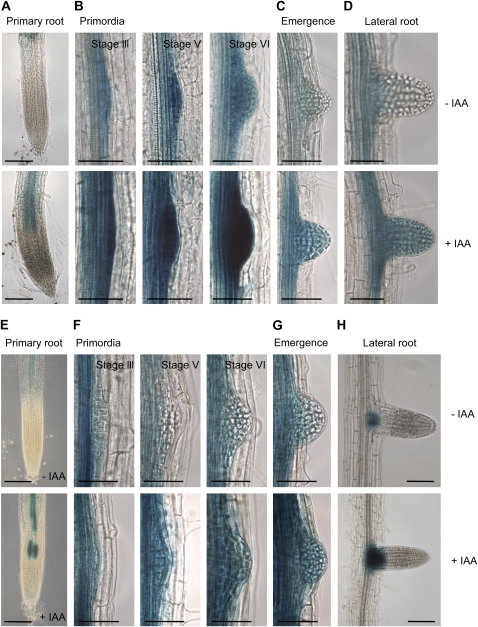
It has been previously reported that in ProLBD16:GUS seedlings, strong GUS activity was detected in the root stele and the lateral root primordia (Okushima et al., 2007). We additionally assessed the expression of GUS at three different stages of lateral primordium formation (Malamy and Benfey, 1997) and during lateral root emergence in transgenic Arabidopsis harboring the 1.4-kb LBD16 promoter-GUS fusion construct (ProLBD16:GUS) that we generated (Fig. 3, A–D). We noted strong GUS expression not only in the root stele (Fig. 3, A–D) and the lateral root primordia (Fig. 3B) but also in the developing (Fig. 3B), emerged (Fig. 3C), and mature (Fig. 3D) lateral roots in 7-d-old light-grown ProLBD16:GUS seedlings. Upon treatment with the auxin IAA, strongly enhanced GUS expression was detected in the primary roots and lateral roots (Fig. 3, A and D, bottom panels). We noted intense GUS expression in developing primordia (Fig. 3B, bottom panels). In order to understand the role of LBD18 in lateral root formation, we generated transgenic Arabidopsis harboring the 2.0-kb LBD18 promoter-GUS fusion construct (ProLBD18:GUS) and examined GUS expression during lateral primordium formation and lateral root emergence (Fig. 3, E–H). In 7-d-old light-grown ProLBD18:GUS seedlings, significant GUS activity was noted in the root stele (Fig. 3E), similar to what was noted in the ProLBD16:GUS seedlings. The GUS staining patterns of ProLBD18:GUS seedlings were also assessed for three different stages of lateral root development. GUS activity was observed in the lateral root primordia as well as the emerging lateral root (Fig. 3, F and G). However, no GUS activity was detected in the lateral root stele (Fig. 3H). Treatment with the auxin IAA resulted in enhanced GUS expression in the primary and lateral roots (Fig. 3, E–H, bottom panels). These GUS expression patterns are consistent with the role of LBD18 in lateral root formation during the auxin response. Overlapping GUS expression patterns of ProLBD16:GUS and ProLBD18:GUS during the formation of lateral root primordium and during lateral root emergence suggest a common function in lateral root initiation and emergence.
Figure 3.
GUS expression in ProLBD16:GUS and ProLBD18:GUS transgenics. A to D, GUS expression in 7-d-old light-grown ProLBD16:GUS seedlings. Seedlings were incubated without or with 20 μm IAA for 4 h. Images were obtained after 8 h of incubation with 5-bromo-chloro-3-indolyl glucuronide. E to H, GUS expression in 7-d-old light-grown ProLBD18:GUS seedlings. Seedlings were incubated without or with 20 μm IAA for 8 h. Images were obtained after 16 h of incubation with 5-bromo-chloro-3-indolyl glucuronide. A and E, GUS expression of a primary root without or with IAA. Bars = 100 mm. B and F, GUS expression of primordia of the developing lateral root without or with IAA. C and G, GUS expression of an emerged lateral root without or with IAA. D and H, GUS expression of a lateral root without or with IAA. Stages of lateral roots were based on Malamy and Benfey (1997). Bars = 50 mm.
Analysis of Primordia and Emerged Lateral Roots in lbd16, lbd18, and lbd16 lbd18 Mutants
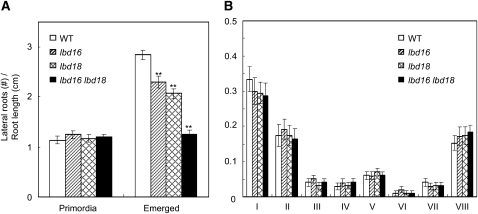
Lateral root development can be divided into four steps: stimulation and dedifferentiation of pericycle cells, ordered cell division and redifferentiation to generate a highly organized lateral root primordium, emergence of the lateral root primordium via cell expansion, and activation of the lateral root meristem to permit the continued growth of the organized lateral root (Malamy and Benfey, 1997; Casimiro et al., 2003). In order to determine the step of lateral root formation in which LBD16 and LBD18 act, we enumerated the lateral root primordia and emerged lateral roots of lbd16-1, lbd18-1, and lbd16-1 lbd18-1 mutants by photographing the roots with a light microscope equipped with a camera. As shown in Figure 4, the numbers of primordia in the lbd16 and lbd18 single or double mutants were similar to the numbers observed in the wild type. However, the numbers of emerged lateral roots of lbd16 and lbd18 single mutants were significantly reduced as compared with the wild type. The lbd16 lbd18 double mutants exhibited additively reduced numbers of emerged lateral roots compared with the single mutants. We further analyzed the effects of lbd16 and lbd18 mutations on primordium development by counting the numbers of primordia from stage I to stage VIII on the basis of the classification made by Malamy and Benfey (1997), showing that primordium development was not significantly affected at every stage examined (Fig. 4B). These results suggest that LBD16 and LBD18 may be involved in the initiation and emergence of lateral roots.
