Abstract
In eukaryotes, alternative splicing of pre-mRNAs contributes significantly to the proper expression of the genome. However, the functions of many auxiliary spliceosomal proteins are still unknown. Here, we functionally characterized plant homologues of nematode suppressors of mec-8 and unc-52 (smu). We compared transcript profiles of maize (Zea mays) smu2 endosperm with those of wild-type plants and identified pre-mRNA splicing events that depend on the maize SMU2 protein. Consistent with a conserved role of plant SMU-2 homologues, Arabidopsis (Arabidopsis thaliana) smu2 mutants also show altered splicing of similar target pre-mRNAs. The Atsmu2 mutants occasionally show developmental phenotypes, including abnormal cotyledon numbers and higher seed weights. We identified AtSMU1 as one of the SMU2-interacting proteins, and Atsmu1 mutations cause similar developmental phenotypes with higher penetrance than Atsmu2. The AtSMU2 and AtSMU1 proteins are localized to the nucleus and highly prevalent in actively dividing tissues. Taken together, our data indicated that the plant SMU-1 and SMU-2 homologues appear to be involved in splicing of specific pre-mRNAs that affect multiple aspects of development.
Pre-mRNA splicing is an essential process where the spliceosome removes introns from pre-mRNAs, and it can lead to more than one splice variant (SV), resulting in the production of different proteins. Alternative splicing has been found to be an important regulatory process in global gene expression, since the splicing pattern of a pre-mRNA can vary in different cell types and at different developmental stages as well as in response to environmental cues (Black, 2003). A model of combinatorial control for pre-mRNA splicing was proposed to explain the apparent complexity of splicing, as the protein complex controlling splice site selection consists of positive- and negative-acting factors (e.g. Ser/Arg-rich [SR] proteins and heterogeneous nuclear ribonucleoproteins [hnRNPs], respectively, that have synergistic and antagonistic interactions; Smith and Valcarcel, 2000; Black, 2003). The balance between splicing factors determines the pattern of pre-mRNA splicing, but tissue-specific regulators appear to be crucial for determining alternative splicing patterns between tissues.
The spliceosome consists of a group of small nuclear RNAs (snRNAs) and proteins that are recruited to pre-mRNAs to remove introns (Jurica and Moore, 2003). Recent proteomic analyses of human spliceosomal complexes (Neubauer et al., 1998; Hartmuth et al., 2002; Jurica et al., 2002; Makarov et al., 2002; Rappsilber et al., 2002; Zhou et al., 2002; Bessonov et al., 2008) revealed nearly 300 putative spliceosomal proteins. Only a fraction of these proteins constitute the catalytic core components for pre-mRNA splicing, while the others are believed to be auxiliary factors. Some of these auxiliary proteins appear to be sequence-specific splicing factors (i.e. recruited to certain sets of pre-mRNAs) or their associated proteins and provide communication links between splicing and other processes in pre-mRNA and mRNA metabolism, such as transcription, capping, and 3′ end formation (Jurica and Moore, 2003). However, the biochemical functions of many such auxiliary factors still remain unknown.
Mechanisms of pre-mRNA splicing in plants are assumed to be similar to those in animals, as the consensus sequences around 5′ and 3′ splice sites and branch sites are similar in plants and animals (Brown and Simpson, 1998) and components of animal spliceosomes are conserved in plant genomes (Reddy, 2001). Likewise, precise control of splice site selection during alternative splicing appears to be common and essential in all higher eukaryotes, including plants. The percentage of Arabidopsis (Arabidopsis thaliana) genes with alternative splicing is currently estimated to be about 22% (Wang and Brendel, 2006), a value much smaller than is found for humans. But the Arabidopsis genome contains at least 19 genes for SR proteins (Reddy, 2004), almost twice the number of known human SR proteins (Graveley, 2000). SR proteins and some spliceosomal proteins in plants appear to form a functional complex through their physical interactions (Reddy, 2004).
Despite conserved cis-elements and trans-acting factors, the precise function of plant splicing factors is poorly defined. This is partly because an in vitro splicing assay using plant cell extracts is currently unavailable, and a surprisingly small number of mutations in genes encoding putative plant spliceosomal proteins, such as the supersensitive to ABA and drought1 (sad1) and stabilized1-1 (sta1-1) mutants of Arabidopsis (Xiong et al., 2001; Lee et al., 2006), have been reported. SAD1 and STA1 encode proteins similar to a human Sm-like snRNP and a human U5 snRNP-associated protein, respectively. Both sad1 and sta1-1 manifest altered stress responses as well as morphological defects and reduced growth, indicating that these mutations have pleiotropic effects on plant development. Similarly, developmental defects were obvious in transgenic plants where the production of SR proteins was deregulated. Overexpression of Arabidopsis SR proteins led to pleiotropic effects on developmental programs and influenced splice site selection among SVs encoding the SR proteins themselves as well as other splicing factors (Lopato et al., 1999; Kalyna et al., 2003). More recently, it was found that SR proteins in rice (Oryza sativa), when overexpressed, also affect alternative splicing of their own transcripts and those of other SR protein genes (Isshiki et al., 2006).
We isolated an RNA-splicing maize (Zea mays) mutant with a variety of phenotypes, including a reduced level of seed storage proteins and defective development of embryos and flowers (Chung et al., 2007). The mutation results from insertion of a transposable element in the first exon of a gene encoding maize ZmSMU2, a homologue of nematode SMU-2 (Spartz et al., 2004), and a human spliceosomal protein (Neubauer et al., 1998; Makarov et al., 2002; Rappsilber et al., 2002; Zhou et al., 2002). Mutations in the nematode smu-2 gene are associated with altered splicing for unc-52 pre-mRNA, supporting a role for SMU-2 in pre-mRNA splicing (Spartz et al., 2004). Nevertheless, it is not clear how SMU-2 homologues regulate pre-mRNA splicing. The human SMU-2 homologue does not appear to be a core component of the spliceosome, since it was not uniformly detected in different spliceosomal complexes (Jurica and Moore, 2003; Bessonov et al., 2008). Therefore, it is likely that SMU-2 homologues are auxiliary factors of spliceosomes. Since SMU-2 homologues lack any known RNA-binding domain, these proteins may participate in pre-mRNA splicing by interacting with other spliceosomal proteins.
Based on the sequence homology and pleiotropic nature of the maize mutant, we proposed that ZmSmu2 plays a role in pre-mRNA splicing. One important prediction from this hypothesis is that one should observe altered splicing of pre-mRNAs in Zmsmu2 mutants compared with wild-type splicing patterns. Here, we report data supporting this hypothesis. Transcript profiling and reverse transcription (RT)-PCR experiments revealed several potential pre-mRNA targets for ZmSMU2. We further examined the role of other plant SMU-2 homologues using Arabidopsis. We characterized the AtSMU2 gene and obtained additional evidence supporting the role of SMU-2 homologues in pre-mRNA splicing. We found interactions of ZmSMU2 and AtSMU2 with proteins that imply a link with pre-mRNA splicing and other mRNA metabolic processes. We identified an interaction of AtSMU2 with AtSMU1, the homologue of the nematode SMU-1 protein that was previously shown to interact with SMU-2 (Spartz et al., 2004). Our data suggest that SMU-l and SMU-2 homologues cooperate with other proteins to regulate the splicing of specific pre-mRNAs.
RESULTS
Altered Pre-mRNA Splicing in Zmsmu2 Mutant Endosperms
To investigate the molecular mechanisms that account for the phenotypes of Zmsmu2-1 endosperms (Chung et al., 2007), microarray hybridization of RNA from homozygous wild-type and Zmsmu2-1 endosperms was performed (Supplemental Protocol S1; Supplemental Table S1; Supplemental Fig. S1). Data from this experiment can be summarized as three major observations (for full description, see Supplemental Protocol S1). First, many genes encoding rRNA processing factors and ribosomal proteins are up-regulated in the mutant (Supplemental Figs. S1F and S2), which appears to result from a feedback mechanism that compensates for defective rRNA processing and inefficient protein synthesis in Zmsmu2 mutant endosperm (Chung et al., 2007). Second, genes involved in the biosynthesis of seed storage proteins are mostly down-regulated in the mutant (Supplemental Figs. S1F and S2), which also explains how the level of seed storage proteins is reduced in the mutant endosperm (Chung et al., 2007). Lastly, it was revealed that many probes with differential gene expression values in the mutant were derived from intronic and alternative exonic sequences (Supplemental Fig. S1E). Furthermore, about 8% of the probes with significantly lower gene expression values in the mutant were estimated to hybridize with specific SVs, while only 4% of randomly chosen probes appeared to be SV specific (Supplemental Tables S2–S4; Supplemental Protocol S1). These results strongly suggested altered splicing in Zmsmu2-1 endosperm.
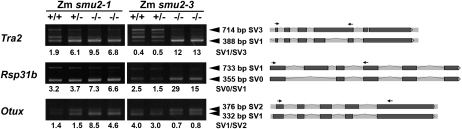
We conducted RT-PCR analysis of selected gene transcripts to compare pre-mRNA splicing in wild-type, Zmsmu2-1, and Zmsmu2-3 endosperms. By an RT-PCR experiment designed to identify SVs, we confirmed at least three pre-mRNAs, SVs of which were differentially accumulated in wild-type and Zmsmu2-1 endosperm in the microarray experiment. Gene models corresponding to these SVs were manually constructed, based on EST evidence, similarity to conserved protein sequences, and intronic sequence consensus, as described in Supplemental Protocol S1 and Supplemental Figure S1A. These three genes encode TRA2 (an SR-like protein similar to Drosophila Transformer2 [Amrein et al., 1988], encoded by GRMZM2G073567 and found on the bacterial artificial chromosome [BAC] clone AC187469.3 at location 124,155–127,755), RSp31B (an SR protein [Gupta et al., 2005], encoded by GRMZM2G021272 and found on the BAC clone AC204569.4 at location 146,139–148,813), and OTUX (an OTU-like protease [Makarova et al., 2000], encoded by GRMZM2G047838 and found on the BAC clone AC232266.1 at location 71,091–75,367). RT-PCR analysis of transcripts for these genes revealed one or more SVs that are preferentially increased or decreased in Zmsmu2-1 endosperm compared with the wild type (Fig. 1, left panels labeled Tra2, Rsp31b, and Otux), and the relative abundance of these SVs was also found to be affected by the Zmsmu2-3 mutation (Fig. 1, right panels). For example, the ratio of Tra2 SV1 to SV3 is higher in Zmsmu2-1 endosperm than in wild-type or heterozygous siblings (Fig. 1, left panel labeled Tra2). Similarly, the ratio of Rsp31b SV0 to SV1 is higher in Zmsmu2-1 than in wild-type or heterozygous siblings (Fig. 1, left panel labeled Rsp31b). The altered splicing pattern of Tra2 and Rsp31b pre-mRNAs in Zmsmu2-3 is very similar to that in Zmsmu2-1 (Fig. 1). In contrast, the splicing pattern of Otux pre-mRNA in Zmsmu2-3 endosperm is opposite to that in Zmsmu2-1: the ratio of Otux SV1 to SV2 is lower in Zmsmu2-3 than in the wild type or a heterozygote, but the ratio is higher in Zmsmu2-1 than in wild-type or heterozygous sibling endosperms (Fig. 1).
Figure 1.
Altered RNA splicing in Zmsmu2-1 and Zmsmu2-3 endosperm. Heterozygous (smu2-1/+ or smu2-3/+) plants were self-pollinated, and kernels were dissected into embryo and endosperm tissues at 16 d after pollination. Embryo DNA was used to genotype the Zmsmu2 locus, and RNA was extracted from homozygous wild-type, heterozygous, and homozygous mutant endosperms and used for semiquantitative RT-PCR. +/+, Endosperm with a homozygous wild-type embryo; +/−, endosperm with a heterozygous embryo; −/−, endosperm with a homozygous smu2-1 mutant embryo. Arrowheads indicate SVs with different band intensities between the wild type and mutants. Pre-mRNA splicing patterns of SVs are shown at right, with RT-PCR primers indicated by arrows. Ratios of differentially accumulated SVs are shown below the panels.
Taken together, the microarray and RT-PCR analyses clearly demonstrated that ZmSMU2 affects alternative splicing of specific pre-mRNAs in developing endosperm, supporting the proposed role of ZmSMU2.
Characterization of smu2 Mutants in Arabidopsis
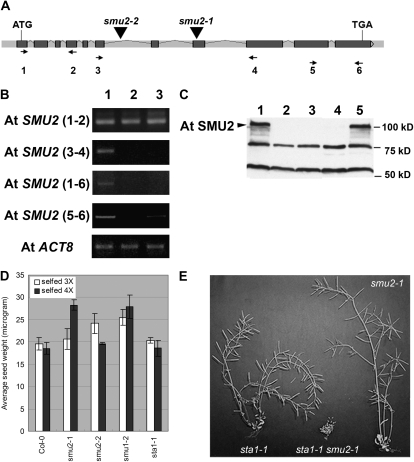
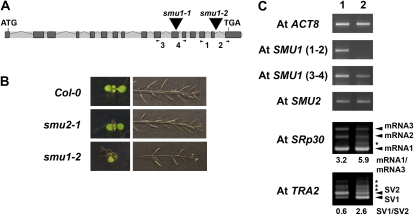
To functionally analyze the Arabidopsis homologue of ZmSMU2, we searched the SIGnAL T-DNA Express database (http://signal.salk.edu/cgi-bin/tdnaexpress; Alonso et al., 2003) for mutants with T-DNA insertions in the AtSMU2 gene (At2g26460). Two putative mutants were identified and designated Atsmu2-1 and Atsmu2-2, respectively. Analysis of the T-DNA-flanking sequences revealed that the Atsmu2-1 T-DNA was in the eighth exon of the gene (Fig. 2A). In Atsmu2-2, a T-DNA insert was confirmed in the sixth intron of the gene (Fig. 2A). To investigate the effect of these mutations on AtSMU2 gene expression, RT-PCR analysis of RNA from seedlings was performed using different combinations of primers (for the locations of the primers, see Fig. 2A). The Atsmu2-1 (Fig. 2B, lane 2) and Atsmu2-2 (Fig. 2B, lane 3) homozygous seedlings did not show significant differences in RNA levels corresponding to the 5′ portion of the AtSMU2 coding sequence [Fig. 2B, AtSMU2 (1–2)] as compared with wild-type seedlings (Fig. 2B, lane 1). However, when primers flanking the T-DNA insertion sites were used, SMU2 transcripts were not detected in the mutants [Fig. 2B, AtSMU2 (3–4)], indicating that the T-DNA insertions disrupted proper transcription, pre-mRNA splicing, or RNA stability. Thus, Atsmu2-1 and Atsmu2-2 homozygous mutant plants are unlikely to produce a wild-type AtSMU2 protein. However, RT-PCR analysis using primers downstream to the T-DNA insertion sites indicated a low level of detectable RNA in the Atsmu2-2 mutant plants [Fig. 2B, AtSMU2 (5–6)]. This suggests that the T-DNA insertion in Atsmu2-2 may create a downstream, cryptic transcription start site that can lead to the production of a partial AtSMU2 mRNA.
Figure 2.