Figure 4.
Numbers of lateral roots of lbd16, lbd18, and lbd16 lbd18 mutants. A, Numbers of primordia of lateral roots or emerged lateral roots. Plants were grown vertically for 8 d after germination. Primordia or emerged lateral roots were photographed with a camera affixed to a Leica CME trinocular light microscope at 100- or 400-fold magnification and counted on the basis of Malamy and Benfey (1997). n > 10 per column. Asterisks denote statistical significance at P < 0.01. WT, Wild type. B, Numbers of primordia at given stages before emergence of lateral roots. Stages I to VIII of primordia were based on the classification by Malamy and Benfey (1997).
Gain-of-Function Analysis of LBD16 and LBD18
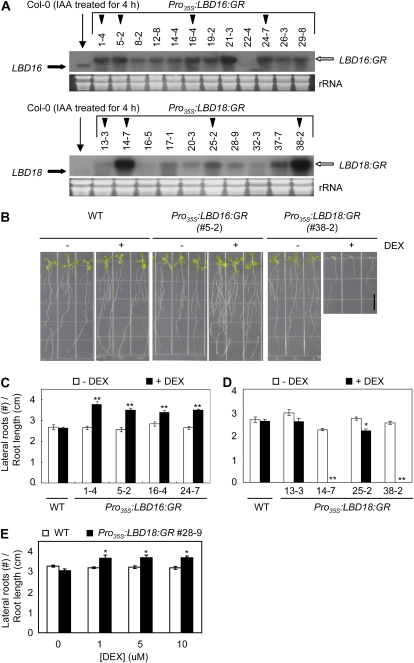
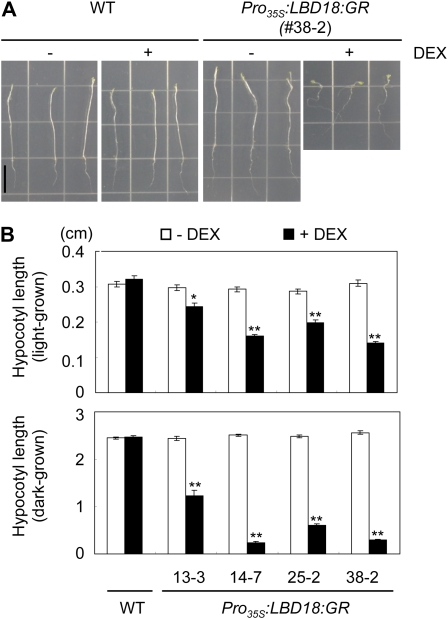
GFP fusion proteins LBD16 and LBD18 are localized in the nucleus in protoplasts isolated from Arabidopsis mesophyll cells (Fig. 5). To evaluate the gain-of-function phenotypes of LBD16 and LBD18 in the nucleus, we utilized a steroid regulator-inducible system via fusion of the full-length coding region of LBD16 or LBD18 in-frame with the hormone-binding domain of the GR to generate transgenic Arabidopsis (Pro35S:LBD16:GR or Pro35S:LBD18:GR) in the ecotype Columbia (Col-0) wild-type background. As observed in the RNA gel-blot analysis of these transgenic lines, two bands corresponding to endogenous LBD16 or LBD18 and LBD16:GR or LBD18:GR transcripts were detected, and the transgenic lines exhibiting high expression ratios of the GR fusion transcripts over the endogenous LBD16 or LBD18 transcripts (indicated by black triangles) were selected for phenotype analysis (Fig. 6A). DEX treatment significantly stimulated lateral root numbers in all four lines of Pro35S:LBD16:GR transgenic plants when compared with the wild-type plants as well as in the absence of DEX (Fig. 6, B and C). This observation is similar to a previous report describing the stimulation of lateral root formation via LBD16 overexpression in arf7 arf19 mutants (Okushima et al., 2007). In contrast, two lines (lines 14-7 and 38-2) of Pro35S:LBD18:GR transgenic plants in the presence of DEX exhibited severely reduced root length and lateral root numbers, and the 25-2 line showed some degree of reduction in both root length and lateral root number (Fig. 6, B and D; Supplemental Fig. S2). When the DEX concentrations were varied from 0.25 to 5 μm with the 38-2 and 25-2 lines, similar results showing dose-response curves were generated (Supplemental Fig. S3). The 13-3 line, which expresses the lowest levels of the LBD18:GR transcripts among the lines tested, showed insignificant changes in both root length and lateral root number upon treatment with DEX. These findings demonstrated that the expression levels of the LBD18:GR transcripts are correlated with phenotype severity in the roots. However, when we examined the 28-9 line, which expresses lower levels of the LBD18:GR transcripts than both the 14-7 and 38-2 lines but higher levels of the transcripts than the 13-3 line, we noted statistically significant stimulations of lateral root number by various concentrations of DEX (Fig. 6E). These results indicate that appropriate levels of LBD18 expression may be required for the stimulation of lateral root formation, but higher levels of LBD18 expression could result in the inhibition of primary and lateral roots. These results also showed that LBD18 as well as LBD16 function in the nucleus to regulate lateral root formation. In addition, consistent with the observed inhibition of root growth with high levels of LBD18 expression, the hypocotyl lengths of four different lines of Pro35S:LBD18:GR transgenic plants were reduced significantly by DEX treatment (Fig. 7).
Figure 5.