A, Structure of the AtSMU2 gene. Dark gray boxes and light gray bars between the boxes represent exons and introns, respectively. Triangles indicate T-DNA insertion sites in the Atsmu2-1 and Atsmu2-2 mutants. B, RT-PCR analysis of AtSMU2 transcripts. Four pairs of primers were used for amplifying different regions of the AtSMU2 mRNA sequence prepared from wild-type 14-DAG Col-0 (lane 1), smu2-1 (lane 2), and smu2-2 (lane 3) seedlings. Numbers in parentheses indicate the primers used for RT-PCR, and their positions are shown as arrows in A. AtACT8 transcript encoding an actin was used as an internal control. C, Immunoblot analysis of AtSMU2 using protein extracts from four 7-DAG Col-0 (lane 1), smu2-1 (lane 2), smu2-2 (lane 3), smu1-2 (lane 4), and sta1-1 (lane 5) seedlings as described by Chung et al. (2007). The arrowhead indicates the AtSMU2 protein. D, Phenotypic analyses of various mutants. Homozygous mutant plants (smu2-1, smu2-2, smu1-2, and sta1-1) were identified among segregating populations and were subsequently self-pollinated two or three times. Phenotypic analysis was conducted as described in “Materials and Methods.” E, Dry sta1-1 (left), smu2-1 sta1-1 (middle), and smu2-1 (right) plants.
To determine whether the mutant plants lacked a full-length AtSMU2 protein, an immunoblot analysis of extracts from wild-type and Atsmu2-1 and Atsmu2-2 mutant plants was conducted. We previously reported that an AtSMU2 protein with a glutathione-S-transferase (GST) tag showed strong cross-reaction with an antiserum prepared against ZmSMU2 (Chung et al., 2007). Of the three prominent bands detected in wild-type Arabidopsis seedlings, the largest band corresponding to a 100-kD protein was absent in the mutant extracts (Fig. 2C), indicating that it corresponds to the AtSMU2 protein. Furthermore, this result showed that Atsmu2-1 and Atsmu2-2 mutant plants do not produce a full-length AtSMU2 protein, suggesting that the two alleles are null for AtSMU2 function.
In order to understand the function of the AtSMU2 gene during development, we analyzed the phenotypes of the smu2-1 and smu2-2 plants. The first generation homozygous mutant seedlings appeared to grow slower than wild-type seedlings, although their overall architecture was indistinguishable from that of wild-type plants (data not shown). On the other hand, we observed the appearance of abnormal phenotypes after further selfing of the mutant alleles. For example, the weight of seeds produced in the F4 populations (self-pollinated three times) of the smu2-1 plants was not significantly different from that of the wild-type seeds, while that of the F5 populations (self-pollinated four times) was considerably greater (Fig. 2D). Homozygous smu2-2 F4 seeds were significantly heavier than wild-type seeds (Fig. 2D), although the F5 seeds were similar in weight to wild-type seeds (Fig. 2D). In addition, smu2-1 and smu2-2 plants produced a low frequency of abnormal seedlings with one, three, or more cotyledons in some F3 and F4 populations. For example, 0.4% of smu2-1 F4 seedlings (n = 744) showed abnormal cotyledon number as compared with no abnormal seedlings (n = 1,443) observed from wild-type ecotype Columbia (Col-0) plants (Table I). However, these phenotypes were not completely penetrant and varied depending on the individual parental plants tested (effectively preventing a genetic complementation of the mutant). Taken together, the genetic evidence indicates that the AtSMU2 gene may play a subtle role in seed development under certain conditions.
Table I.
Arabidopsis mutant phenotypes in seedling development
Values are presented as means ± sd.
| Genotype | Generation | Abnormal Cotyledon Numbera | Germination Failure or Arrested Growtha |
|---|---|---|---|
| % | % | ||
| Col-0 | F4 | 0 ± 0 | 0.2 ± 0.3 |
| F5 | 0 ± 0 | 0.1 ± 0.2 | |
| smu2-1 | F4 | 0.4 ± 0.5 | 0.8 ± 1.0 |
| F5 | 0 ± 0 | 0.1 ± 0.3 | |
| smu2-2 | F4 | 0.3 ± 0.4 | 0.6 ± 0.4 |
| F5 | 0.2 ± 0.3 | 0.8 ± 0.7 | |
| smu1-2 | F4 | 45.8 ± 4.8 | 13.9 ± 8.6 |
| F5 | 10.0 ± 2.0 | 10.5 ± 8.8 | |
| SMU1Bar/_; smu1-2 | T2 | 0.4 ± 0.5 | 0 ± 0 |
| SMU1-GFPBar/_; smu1-2 | T2 | 2.3 ± 1.4 | 0.3 ± 0.8 |
| sta1-1 | F4 | 2.8 ± 1.0 | 0.5 ± 0.7 |
| F5 |
0.3 ± 0.3 |
0.2 ± 0.3 |
Phenotypes were scored as described in “Materials and Methods.”
Genetic Interaction between Atsmu2 and sta1
To investigate any role for SMU2 in pre-mRNA splicing in Arabidopsis, we created double-mutant combinations between Atsmu2 alleles and a mutation in the STA1 gene, which encodes a conserved protein with high similarity to the human U5 snRNP-associated 102-kD protein and the yeast proteins PRP1p and Prp6p (Lee et al., 2006). The sta1-1 mutant used here is likely a partial loss-of-function allele, because it contains a two-codon deletion in the coding region. Homozygous sta1-1 plants exhibit mild developmental defects, including delayed growth and serrated leaf margins (Lee et al., 2006). We observed that although the size of the mutant plants was typically smaller than that of wild-type plants, they often showed more shoots per plant and produced a comparable number of seeds as wild-type plants (Fig. 2E). The weight of the seeds obtained from sta1-1 mutants was not significantly different from that of wild-type plants (Fig. 2D). The sta1-1 mutant plants also showed several additional phenotypes, including an abnormal number of cotyledons (n = 984; Table I).
No double mutants were identified among 114 F2 plants analyzed from a cross of homozygous Atsmu2-1 and sta1-1 plants. Further genotyping of 52 F3 plants that were homozygous for smu2-1 and segregating for sta1-1 and STA1 resulted in the identification of four smu2-1 sta1-1 double mutant plants, three of which produced three cotyledons while one produced two cotyledons of different sizes. Three of the double mutant plants died before flowering, but one with three cotyledons developed numerous small leaves and short inflorescences bearing only one or two short siliques (Fig. 2E) with one or two seeds per silique. None of the 15 seeds obtained from this plant germinated. Similar results were obtained using the smu2-2 allele in combination with sta1-1. No double mutant plants were obtained from F2 plants segregating for smu2-2 and sta1-1, while three double mutants were identified from F3 populations. All three smu2-2 sta1-1 double mutant seedlings ceased to grow at the cotyledon stage or before flowering. Plants that are either homozygous for sta1-1 and heterozygous for smu2-2 or heterozygous for sta1-1 and homozygous for smu2-2 produced partially filled siliques, suggesting that most of the double mutants are aborted in early seed development. Thus, compared with the single mutant parental plants (Fig. 2E), the smu2 sta1 double mutant plants exhibited a more severe set of developmental defects. Together with the observation that smu2 single mutants are phenotypically distinct from sta1-1, this genetic interaction indicated that AtSMU2 has at least some nonoverlapping functions with STA1.
The Atsmu2 Mutations Cause Pre-mRNA Splicing Defects
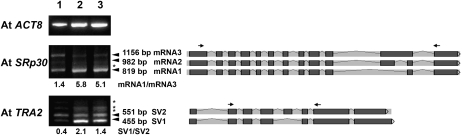
To investigate whether AtSMU2 functions in pre-mRNA splicing, we analyzed Atsmu2 mutant seedlings for genes previously known to produce SVs. Using RT-PCR, we detected three previously described SVs (mRNA1, -2, and -3) for the At1g09140 (AtSRp30) gene (Lopato et al., 1999) in both wild-type and mutant plants (Fig. 3). However, the ratio of mRNA1 to mRNA3 was higher in the two mutants than in wild-type seedlings (Fig. 3). Furthermore, an additional SV candidate was detected specifically in the mutant seedlings (Fig. 3, asterisks). Analysis of other genes known to undergo alternative splicing, however, did not indicate any differential accumulation of their SVs in the mutant seedlings (data not shown), suggesting that only a certain number of alternative splicing events were affected by the Atsmu2-1 mutation.