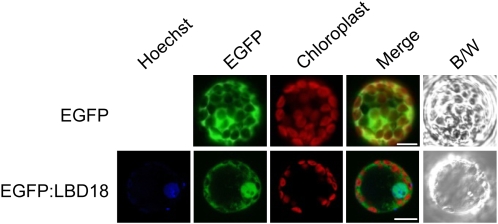
Nuclear localization of EGFP fusion proteins of LBD18. Images represent epifluorescence (EGFP), autofluorescence (chloroplast), merged, and black and white (B/W) field images of mesophyll protoplasts transfected with plasmid DNAs. Bars = 10 μm.
Figure 6.
Analysis of lateral root numbers and primary root lengths in Pro35S:LBD16:GR and Pro35S:LBD18:GR transgenic plants. A, RNA gel-blot analysis of LBD16, LBD18, LBD16:GR, and LBD18:GR expression in wild-type, Pro35S:LBD16:GR, and Pro35S:LBD18:GR transgenic plants. Numbers above the top gels indicate the line numbers of homozygous transgenic Arabidopsis. Twenty micrograms of total RNA isolated from 10-d-old light-grown seedlings was subjected to RNA gel-blot analysis using LBD16 or LBD18 DNA probes. Transcripts of LBD16 and LBD18 are indicated by a thick black arrow, and the corresponding GR fusion transcripts are indicated by the white arrow. The black triangles indicate the Arabidopsis transgenic lines used for phenotypic analysis. Total RNA from wild-type plants was isolated from plants treated for 4 h with 20 μm IAA. B, Representative seedlings of Pro35S:LBD16:GR and Pro35S:LBD18:GR treated without or with DEX. Numbers in parentheses indicate the line numbers of Arabidopsis transgenic plants. Plants were grown vertically for 8 d in the absence (−) or presence (+) of 10 μm DEX. WT, Wild type. C, Lateral root numbers of various lines of Pro35S:LBD16:GR transgenic plants without or with DEX. Plants were grown vertically for 8 d in the absence or presence of 10 μm DEX, and lateral root numbers per unit root length (cm) measured were plotted. Error bars indicate se. n > 20. Asterisks denote statistical significance at P < 0.01. D, Lateral root numbers of various lines of Pro35S:LBD18:GR transgenic plants without or with DEX. Plants were treated as described in the legend of Figure 7C. n > 20. Asterisks denote statistical significance at P < 0.05. Numbers below the columns in C and D indicate the line numbers of Arabidopsis transgenic plants. E, Lateral root numbers of line 28-9 of Pro35S:LBD18:GR transgenic plants without or with DEX. Plants were grown vertically for 8 d with varying concentrations of DEX, and lateral root numbers per unit root length (cm) measured were plotted. n > 21. [See online article for color version of this figure.]
Figure 7.
Hypocotyl lengths of Pro35S:LBD18:GR transgenic plants. A, Representative plants of 7-d-old dark-grown Pro35S:LBD18:GR plants treated without or with DEX. B, Hypocotyl lengths of 7-d-old light- or dark-grown Pro35S:LBD18:GR plants treated without or with DEX. n > 20. WT, Wild type. Error bars indicate se. Asterisks denote statistical significance at P < 0.05 (*) and P < 0.01 (**). [See online article for color version of this figure.]
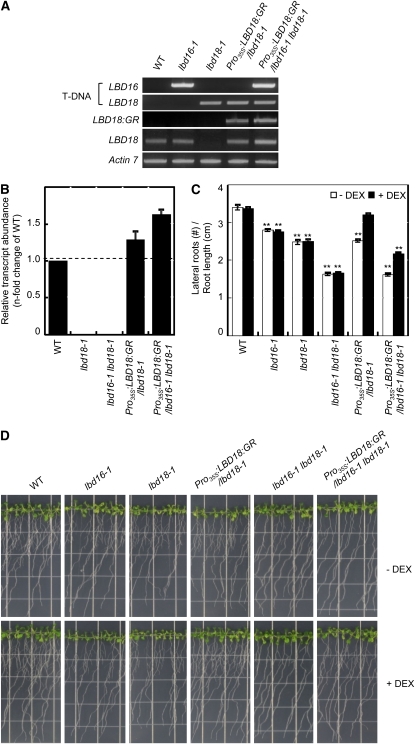
Overexpression of LBD18 Rescues Lateral Root Formation of lbd18 Single Mutants and lbd16 lbd18 Double Mutants
Although we have observed statistically significant increases in the numbers of lateral roots in Pro35S:LBD18:GR transgenic plants (the 28-9 line) with various concentrations of DEX (Fig. 6E), other lines evidenced severe root growth inhibition phenotypes. To address this mixed phenotype problem, we attempted to determine whether LBD18 could rescue the wild-type phenotype in lbd18 and lbd16 lbd18 mutants using Pro35S:LBD18:GR. Transgenic lbd18 or lbd16 lbd18 mutants expressing LBD18:GR transcripts were generated by crossing the mutants with Pro35S:LBD18:GR, as demonstrated by the T-DNA insertions and expression patterns of LBD18:GR and LBD18 (Fig. 8A). Quantitative RT-PCR analysis of the LBD18 transcripts demonstrated that the transgenic mutant plants express the LBD18:GR transcripts to a level comparable to the LBD18 transcript level of the wild-type plants (Fig. 8B). DEX treatment of Pro35S:LBD18:GR in the lbd18 mutant background resulted in a significant increase in the number of lateral roots (Fig. 8, C and D). A similar level of induction of lateral root formation was observed in the lbd16 lbd18 double mutant background. No other significant phenotypes were detected with these transgenic mutants. These results show that LBD18 is responsible for its mutant phenotype and also that LBD18:GR is functional in lateral root formation. We found that while DEX treatment of Pro35S:LBD18:GR in the lbd18 mutant background can complement lbd18 at almost a wild-type level, that of Pro35S:LBD18:GR in lbd16 lbd18 can induce lateral root formation at the same level as can be achieved by DEX treatment of Pro35S:LBD18:GR in lbd18 but cannot fully complement the lbd16 lbd18 double mutants. These findings suggest that while LBD16 and LBD18 play roles in lateral root formation, they may also perform a distinctive role in a different pathway.