Figure 3.
RT-PCR detection of SVs in Atsmu2-1 and Atsmu2-2. RT-PCR was performed using total RNA from 14-DAG Col-0 (lane 1), smu2-1 (lane 2), and smu2-2 (lane 3) seedlings. Arrowheads indicate the positions of known SVs of At1g09140 (AtSRp30) and At1g07350 (AtTRA2), while asterisks correspond to putative SVs first observed here. Pre-mRNA splicing patterns are shown on the right side, with dark gray boxes and light gray bars between the boxes representing exons and introns, respectively. Arrows depict primers used in each reaction. Ratios of differentially accumulated SVs are shown below the panels.
Interestingly, AtSRp30 encodes a protein showing sequence similarity to ZmRSp31B. Based on this, we speculated that Zmsmu2-dependent alternative splicing events might be evolutionarily conserved if they are functionally significant for the two species. A search of the Arabidopsis protein sequence database identified At5g04250 (OTUX-like) and At1g07350 (AtTRA2) as putative homologues of OTUX and TRA2, respectively. Analysis of the AtTRA2 mRNA from wild-type plants indicated the presence of four SVs (Fig. 3), two of which (SV1 and -2) were previously annotated in The Arabidopsis Information Resource (http://www.arabidopsis.org). As shown in Figure 3, the accumulation of SV1 relative to SV2 was elevated in Atsmu2-1 and Atsmu2-2 seedlings as compared with wild-type seedlings. These data indicate that the Atsmu2 mutations affect alternative splicing of specific pre-mRNAs. Thus, AtSMU2 may function as a pre-mRNA splicing factor, similar to the role played by its homologues in nematodes and maize.
Expression of AtSMU2 during Development
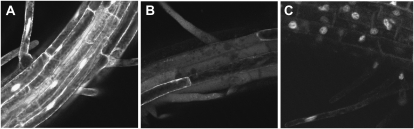
Involvement of AtSMU2 in pre-mRNA splicing would suggest that the protein is localized to the nucleus. To test this, we generated transgenic Arabidopsis plants that produced an AtSMU2-GFP fusion product under the control of a modified cauliflower mosaic virus 35S gene promoter/enhancer sequence. As shown in Figure 4C, the AtSMU2-GFP fusion protein was localized exclusively to the root cell nuclei. In contrast, a nonconjugated GFP protein control was detected in both cytoplasm and the nucleus (Fig. 4A) and a GUS-GFP fusion protein control was detected exclusively in the cytoplasm (Fig. 4B). Therefore, the nuclear localization of AtSMU2 corresponds to its putative function in pre-mRNA splicing.
Figure 4.
Subcellular localization of AtSMU2 fusion protein. A, Arabidopsis root cells overexpressing GFP showed a diffused localization pattern in the cytosol and the nuclei. B, GUS-GFP in Arabidopsis root cells was exclusively localized in the cytosol. C, Arabidopsis root cells overexpressing AtSMU2-GFP exhibited nucleus-specific localization.
Immunoblot analysis of ZmSMU2 in various maize tissues indicated the highest levels of expression in rapidly developing tissues or organs, such as the developing endosperm, embryo, shoot apex, and flower (Chung et al., 2007). To identify the highest levels of protein accumulation for AtSMU2 during development, we extracted proteins from various Arabidopsis organs and performed immunoblot analysis using anti-ZmSMU2 antibodies. As shown in Figure 5, the highest levels of AtSMU2 protein was detected in seedlings at 7 d after germination (DAG; lane 1), young flowers before anthesis (lane 6), and developing siliques less than 5 mm in length (lane 8). Lower levels of AtSMU2 accumulation were detected in roots (lane 2), expanding cauline leaves (lane 4), open flowers (lane 7), and dry seeds (lane 9), while the inflorescence (lane 5) contained only a trace amount of the protein, which was only detectable when the immunoblot was overexposed. No protein was detected in senescing rosette leaves (lane 3). Thus, the AtSMU2 protein, like ZmSMU2 (Chung et al., 2007), appears to be present throughout the plant, although its abundance varied, with the highest level of accumulation in mitotically active tissues and organs.
Figure 5.
Immunoblot analysis of AtSMU2 showing accumulation of AtSMU2 protein in various organs and structures of Arabidopsis. Each lane contained approximately 10 μg of proteins from reproductive and vegetative organs of Col-0 plants. Lane 1, Seedlings at 7 DAG; lane 2, roots of 30-DAG plants; lane 3, senescing rosette leaves; lane 4, young cauline leaves; lane 5, inflorescence; lane 6, immature flowers; lane 7, open flowers; lane 8, developing siliques (less than 5 mm in length); lane 9, dry seeds.
ZmSMU2 and AtSMU2 Interact with AtSMU1
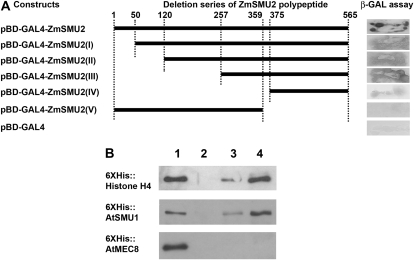
To gain additional insight into the molecular functions of plant SMU-2 homologues, we used the yeast two-hybrid (Y2H) system to identify proteins that interacted with ZmSMU2. Transformants containing a GAL4 binding domain fused to a full-length ZmSMU2 protein exhibited self-activation (Fig. 6A). Removal of the N terminus (residues 1–374, construct IV) or C terminus (residues 360–565, construct V) substantially reduced the self-activation (Fig. 6A), allowing us to create a fusion protein bait to screen two maize libraries and one Arabidopsis library. In a screen of a maize endosperm library using construct IV as a bait, six colonies were obtained, all of which encoded a full-length histone H4 cDNA. To expand the repertoire of ZmSMU2-interacting proteins, we used construct V as a bait to screen a cDNA library prepared from young maize ears. This analysis resulted in the identification of six ZmSMU2-interacting proteins represented by 38 clones (Table II, clone identifiers starting with Zm), including an SMU-1-like protein containing WD40 repeats (Spartz et al., 2004). Three additional putative ZmSMU2-interacting proteins were identified from a screen of an Arabidopsis library of 3-DAG seedlings using construct V as a bait (Table II, clone identifiers starting with At).
Figure 6.
Interactions of ZmSMU2 and AtSMU2 with other proteins. A, Y2H bait constructs were tested for self-activation and the corresponding β-GAL assays. A construct containing the full-length ZmSMU2 protein (pBD-GAL4-ZmSMU2; top) exhibited strong self-activation of the β-GAL reporter gene when introduced into Y190 cells. N-terminal deletions of ZmSMU2 (bait constructs I–IV) also showed self-activation, albeit at lower levels than for the full-length protein. Only a C-terminal deletion (bait construct V) and a negative control (pBD-GAL4; bottom) did not activate the reporter gene. Black bars represent the ZmSMU2 polypeptides. Numbers above the bars indicate amino acid residues corresponding to the full-length ZmSMU2 polypeptide. B, Confirmation of Y2H screen by in vitro pull-down assay using GST or GST fusion protein as a bait. GST (lane 2), GST-AtSMU2 (lane 3), or GST-ZmSMU2 (lane 4) bound to glutathione-agarose beads was used to pull down 6XHis-histone H4, 6XHis-AtSMU1, or 6XHis-AtMEC8. Lane 1, Ten percent input; lanes 2, 3, and 4, 30% pull-down by GST, GST-AtSMU2, and GST-ZmSMU2, respectively.
Table II.
Summary of a Y2H screen for proteins interacting with ZmSMU2
| Clone Identifier | TopBLASTNa | Percentage Identityb | Predicted Proteinc |
|---|---|---|---|
| Zm6-1 | DV495718.1 | 100 | Rasputin-like protein (Pazman et al., 2000) |
| Zm8-1 | BU051217.1 | 98 | Histone H4 |
| Zm17-3 | DN226990.1 | 97 | SMU-1-like protein (Spike et al., 2001) |
| Zm31-7 | DN218694.1 | 96 | VOZ-like protein (Mitsuda et al., 2004) |
| Zm33-8 | CA404370.1 | 97 | EF-1β |
| Zm59-20 | DT654009.1 | 96 | Glycerol kinase-like protein |
| At1-1 | NM_103009.3 | 97 | TAFII250-like HAF01, partial (Bertrand et al., 2005) |
| At2-3 | NM_118947.2 | 98 | TPR protein, partial |
| At3-3 |
NM_117736.2 |
99 |
DUF751-containing protein |
GenBank accession numbers with the highest nucleotide sequence similarity to the Y2H clone shown in the left column.
The percentage of nucleotide sequence identity based on BLASTN analysis.
Based on the BLASTX result for the TopBLASTN sequences.
We primarily focused on the interaction of AtSMU2 and ZmSMU2 with the SMU-1-like protein, because previous findings indicated that SMU-1 and SMU-2 homologues are likely partners in the formation of a functional spliceosome complex. The Caenorhabditis elegans SMU-1, which also contains WD40 repeat motifs, interacts with SMU-2 (Spartz et al., 2004), and the human SMU1 and SMU2 proteins were found within human spliceosomal complexes (Jurica and Moore, 2003). Therefore, we used in vitro pull-down assays to confirm the Y2H interactions indicated above. As shown in Figure 6B, the GST-AtSMU2 and GST-ZmSMU2 recombinant proteins when used as baits were capable of pulling down His-tagged AtSMU2 and histone H4 proteins in vitro. No interaction was detected when GST alone was used to pull down the target proteins (Fig. 6B, lane 2). In addition, a His-tagged AtMEC8 used as a control also did not show interaction with the GST-AtSMU2 and GST-ZmSMU2 proteins. The AtMEC8 protein is an Arabidopsis counterpart of the nematode MEC-8 that contains two RNA recognition motifs and has been shown to regulate alternative splicing of the unc-52 transcripts (Lundquist et al., 1996). Taken together, these data indicate that the AtSMU2 and ZmSMU2 proteins interact with SMU1, a highly conserved component of the eukaryotic spliceosome.
Expression of AtSMU1 during Development
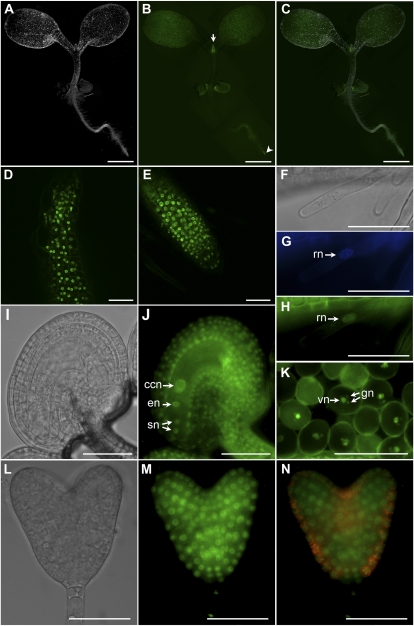
To examine the expression of AtSMU1 during development, we generated a GFP fusion construct under the control of the endogenous promoter (AtSMU1-GFPKan) and introduced it into Arabidopsis plants. The AtSMU1-GFP fusion protein was detected mainly in actively dividing cells, such as the newly emerged leaf and the root tip of seedlings (Fig. 7, A–C). A close examination of these structures using confocal microscopy revealed that the fusion protein was likely nucleus localized (Fig. 7, D and E). This was further confirmed in the root hair cells using colocalization of 4′,6-diamino-phenylindole (DAPI) and GFP signals (Fig. 7, F–H). GFP expression was detected during embryogenesis including the heart stage of embryo development (Fig. 7, L–N). The AtSMU1-GFP fusion product was also detected in the nuclei of all cell types within the female gametophyte, including egg cell, central cell, synergid cells (Fig. 7J), and the antipodal cells (data not shown). Within the mature pollen, GFP signal was localized to both vegetative and generative nuclei (Fig. 7K). These results indicate that the subcellular localization and distribution of AtSMU1 protein are similar to those of AtSMU2.
Figure 7.
Expression of SMU1-GFPkan during plant development. A to C, Dark-field, epifluorescence GFP, and merged images of a kanamycin-resistant seedling. The arrow points to a newly emerged leaf. The arrowhead points to the root tip. D, Confocal image of the area indicated by the arrow in B. E, Confocal image of the area indicated by the arrowhead in B. F to H, Bright-field, epifluorescence DAPI, and GFP images of a root hair. I and J, Bright-field and epifluorescence GFP images of a flower stage 13 ovule. K, Epifluorescence GFP image of mature pollen. L to N, Bright-field, epifluorescence GFP, and fluorescein isothiocyanate images of a heart-stage embryo. ccn, Central cell nucleus; en, egg cell nucleus; gn, generative nuclei; rn, root hair nucleus; sn, synergid nuclei; vn, vegetative nucleus. Bars = 1 mm in A to C and 50 μm in D to N.
Molecular and Genetic Characterization of AtSMU1
Identification of ZmSMU1 and AtSMU1 proteins as interacting partners for ZmSMU2 and AtSMU2 prompted us to investigate the phenotype of smu1 loss-of-function alleles. Like AtSMU2, SMU1 appeared to be a single-copy gene in the Arabidopsis genome. We identified three putative T-DNA insertional mutant alleles in the AtSMU1 gene (At1g73720) and designated them smu1-1 (SALK_123852), smu1-2 (SALK_051163), and smu1-3 (SAIL_95_E04). Sequencing of the genomic DNA flanking these insertions revealed that the T-DNAs in the smu1-1 and smu1-2 alleles were located within exon 11 and intron 15, respectively (Fig. 8A). By contrast, the smu1-3 allele contained a T-DNA insertion in the promoter region. Analysis of the individual segregants from selfing populations of heterozygous smu1-1/+, smu1-2/+, or smu1-3/+ plants indicated that AtSMU1 may be required for plant viability. Homozygous smu1-1 seedlings could not be maintained under normal growth conditions, as they were extremely dwarfed (1–5 mm in diameter), developed many small leaves, and did not produce any flowers (data not shown). This suggested that in comparison with the smu1-2 allele (see below), smu1-1 is likely a complete loss-of-function allele. On the other hand, homozygous smu1-2 plants were generally more viable and reached the flowering stage despite exhibiting a syndrome of developmental abnormalities including (but not limited to) abnormal cotyledon number (Table I; Fig. 8B), increased seed weight (Fig. 2D), abnormal flower positions (node spacing; Fig. 8B), and seed germination defects (Table I). However, some of these phenotypes were associated with a reduced penetrance that varied significantly from generation to generation (Table I). The reduced severity of the phenotypes observed in smu1-2 plants was consistent with the intronic T-DNA insertion site in this allele, indicating that smu1-2 is a weaker allele than smu1-1. Finally, in contrast to the smu1-1 and smu1-2 alleles, smu1-3 did not exhibit any phenotypic changes, indicating that the upstream T-DNA insertion in this allele did not affect AtSMU1 gene expression.
Figure 8.