Figure 8.
Complementation of lbd18 or lbd16 lbd18 by LBD18:GR. A, PCR analysis of Pro35S:LBD18:GR/lbd18-1 and Pro35S:LBD18:GR/lbd16-1 lbd18-1 transgenic mutants compared with lbd18-1 and lbd16-1 lbd18-1 mutants. Seven-day-old plants grown on Murashige and Skoog plates were harvested for genomic DNA followed by PCR analysis for T-DNA insertion, and total RNA was also isolated followed by RT-PCR analysis using the primers for the LBD18:GR fusion transcripts or the LBD18 transcripts. ACTIN7 mRNA was used as a loading control. WT, Wild type. B, Quantitative RT-PCR analysis of the LBD18 transcripts in Pro35S:LBD18:GR/lbd18-1 and Pro35S:LBD18:GR/lbd16-1 lbd18-1 transgenic mutants compared with lbd18-1 and lbd16-1 lbd18-1 mutants. Relative abundance of the LBD18 transcripts is shown compared with the wild type. C, Lateral roots of Pro35S:LBD18:GR/lbd18-1 and Pro35S:LBD18:GR/lbd16-1 lbd18-1 transgenic mutants compared with lbd18-1 and lbd16-1 lbd18-1 mutants without or with DEX. Plants were incubated and treated as described in Figure 6C, but for 12 d. Error bars indicate se. n > 24. Asterisks denote statistical significance at P < 0.01. D, Lateral roots of transgenic mutants and wild-type plants. Photographs were taken 12 d after germination. [See online article for color version of this figure.]
LBD18 Induces the Formation of Lateral Roots in arf7 arf19 Mutants
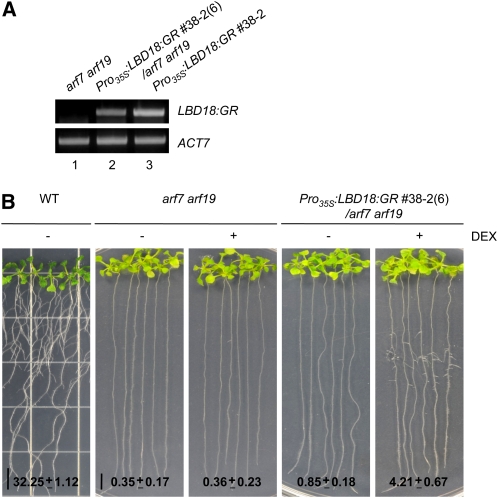
The results of our earlier study demonstrated that the auxin up-regulated expression of LBD16, LBD18, and LBD29 genes was repressed dramatically by DEX treatment and was inhibited completely by double mutations of ARF7 and ARF19, thereby indicating that these LBD genes might be regulated by ARF7 and ARF19 in auxin signaling (Lee et al., 2009). It has also been previously reported that LBD16 and LBD29 overexpression induces lateral root formation in the absence of ARF7 and ARF19 and that dominant repression of LBD16 inhibits lateral root formation (Okushima et al., 2007). To further demonstrate the positive role played by LBD18 in lateral root formation, we constructed DEX-regulated LBD18-transgenic plants in the arf7-1 arf19-1 mutant background (Pro35S:LBD18:GR/arf7 arf19) by crossing these transgenic plants and mutants. The RT-PCR analysis of the LBD18:GR transcripts revealed fewer transcripts in Pro35S:LBD18:GR/arf7 arf19 compared with Pro35S:LBD18:GR used for the construction of this transgenic mutant (Fig. 9A). As shown in Figure 9B, we noted a significant induction of lateral root numbers in Pro35S:LBD18:GR/arf7 arf19 after 12 d of DEX treatment, as compared with what was observed in the absence of DEX and in both the presence and absence of DEX in the arf7 arf19 mutants. DEX treatment of wild-type plants did not affect lateral root number (data not shown). These results reveal that LBD18 positively regulates lateral root formation downstream of ARF7 and ARF19.
Figure 9.
Induction of lateral roots in arf7 arf19 mutants by activation of LBD18:GR with DEX treatment. A, RT-PCR analysis of Pro35S:LBD18:GR/arf7-1 arf19-1 transgenic mutants compared with arf7 arf19 and Pro35S:LBD18:GR for the LBD18:GR transcripts. Seven-day-old plants grown on Murashige and Skoog plates were harvested for total RNA followed by RT-PCR analysis using the primers for the LBD18:GR fusion transcripts. ACTIN7 mRNA was used as a loading control. B, Lateral roots of Pro35S:LBD18:GR/arf7-1 arf19-1 transgenic mutants compared with arf7 arf19 without or with DEX. Plants were incubated and treated as described in Figure 6C, but for 12 d. The numbers at bottom indicate average lateral root numbers ± se. n > 18. WT, Wild type.
DISCUSSION
Recent studies have demonstrated the functions of some LBD genes in lateral organ development in Arabidopsis and in crop plants, such as rice and maize (Zea mays). For many Arabidopsis LBD genes, loss-of-function mutation phenotypes were not apparent with the exception of LBD6, which performs a role in leaf development (Semiarti et al., 2001; Xu et al., 2003). However, several loss-of-function mutants of rice and maize LBD genes have been reported to evidence clear phenotypes (Inukai et al., 2005; Liu et al., 2005; Bortiri et al., 2006; Evans, 2007; Taramino et al., 2007). The ectopic expression of several LBD genes, as wild type or as proteins fused to a transcriptional repression domain, yielded morphological phenotypes, providing clues to the biological functions of these LBD genes (Shuai et al., 2002; Chalfun-Junior et al., 2005; Borghi et al., 2007; Okushima et al., 2007). For example, the overexpression of LBD16 or LBD29 induced lateral root formation in the absence of ARF7 and ARF19, and the dominant repression of LBD16 activity inhibited lateral root formation; these findings suggest that these LBDs function downstream of ARF7- and ARF19-dependent auxin signaling in lateral root formation (Okushima et al., 2007). In this study, we demonstrated that LBD16 and LBD18 are involved in lateral root formation at the emergence step, based on the results of analysis of lbd16 and lbd18 single or double loss-of-function mutants. LBD18 overexpression using a DEX-inducible system was shown to complement lateral root formation in lbd18 as well as the lbd16 lbd18 mutants, without inducing any other phenotypes. LBD18 overexpression also induced lateral root formation in arf7 arf19 mutants with blocked lateral root formation. Collectively, these results suggest that LBD18 performs a function in lateral root formation, particularly during the initiation and emergence steps, in conjunction with LBD16 downstream of ARF7 and ARF19.
The lbd16 lbd18 double mutants exhibited substantially reduced numbers of lateral roots compared with the lbd16 and lbd18 single mutants (Fig. 2), thereby suggesting that LBD16 and LBD18 might function in a combinatorial manner in lateral root formation. Because we failed to detect a protein-protein interaction between LBD16 and LBD18 in a yeast two-hybrid assay (data not shown), LBD16 and LBD18 might function redundantly in different pathways or might require auxiliary factors for their functional interaction. Some degree of lateral root formation could still be detected in the lbd16 lbd18 double mutants, thereby indicating the existence of additional components for lateral root formation.
In the transgenic line expressing appropriate levels of the LBD18:GR transcripts in the wild-type background, a statistically significant increase in lateral root number was induced by treatment with various concentrations of DEX (Fig. 6E). In contrast, the transgenic lines expressing higher levels of the LBD18:GR transcripts exhibited significantly halted root growth, significantly decreased numbers of lateral roots, and reduced hypocotyl length (Figs. 6 and 7; Supplemental Figs. S2 and S3). These strong phenotypes may be related to the effects of ectopic tracheary element-like cells in nonvascular cells, as reported previously (Soyano et al., 2008). These results also indicate that appropriate LBD18 expression levels might be necessary for the induction of lateral root formation in wild-type plants. In order to further demonstrate that increased LBD18 expression can stimulate lateral root formation and that LBD18 is responsible for the mutant phenotype, we generated the transgenic mutants Pro35S:LBD18:GR in the lbd18 mutant background as well as in the lbd16 lbd18 mutant background that express the LBD18:GR transcripts at the wild-type level or slightly higher. We noted that DEX treatment can induce lateral root formation in both lbd18 and lbd16 lbd18 mutant backgrounds (Fig. 8, C and D), thus clearly demonstrating that LBD18 plays a role in lateral root formation. GUS expression detected in the developing lateral roots of ProLBD18:GUS seedlings (Fig. 3) is also consistent with the role of LBD18 in lateral root formation. In previous studies, it has been determined that in ProLBD16:GUS seedlings, GUS expression can be detected in the lateral root primordium (Okushima et al., 2007). We determined that while the GUS expression of ProLBD16:GUS seedlings is strong in the root stele and the developing and fully emerged lateral roots, that of ProLBD18:GUS is restricted to the primordia and the emerging lateral roots and is not detected in the lateral root stele. These GUS expression patterns suggest that LBD16 and LBD18 may have overlapping functions in the formation of lateral roots and that LBD16 may play an additional role in the continued growth of lateral roots compared with LBD18.
Lateral roots are formed from mature root pericycle cells located adjacent to the proxylem poles of the parent root (Casimiro et al., 2003). The developmental events of lateral root formation include priming, initiation, primordium development, and the emergence of lateral roots (Fukaki and Tasaka, 2009). Recent studies with Arabidopsis have shown that auxin transport and signaling are necessary for lateral root initiation and primordium development. The auxin influx carrier, AUX1-dependent basipetal auxin transport, regulates the initiation of the lateral root (De Smet et al., 2007). The AUX1-like auxin influx carrier, LAX3 (for Like AUX1), promotes lateral root emergence (Swarup et al., 2008). Multiple pin mutations induced dramatic defects in root patterning, including the development of the lateral root primordium (Benková et al., 2003), thereby suggesting that auxin efflux regulated by PIN proteins is also required for lateral root formation. Auxin signaling mediated by the Aux/IAA and ARF families of transcriptional regulators has been determined to be required for lateral root formation. Various gain-of-function mutants of Aux/IAA genes, slr-1, shy2/iaa3, msg2/iaa19, axr5/iaa1, iaa28, and crane/iaa18, yielded dramatically reduced numbers of lateral roots (Tian and Reed, 1999; Rogg et al., 2001; Fukaki et al., 2002; Tatematsu et al., 2004; Yang et al., 2004; Uehara et al., 2008). Whereas mutations in ARF19 had little effect on their own and ARF7 mutations resulted in impairments in hypocotyl phototropism, a variety of phenotypes that were not detected in these single mutants were observed in arf7 arf19 double mutants, including reduction in lateral root formation (Harper et al., 2000; Okushima et al., 2005; Wilmoth et al., 2005), suggesting that ARF7 and ARF19 regulate lateral root formation in a redundant fashion. A novel regulator of lateral root primordium development, PUCHI, which encodes AP2/EREBP, has been identified and shown to play a role in lateral root morphogenesis by altering the pattern of cell division during the early stages of primordium development (Hirota et al., 2007). It has been suggested that PUCHI functions downstream of auxin signaling.