Analysis of Atsmu1 mutant alleles. A, Structure of the AtSMU1 gene and the T-DNA insertions in the Atsmu1-1 and Atsmu1-2 mutants (triangles). B, Phenotypes of Col-0 wild-type, Atsmu2-1 (F4), and Atsmu1-2 (F3) plants. Seedlings of smu1-2 at 7 DAG often showed an abnormal number of cotyledons (left panels; see Table I) and arrangement of flowers/siliques in the primary inflorescence (right panels). C, RT-PCR comparison of AtSMU2, AtSMU1, AtSRp30, and AtTRA2 transcripts in Col-0 and smu1-2. Two pairs of primers were used to amplify different regions of the AtSMU1 cDNA prepared from 14-DAG seedlings of Col-0 (lane 1) and smu1-2 (lane 2) plants. Numbers in parentheses indicate the primers used for the RT-PCR; the positions of the primers are shown as arrowheads in A. Arrowheads indicate the positions of known SVs of SRp30 and TRA2, while asterisks correspond to the potentially novel SVs. Ratios of differentially accumulated SVs are shown below the panels. [See online article for color version of this figure.]
To see if the smu1-2 phenotypes are caused by T-DNA insertion in the corresponding gene, we performed complementation of the mutant with the wild-type AtSMU1 gene. In addition to construct AtSMU1-GFPKan, we also generated construct AtSMU1-GFPBar and AtSMU1Bar and transformed smu1-2 heterozygous plants. For construct AtSMU1-GFPBar, we obtained 41 T1 transformants, and five of them were homozygous for the smu1-2 mutant allele. The GFP expression pattern of AtSMU1-GFPBar was similar to that of AtSMU1-GFPKan. For construct AtSMU1Bar, we obtained 21 T1 transformants, and seven of them were homozygous for the smu1-2 mutant allele. The T1 transformants homozygous for smu1-2 germinated and grew normally, developed two cotyledons, and showed normal node spacing in the inflorescence. The T2 populations segregating for the transgenes displayed close to normal germination rates and cotyledon morphology (Table I). This phenotypic rescue by the transgenes clearly demonstrates that the Atsmu1-2 phenotypes are caused by the T-DNA insertion in the AtSMU1 gene.
To confirm that the T-DNA insertion in smu1-2 affected the expression of AtSMU1, we used RT-PCR to amplify AtSMU1 RNA in wild-type and mutant plants. As shown in Figure 8C, the RT-PCR product obtained with AtSMU1-specific primers indicated that the transcript in smu1-2 seedlings lacked the 3′ terminal region of the coding sequence. The RT-PCR product with another pair of AtSMU1-specific primers [Fig. 8C, AtSMU1 (3–4)] showed that the mutant seedlings accumulated reduced levels of AtSMU1 transcript compared with the wild type. The smu1-2 mutation did not appear to affect AtSMU2 mRNA levels (Fig. 8C, AtSMU2). Nevertheless, AtSMU2 protein was not detected in smu1-2 mutant extract (Fig. 2C, lane 4), whereas its level in the sta1-1 mutant was comparable to that of the wild type (Fig. 2C, lanes 1 and 5). These data indicate that the expression of AtSMU1 is required for proper accumulation of the AtSMU2 protein in the cell.
Protein interaction studies (see above) indicated that AtSMU1 may function in pre-mRNA splicing in a complex with AtSMU2. Therefore, we predicted that the smu1-2 mutant would manifest altered splicing of AtTRA2 and SRp30 pre-mRNAs, as was observed in Atsmu2-1 and Atsmu2-2 mutant plants. As shown in Figure 8C, the relative abundance of AtTRA2 and SRp30 SVs in smu1-2 resembled those seen in smu2-1 and smu2-2 mutant plants (Fig. 3). These data indicate that AtSMU1 and AtSMU2 are involved in the alternative splicing of common pre-mRNAs.
DISCUSSION
We hypothesized that ZmSMU2 is a pre-mRNA splicing factor (Chung et al., 2007), and data presented in this paper provide additional evidence supporting this hypothesis. We found an evolutionarily conserved protein interaction between SMU-1 and SMU-2 homologues (Fig. 6) and nuclear localization of AtSMU2-GFP (Fig. 4) and AtSMU1-GFP (Fig. 7). Intriguingly, Zmsmu2, Atsmu2, and Atsmu1 mutants showed an altered pattern of pre-mRNA splicing (Figs. 1, 3, and 8). It is known that overexpression of splicing regulators in plants and animals, including SR proteins, often leads to altered splicing of their own RNAs and that of other splicing regulators (Bell et al., 1991; Jumaa and Nielsen, 1997; Lopato et al., 1999; Kalyna et al., 2003; Isshiki et al., 2006). In this regard, the altered splicing patterns of Rsp31b and Tra2 in Zmsmu2-1 and Zmsmu2-3 endosperm (Fig. 1) are particularly interesting, because these genes encode an SR and an SR-related protein, respectively. The altered splicing that occurs when splicing regulators are overexpressed may be due to an autoregulatory mechanism of the splicing regulatory network. This mechanism, although not understood, is being actively investigated (Wollerton et al., 2004; Kumar and Lopez, 2005). Altered splicing of Tra2 and Rsp31b pre-mRNAs in Zmsmu2-1 could also be a secondary consequence of the Zmsmu2-1 and Zmsmu2-3 mutations (i.e. Tra2 and Rsp31b might not normally be primary targets of the ZmSMU2 protein). Consistent with this, the microarray data indicated that expression of genes encoding splicing regulators was down- or up-regulated in Zmsmu2-1 endosperm (Supplemental Table S1). Among these putative splicing regulators are an SRp75-like protein, a KH domain-containing protein, a TIA-1-like oligouridylate-binding protein, and a hnRNP A2/B1-like protein (Supplemental Table S1).
In contrast to SVs for Tra2 and Rsp31b genes, Zmsmu2-1 and Zmsmu2-3 endosperms showed opposite effects on the splicing pattern in Otux pre-mRNA (Fig. 1). Importantly, the ZmSMU2 protein level is higher in smu2-1 mutant endosperm than in its wild-type sibling, while it is lower in smu2-3 than in the corresponding wild type (Chung et al., 2007). Therefore, if the action of ZmSMU2 as a splicing factor follows a model of combinatorial control for pre-mRNA splicing (Smith and Valcarcel, 2000; Black, 2003), Otux pre-mRNA may represent a true target of the ZmSMU2 splicing factor, since its splicing depends on the concentration of ZmSMU2 protein.
Our genetic data also indicate that AtSMU1 and AtSMU2 function in a similar pathway to control pre-mRNA splicing in Arabidopsis. Similar to Zmsmu2 mutants, Atsmu2-1, Atsmu2-2, and Atsmu1-2 showed alterations in AtTRA2 and SRp30 splicing patterns (Figs. 3 and 8C). Consistent with the proposed role of the plant SMU-2 homologues in pre-mRNA splicing, the Zmsmu2, Atsmu2, and Atsmu1 mutants are all pleiotropic. The Zmsmu2-1 mutant embryos often produce twin shoots and roots (Chung et al., 2007). Similarly, the Atsmu1-2 mutation is associated with an abnormal number of cotyledons and irregular inflorescence architecture (Fig. 8), indicating an abnormal meristematic function. Multiple developmental phenotypes are also associated with Atsmu2 mutations, albeit low in penetrance. In addition, the Atsmu1 and Atsmu2 mutants showed similar phenotypes, including increased seed weight (Fig. 2D) and abnormal cotyledon number (Table I). Increased seed weight was not associated with a mutation in the STA1 gene, which presumably encodes a core spliceosomal protein, although sta1-1 mutant seedlings have similar defects in germination and cotyledon development. Furthermore, Atsmu2 sta1 double mutants showed very severe developmental defects (Fig. 2E), suggesting that the targets of STA1 may not overlap with those of the AtSMU2 gene in the main.