LBD16 has been previously proposed to be involved in the initiation step of lateral root formation, based on the observation that the dominant repression of LBD16 profoundly inhibited lateral root formation (Okushima et al., 2007). Our study involving loss-of-function mutant analysis demonstrated that, while the numbers of primordia of lbd16 and lbd18 single and double mutants were similar to those of the wild-type plants, the numbers of emerged lateral roots of the single mutants were reduced significantly compared with the wild type (Fig. 4A). In the lbd16 lbd18 double mutants, additively reduced numbers of emerged lateral roots were noted as compared with the single mutants. Moreover, the numbers of primordia at stage I to stage VIII were not affected by lbd16 and lbd18 mutations (Fig. 4B). These findings reveal that LBD16 and LBD18 are likely to be involved in the initiation and emergence of lateral roots and to function additively in a different pathway. Recent study showed that GUS of ProLBD18:GUS transgenics is expressed in immature tracheary elements and that LBD18 functions in the differentiation of tracheary elements (Soyano et al., 2008). Therefore, it is possible that the phenotype of lateral root emergence in the lbd16 lbd18 mutant might be related to the vascular differentiation in the lateral root primordium.
The results of microarray analysis demonstrated that the loss of ARF7 and ARF19 function completely suppressed the expression of LBD16, LBD18, and LBD29 in response to auxin (Okushima et al., 2005). The ectopic expression of LBD16 or LBD29 in arf7 arf19 mutants induced an increase in lateral root numbers (Okushima et al., 2007). We have also determined that ectopic LBD18 expression in arf7 arf19 mutants as the result of DEX treatment induced a significant increase in lateral root numbers (Fig. 9). Thus, we propose that LBD16, LBD18, and LBD29 might act in a combinatorial fashion to regulate lateral root formation downstream of ARF7 and ARF19 during the auxin response. The results of our previous study demonstrated that the DEX-regulated expression of iaa1 with a domain II mutation resulted in a severe inhibition of lateral root formation and the suppression of LBD16 and LBD18 expression in response to auxin (Park et al., 2002; Lee et al., 2009). Reduced lateral root formation has also been noted in endogenous gain-of-function iaa1/axr5 mutants (Yang et al., 2004). The arf7 or arf19 mutation resulted in a significant suppression of auxin-mediated LBD16 expression (Lee et al., 2009). The expression of LBD18 and LBD29 in response to auxin in the arf7 or arf19 mutant was inhibited almost to basal levels (Lee et al., 2009). In the arf7 arf19 double mutants, LBD16, LBD18, and LBD29 expression was inhibited completely in response to auxin. In ProIAA1:GUS 7-d-old light-grown seedlings, we have detected strong GUS expression in various tissues, including the lateral roots (Lee et al., 2009). In ProARF7:GUS and ProARF19:GUS seedlings, GUS expression was also detected in various tissues, including the lateral roots, but the expression patterns of these two GUS fusion genes are distinct. Partial overlap in the GUS of ProARF7:GUS seedlings was detected in the root stele and in the developing lateral roots, whereas the GUS of ProARF19:GUS seedlings was shown to be expressed in the entire region of the primary and lateral roots (Okushima et al., 2005). Collectively, these results indicate that LBD16 and LBD18 might be regulated downstream of the IAA1-ARF7/ARF19 transcriptional regulator system in auxin signaling to modulate lateral root formation at the initiation and emergence steps.
MATERIALS AND METHODS
Plant Growth and Tissue Treatment
Arabidopsis (Arabidopsis thaliana) seedlings were grown under a 16-h photoperiod and treated as described previously (Park et al., 2002).
Plasmid Construction and Arabidopsis Transformation
The promoter region of LBD16 or LBD18, which encompasses 1,416 bp from −1,436 to −20 bp or 2,034 bp from −2,054 to −20 bp relative to the AUG initiation codon, respectively, was isolated with PCR primers harboring HindIII (N terminus) and XmaI (C terminus) sites for LBD16 and XbaI (N terminus) and XmaI (C terminus) sites for LBD18 using the Pfu DNA polymerase (Stratagene) from the genomic DNA of Arabidopsis Col-0. These PCR products were inserted into pGEM-T Easy vector (Promega). The DNA fragments were cut with their corresponding restriction enzymes and subcloned into pBI101 (Clontech) in place of the cauliflower mosaic virus 35S promoter. The full-length LBD16 and LBD18 coding regions were synthesized via PCR using primers harboring XbaI (N terminus) and XhoI (C terminus), then ligated into pBI-deltaGR (Lloyd et al., 1994) as translational fusions with the GR hormone-binding domain to generate the constructs Pro35S:LBD16:GR and Pro35S:LBD18:GR, respectively. Transgenic Arabidopsis plants harboring these constructs (Pro35S:LBD16:GR and Pro35S:LBD18:GR) were then generated via Agrobacterium tumefaciens-mediated transformation (Bechtold et al., 1993). T3 homozygous transformants were generated and amplified (Park et al., 2002). All constructs were confirmed via DNA sequencing prior to plant transformation. The oligonucleotides utilized for PCR and the PCR conditions are provided in Supplemental Table S1.
Isolation and Phenotypic Analysis of Arabidopsis lbd16 and lbd18 Single and lbd16 lbd18 Double T-DNA Insertion Mutants
Arabidopsis lbd16-1 (SALK_095791) and lbd18-1 (SALK_038125) T-DNA insertion mutants from the Arabidopsis Biological Resource Center (ABRC) were verified by PCR with primers designed with the T-DNA primer design program, which is available from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/). The homozygous T-DNA insertion mutant lines were isolated with the primers shown in Supplemental Table S1. Double lbd16 lbd18 mutants were generated by crossing of lbd16-1 (female) with lbd18-1 (male), and the resultant homozygous lines isolated were verified by PCR. Single-copy T-DNA in lbd16 and lbd18 was verified by backcrossing these mutants into wild-type plants and assessing the segregation of T-DNA by PCR. The null mutations of lbd16, lbd18, and lbd16 lbd18 were further verified by RT-PCR analysis. Phenotypic analysis of these T-DNA insertion mutants was conducted for root length, hypocotyl length, number of lateral roots, gravitropic response, and morphological changes, as described previously (Park et al., 2002). For the analysis of the effects of 2,4-D on the lateral root number, we used 5-d-old seedlings grown vertically 4 d after transfer to the medium containing the indicated concentrations of 2,4-D (Dharmasiri et al., 2005).
Generation of Pro35S:LBD18:GR Transgenic Arabidopsis in lbd18, lbd16 lbd18, or arf7 arf19 Mutant Backgrounds
Transgenic mutant Pro35S:LBD18:GR/lbd18 or Pro35S:LBD18:GR/lbd16 lbd18 was generated by crossing lbd18-1 or lbd16-1 lbd18-1 (female) with Pro35S:LBD18:GR (male). Homozygous lines were isolated on the basis of genotyping and the PCR detection of genomic DNA for the LBD18:GR transgene. Expression levels of the LBD18:GR transcripts in Arabidopsis overexpressing Pro35S:LBD18:GR in lbd18-1 or lbd16-1 lbd18-1 mutant were determined by RT-PCR analysis.
arf7-1 arf19-1 mutants were acquired from the ABRC and confirmed by genotyping (http://signal.salk.edu) and lack of lateral root phenotype (Okushima et al., 2005). Arabidopsis overexpressing Pro35S:LBD18:GR in arf7-1 arf19-1 mutants was generated by crossing arf7-1 arf19-1 (female) with Pro35S:LBD18:GR (male). Homozygous lines were isolated according to genotype and lack of the lateral root phenotype for arf7-1 arf19-1 and also by PCR detection of genomic DNA for the LBD18:GR transgene. Expression levels of the LBD18:GR transcripts in Arabidopsis overexpressing Pro35S:LBD18:GR in arf7-1 arf19-1 mutants were determined by RT-PCR analysis. The oligonucleotides and PCR conditions utilized are provided in Supplemental Table S1.
RNA Isolation, RNA Gel-Blot Analysis, RT-PCR, and Real-Time RT-PCR
Following treatment, Arabidopsis plants were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated from frozen Arabidopsis using TRI Reagent (Molecular Research Center). Total RNA was separated on a 1.2% agarose gel, transferred to nylon membranes, hybridized for 3 h with 32P-labeled DNA probes at 68°C using 10 mL of QuickHyb solution (Stratagene), and then washed. The blots were subsequently exposed to x-ray film. The DNA probes for RNA gel-blot analysis were RT-PCR amplified, subcloned into pGEM-T Easy vector (Promega), and then confirmed by DNA sequencing. For RT-PCR analysis, total RNA was isolated using an RNeasy Plant Mini kit (Qiagen) and subjected to RT-PCR analysis with an Access RT-PCR System (Promega) according to the manufacturer's instructions. Real-time RT-PCR was conducted with the QuantiTect SYBR Green RT-PCR kit (Qiagen) in a Rotor-Gene 2000 real-time thermal cycling system (Corbett Research). In order to determine the copy number of the transcripts in the treated sample, real-time PCR was performed on each sample with a known quantity of the in vitro transcribed RNA (Promega), yielding specific threshold values (Ct). A standard curve was generated to demonstrate the linear correlation between log(copy numbers of the RNA) and the Ct. The copy numbers of the transcripts of unknown samples were subsequently calculated from this standard curve. RT-PCR conditions, primer sequences, and DNA probes used in this study are provided in Supplemental Table S1.
Nuclear Localization of EGFP:LBD18 Fusion Proteins in Arabidopsis Protoplasts
LBD18 full-length DNA was PCR amplified using Pfu DNA polymerase (Stratagene) and inserted into the Pro35S:EGFP vector (Lee et al., 2008) at the XhoI sites as a translational fusion, thereby yielding the Pro35S:EGFP:LBD18 DNA construct. PCR-amplified DNA sequences were used for subcloning after verification by DNA sequencing. These plasmids were purified using a Qiagen Plasmid Midi kit prior to protoplast transformation. The protoplasts from Arabidopsis mesophyll cells were prepared as described previously (Lee et al., 2008). Protoplasts isolated from the rosette leaves of 2- to 3-week-old Arabidopsis plants grown under a 16-h photoperiod were transfected with plasmid DNA by polyethylene glycol-mediated protoplast transformation and incubated for 18 h in the light at room temperature. The nuclear localization of the GFP fusion proteins was monitored by capturing GFP images with a TCS SP5 AOBS spectral confocal and multiphoton microscope system (Leica Microsystems). Confocal images of the GFP fusion proteins were acquired at the Korea Basic Science Institute. Argon (488 nm) and helium/neon (633 nm) lasers were utilized for GFP excitation and autofluorescence of chlorophyll excitation, respectively. Hoechst 33342 (Sigma) was utilized at 12 ng mL−1 to stain the nuclei of the protoplasts. The protoplasts were incubated with Hoechst 33342 for 15 min at room temperature in darkness and rinsed three times in culture medium, and the excess buffer was drained and replaced with fresh culture medium.