It remains to be seen what pre-mRNA targets for plant SMU-2 homologues are responsible for the developmental phenotypes of Zmsmu and Atsmu mutants, although our microarray analysis of Zmsmu2 endosperm suggested extensive changes in the expression of genes controlling the biosynthesis and perception of plant hormones (Supplemental Table S1). Notably, an abnormal number of cotyledons and twin embryos were also caused by ectopic expression of AtRSZ33, which encodes an Arabidopsis SR protein (Kalyna et al., 2003). Spartz et al. (2004) proposed that SMU-2 may act similarly to SR-related proteins, which also lack RNA-binding motifs. In fact, we observed several features shared by ZmSMU2 and SR proteins. First, Zmsmu2 mutations are associated with altered splicing patterns in multiple pre-mRNAs, including those encoding SR proteins (Fig. 1). This effect has been observed in plant and animal cells overexpressing SR proteins (Mizzen et al., 1996; Jumaa and Nielsen, 1997; Lopato et al., 1999; Kalyna et al., 2003; Kumar and Lopez, 2005). In addition, expression of Arabidopsis SR protein genes appeared to be tissue specific and particularly high in pollen and in actively dividing cells (Lopato et al., 1999, 2002; Kalyna et al., 2003). This pattern of expression is very similar to that observed in AtSMU2 and AtSMU1 genes (Figs. 5 and 7).
Our study of protein interactions with ZmSMU2 and AtSMU2 suggests that plant SMU-2 homologues work in a protein complex to regulate not only pre-mRNA splicing but also other aspects of mRNA metabolism. No ZmSMU2-interacting proteins, except ZmSMU1, have human homologues that were previously identified as spliceosomal proteins or are known to function in pre-mRNA splicing (Table II). However, this does not mean that the observed protein interactions conflict with the proposed function of ZmSMU2 in pre-mRNA splicing. Rather, these interactions suggest that ZmSMU2 could also serve as a link to related processes in mRNA metabolism, such as transcription initiation and chromatin modification. Splicing factors, especially SR proteins, were shown to interact with the preinitiation complex, transcription elongation factors, 5′ and 3′ processing factors, and the mRNA export complex (Maniatis and Reed, 2002). In this regard, it is not surprising that ZmSMU2-interacting proteins include proteins that are found in preinitiation complexes. For instance, a Y2H screen for ZmSMU2-interacting proteins identified a partial HAF01 clone (Bertrand et al., 2005) showing a high degree of sequence identity to human TAFII250, the largest polypeptide in the TFIID complex. Notably, animal homologues of TAFII250 can acetylate histone H4, another ZmSMU2-interacting protein (Mizzen et al., 1996), although it is still unclear whether histone H4 is a genuine in vivo substrate for the histone acetyltransferase activity of TAFII250. One intriguing possibility is that SMU2 may mediate coupling of pre-mRNA splicing with chromatin modification. For example, SMU2 could recruit a chromatin modification complex to an alternative exon. Alternatively, SMU2 could help recognize a specific histone code during pre-mRNA splicing and affect alternative splicing of transcripts from genes with a particular chromatin modification (e.g. preferentially include/exclude introns containing a repetitive sequence).
In addition to interacting with a histone or a histone modification factor, ZmSMU2 might also interact with sequence-specific transcription factors in vivo. One of the ZmSMU2-interacting proteins identified in our Y2H screen encodes a protein containing a zinc-coordinating motif and a conserved basic region with high sequence similarity to the Arabidopsis sequence-specific transcription factors AtVOZ1 and AtVOZ2 (Mitsuda et al., 2004). Taken together, protein interactions identified from the Y2H screen suggest a role for ZmSMU2 in connecting pre-mRNA splicing and transcription initiation.
More recently, a biochemical role for animal and plant SMU-1 homologues has been proposed. The human SMU-1 homologue was identified from immunopurified CUL4B-DDB1 ubiquitin ligase complexes (Higa et al., 2006), along with other proteins with a similar type of WD40 motif or DWD (DDB1 binding WD40) motif. These DWD proteins were found to be specific substrate receptors for the corresponding ubiquitin ligase complex. In addition, a subset of Arabidopsis and rice proteins containing a DWD motif was shown to interact with DDB1 in vitro and in vivo (Lee et al., 2008), although an interaction for AtSMU1 was not tested in this study. Considering the highly conserved interactions of animal and plant DWD proteins with DDB1, plant SMU-1 homologues are most likely involved in the recognition of specific target proteins for ubiquitination. If the proposed CUL4-DDB1-SMU1 ubiquitin ligase complex is involved in proteasome-dependent proteolysis, what can be the target? Probably it is not AtSMU2, since AtSMU2 is unstable in smu1-2 rather than stabilized (Fig. 2C). Alternatively, the CUL4-DDB1-SMU1 complex may be involved in a nonproteolytic function. No matter what biochemical activity SMU-1 homologues have, their target proteins for ubiquitination are most likely to include spliceosomal proteins.
Comparison of the C. elegans smu-1 and smu-2 mutants, the Atsmu1 and Atsmu2 mutants, and the Zmsmu2 mutant indicates that SMU orthologues vary in terms of their dispensability. In the nematode, smu-1 and smu-2 null mutants are viable and do not show a highly penetrant phenotype (Spike et al., 2001; Spartz et al., 2004), indicating that both loci are dispensable. Genetic interaction between smu-1 and smu-2 was also found in C. elegans, where these two loci were originally identified from a genetic screen for recessive, loss-of-function suppressors of mec-8 unc-52 synthetic lethality (Lundquist and Herman, 1994). In the nematode, smu-1 smu-2 double mutants were phenotypically indistinguishable from the single mutants (Spartz et al., 2004). In Arabidopsis, smu2 null mutants are viable, while a strong smu1-1 allele is lethal, and a weak smu1-2 allele has several developmental defects. In contrast, the maize smu2 mutants, which are not null, show severe developmental defects and are sterile (Chung et al., 2007), indicating that Zmsmu2 is an essential gene. Three models could explain the variation among the three species. First, although AtSMU2 and SMU-2 are encoded by single-copy genes, the Arabidopsis and C. elegans genomes may encode genes that function in a partially redundant manner to the SMU-2 homologues. Second, although the amino acid sequences of SMU-1 and SMU-2 homologues are highly conserved, these proteins may have diverged to gain additional functions in various eukaryotic organisms. Third, the SMU-1 and SMU-2 homologues may have a similar biochemical role, but their target pre-mRNAs (and proteins if SMU-1 homologues are involved in ubiquitination of target proteins) may differ among various species.
In the last model, ZmSMU2-dependent pre-mRNA targets would be critical for viability, while AtSMU2- and SMU-2-dependent targets may not be as critical for cellular functions to cause early developmental defects. Alternatively, ZmSMU2 may be required for processing of a larger set of pre-mRNA targets than AtSMU2 and SMU-2, and this could result from differences in the respective genomes. For example, the average size of Arabidopsis, rice, and maize introns is estimated to be 167, 413, and 607 bp, respectively (Haberer et al., 2005). This variation in average intron size is probably due to the frequency of transposon insertions in each genome (Haberer et al., 2005). The higher frequency of transposon-related sequences in maize introns could be responsible for the indispensability of ZmSMU2. Consistent with this, transcript profiling of Zmsmu2-1 endosperm revealed several transposon-like sequences that were differentially expressed in wild-type and smu2-1 mutant endosperm (Supplemental Table S1). In this regard, it would be interesting to know if ZmSmu2 is the endosperm splicing regulator that was proposed to be responsible for differential splicing of waxy pre-mRNA for the wxG allele, which contains a retrotransposon in an intron (Marillonnet and Wessler, 1997). There have been several reports in maize describing similar alternative splicing events that were triggered by insertion of different transposon types, and at least some of the alternative splicing events were found to be tissue specific (Ortiz and Strommer, 1990; Marillonnet and Wessler, 1997; Lal and Hannah, 1999).