Statistical Analysis
Quantitative data on the phenotypes, such as measurements of root length, number of lateral roots, and hypocotyl length, were subjected to statistical analysis for every pair-wise comparison using the software for Student's t test (Predictive Analytics Software for Windows version 17.0).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Dose-response curves for root elongation of lbd16, lbd18, and lbd16 lbd18 mutants on IAA.
Supplemental Figure S2. Lateral roots and primary root lengths of Pro35S:LBD16:GR and Pro35S:LBD18:GR transgenic plants.
Supplemental Figure S3. Root lengths of Pro35S:LBD18:GR transgenic plants in the presence of varying concentrations of DEX.
Supplemental Table S1. Oligonucleotides used for PCR and PCR conditions.
Supplementary Material
Acknowledgments
We thank the ABRC for the T-DNA insertion mutants utilized in this study.
This work was supported by the Plant Diversity Research Center of the 21st Century Frontier Research Program (grant no. PF06302–01), by the Agricultural Plant Stress Research Center (grant no. R11–2001–092–04001–0), and by the World Class University project funded by the Ministry of Education, Science and Technology (grant no. R31–2009–000–20025–0) to J.K.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jungmook Kim (jungmkim@chonnam.ac.kr).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci (Paris) 316 1194–1199 [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Borghi L, Bureau M, Simon R (2007) Arabidopsis JAGGED LATERAL ORGANS is expressed in boundaries and coordinates KNOX and PIN activity. Plant Cell 19 1795–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, Hake S (2006) ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8 165–171 [DOI] [PubMed] [Google Scholar]
- Chalfun-Junior A, Franken J, Mes JJ, Marsch-Martinez N, Pereira A, Angenent GC (2005) ASYMMETRIC LEAVES2-LIKE1 gene, a member of the AS2/LOB family, controls proximal-distal patterning in Arabidopsis petals. Plant Mol Biol 57 559–575 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frey NF, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134 681–690 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jurgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9 109–119 [DOI] [PubMed] [Google Scholar]
- Evans MM (2007) The indeterminate gametophyte1 gene of maize encodes a LOB domain protein required for embryo sac and leaf development. Plant Cell 19 46–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29 153–168 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69 437–449 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A, Kato T, Fukaki H, Aida M, Tasaka M (2007) The auxin-regulated AP2/EREBP gene PUCHI is required for morphogenesis in the early lateral root primordium of Arabidopsis. Plant Cell 19 2156–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands A, Bell EM, Shuai B, Smith HM, Springer PS (2007) LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res 35 6663–6671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H, Hasebe M, Soma T, Ikezaki M, Machida C, et al (2002) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol 43 467–478 [DOI] [PubMed] [Google Scholar]
- Ku SJ, Park JY, Ha SB, Kim J (2009) Overexpression of IAA1 with domain II mutation impairs cell elongation and cell division in inflorescences and leaves of Arabidopsis. J Plant Physiol 166 548–553 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Kim S, Ha YM, Kim J (2008) Phosphorylation of Arabidopsis response regulator 7 (ARR7) at the putative phospho-accepting site is required for ARR7 to act as a negative regulator of cytokinin signaling. Planta 227 577–587 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Park JW, Lee HW, Kim J (2009) Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J Exp Bot 60 3935–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43 47–56 [DOI] [PubMed] [Google Scholar]
- Lloyd AM, Schena M, Walbot V, Davis RW (1994) Epidermal cell fate determination in Arabidopsis: patterns defined by a steroid-inducible regulator. Science 266 436–439 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124 33–44 [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Iwakawa H, Machida Y, Machida C (2009) Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J 58 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17 444–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kim HJ, Kim J (2002) Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J 32 669–683 [DOI] [PubMed] [Google Scholar]
- Rogg LE, Lasswell J, Bartel B (2001) A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 13 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiarti E, Ueno Y, Tsukaya H, Iwakawa H, Machida C, Machida Y (2001) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128 1771–1783 [DOI] [PubMed] [Google Scholar]
- Shuai B, Reynaga-Pena CG, Springer PS (2002) The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol 129 747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Thitamadee S, Machida Y, Chua NH (2008) ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis. Plant Cell 20 3359–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10 946–954 [DOI] [PubMed] [Google Scholar]
- Taramino G, Sauer M, Stauffer JL Jr, Multani D, Niu X, Sakai H, Hochholdinger F (2007) The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J 50 649–659 [DOI] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Reed JW (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126 711–721 [DOI] [PubMed] [Google Scholar]
- Uehara T, Okushima Y, Mimura T, Tasaka M, Fukaki H (2008) Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant Cell Physiol 49 1025–1038 [DOI] [PubMed] [Google Scholar]
- Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43 118–130 [DOI] [PubMed] [Google Scholar]
- Xu L, Xu Y, Dong A, Sun Y, Pi L, Huang H (2003) Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130 4097–4107 [DOI] [PubMed] [Google Scholar]
- Yang X, Lee S, So JH, Dharmasiri S, Dharmasiri N, Ge L, Jensen C, Hangarter R, Hobbie L, Estelle M (2004) The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J 40 772–782 [DOI] [PubMed] [Google Scholar]
- Yang Y, Yu X, Wu P (2006) Comparison and evolution analysis of two rice subspecies LATERAL ORGAN BOUNDARIES domain gene family and their evolutionary characterization from Arabidopsis. Mol Phylogenet Evol 39 248–262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.