MATERIALS AND METHODS
Arabidopsis Mutants
The T-DNA insertional Arabidopsis (Arabidopsis thaliana) mutants smu2-1 (stock identifier SALK_039202), smu2-2 (stock identifier WiscDsLox320H09), smu1-1 (stock identifier SALK_123852), smu1-2 (stock identifier SALK_051163), and smu1-3 (stock identifier SAIL_95_E04) were obtained from the Arabidopsis Biological Resource Center. The ethyl methanesulfonate mutant sta1-1 (Lee et al., 2006) was obtained from Dr. Jian-Kang Zhu (University of California, Riverside). smu2-1 and smu2-2 were crossed to the Col-0 ecotype twice and smu1-2 was crossed once before any phenotypic analyses were carried out. Homozygous mutant plants were identified using PCR analysis of genomic DNA isolated from individual plants (Edwards et al., 1991) with a combination of the following primers: for the AtSMU2-1 allele, 2646Ex7_F_In7 (5′-CCGGTGCCCGAGGTATGTATACTT-3′) and AtMTO_R2 (5′-CTTGGCTCCAAACTGTAAGACCAG-3′); for Atsmu2-1, 2646Ex7_F_In7 and T-DNA_LB_b1 (5′-GCGTGGACCGCTTGCTGCAACT-3′); for AtSMU2-2, AtMTO_F3 (5′-CCAGTGGATTGTTAAGCCTCAGAC-3′) and 2646In7_R_Ex7 (5′-AAGTATACATACCTCGGGCACCGGGCAG-3′); for Atsmu2-2, AtMTO_F3 and pDS-Lox_LB (5′-AACGTCCGCAATGTGTTATTAAGTTGTC-3′); for AtSMU1-2, either AtSMU-1_F3 (5′-AGAGTTTCTCATCCGGTAATAGGGAAG-3′) and AtSMU-1_R1 (5′-TCAGGGCTTCCATAACTTCATAGTG-3′) or SMU-4f (5′-TAAATTTCCAGCAACGGTCTCC-3′) and atsmu1-2r (5′-GACATGGCAATAGTCTGGCTAGTACA-3′); for Atsmu1-2, either AtSMU-1_F3 and T-DNA_LB_b1 or SMU-4f and LBa1 (5′-TGGTTCACGTAGTGGGCCATCG-3′); for STA1-1, AtPRP6_R2 (5′-CCAACTCTCTCACATGCCTCAGCC-3′) and AtPRP6_F2 (5′-AGGGGCTGCGAAGAGTGCCCG-3′); and for sta1-1, AtPRP6_R2 and AtPRP6_F2hc (5′-AGAGGGGCTGCGAAGAGAAAAAT-3′).
Characterizations of Mutant Phenotypes
To measure seed weights, four to six plants of the F3 or F4 generation were self-pollinated. All of the F4 plants analyzed were obtained from one of the F3 plants. At least 200 F4 or F5 seeds harvested from each self-pollinated plant were weighed and counted to calculate average seed weights. Seeds were surface sterilized, placed on solid medium (0.5× Murashige and Skoog salts, 1% [w/v] Suc, and 0.7% [w/v] phytoagar), and stored at 4°C for 3 d. Percentage of germination failure or arrested growth, and the number of cotyledons, were analyzed at 7 DAG.
Reporter Constructs and Plant Transformation
For GFP, GUS-GFP, and AtSMU2-GFP fusion proteins, the cDNA for GFP was amplified by PCR using primers NcoI_gfpF (5′-CTCACCATGGTGAGCAAGGGCGA-3′) and BamHI_gfpR (5′-TAGGATCCTTACTTGTACAGCTCGTC-3′), and the DNA product was digested with NcoI and BamHI and introduced into pRTL2 (Carrington et al., 1990). pRTL2 contains two tandem repeats of the cauliflower mosaic virus 35S promoter and the tobacco etch virus leader sequence upstream of multiple cloning sites. The recombinant expression cassette was transferred into the HindIII site of the binary vector pBIN19 (Bevan, 1984), and the resulting plasmid, pBIN19-35Sp-GFP, was used to transform Arabidopsis Col-0 gl1 using the floral dip method (Clough and Bent, 1998). To create transgenic Arabidopsis plants expressing GUS-GFP, a GUS cDNA was amplified with primers NcoI_gusF (5′-TCTACCATGGTAGATCTGACTAG-3′) and gusRgfp (5′-TTGCTCACCATGCTAGCTTGTTTGCCTC-3′), and the cDNA for GFP was amplified with primers gusFgfp (5′-AACAAGCTAGCATGGTGAGCAAGGGCGA-3′) and BamHI_gfpR. A second PCR was performed using NcoI_gusF and BamHI_gfpR to create the fusion product, GUS-GFP. For a transgenic plant overexpressing AtSMU2-GFP, the AtSMU2 cDNA was amplified with primers F+60BspHI (5′-TTTCATGAAACCTTCAAAATCGCATC-3′) and 2646Rgfp (5′-TTGCTCACCATATGCTTGGATCTCTTAG-3′), and the cDNA for GFP was amplified with primers 2646Fgfp (5′-GATCCAAGCATATGGTGAGCAAGGGCGA-3′) and BamHI_gfpR. A second PCR was performed using F+60BspHI and BamHI_gfpR to make the fusion product, AtSMU2-GFP. The fusion PCR products, GUS-GFP and AtSMU2-GFP, were cloned into the NcoI and BamHI sites of pRTL2, and the expression cassettes were transferred into the HindIII site of a binary vector, pBIN19. The resulting plasmids, pBIN19-35Sp-GUS-GFP and pBIN19-35Sp-AtSMU2-GFP, were used to transform Arabidopsis gl1. Kanamycin-resistant seedlings were rescued, and images of GFP expressed in roots of the T2 plants at 5 DAG were collected on a Bio-Rad MRC 1024 confocal laser scanning microscope and processed with NIH Image software (http://rsb.info.nih.gov/nih-image/) and Adobe Photoshop 6.0 or CS.
For the AtSMU1-GFP translational fusion, a 7.4-kb fragment containing 2.7 kb of 5′ flanking sequence and the coding region of SMU1 was amplified from Col-0 genomic DNA by Phusion polymerase (Finnzymes) with primers SUM1(−2700)Hind-f (5′-GCTGAAGCTTGGGACATTGAGGCAGC-3′) and SUM1-BamStop-r (5′-GAAGGATCCGGGCTTCCATAACTTCATA-3′), restriction digested with HindIII and BamHI, and cloned into the pBN-GFP vector (Wang et al., 2006), resulting in construct pBN-SMU1-GFP (SMU1-GFPKan). The HindIII/EcoRI fragment containing SMU1-GFP and the nopaline synthase polyadenylation sequence (NOS-ter) was subcloned from pBN-SMU1-GFP into pGPTV-BAR vector (Becker et al., 1992), resulting in construct pGPTV-SMU1-GFP (SMU1-GFPBar).
For complementation of the Atsmu1-2 mutant, a 7.8-kb fragment containing 2.7 kb of 5′ flanking sequence, the coding region, and 433 bp of 3′ flanking sequence of SMU1 was amplified from Col-0 genomic DNA by Phusion polymerase with primer SUM1(−2700)Hind-f (5′-GCTGAAGCTTGGGACATTGAGGCAGC-3′) and SUM1-Bam-r (5′-CTGCGGATCCCGTTGTAATGGTCTCTA-3′), restriction digested with HindIII and BamHI, and cloned into a modified pBI101 vector where the NOS-ter and GUS coding regions were replaced with a synthetic linker (5′-GAATTCGAGCTCGGTACCCGGGGATCC-3′), resulting in construct pBI-SMU1 (SMU1Kan). The HindIII/EcoRI fragment containing SMU1 and NOS-ter was subcloned from pBI-SMU1 into pGPTV-BAR vector (Becker et al., 1992), resulting in construct pGPTV-SMU1 (SMU1Bar).
The standard flower dip method (Clough and Bent, 1998) was used to transform Arabidopsis ecotype Col-0 or smu1-2/+ plants with Agrobacterium tumefaciens strain GV3101 pMP90 (Koncz and Schell, 1986) containing appropriate constructs. T1 transformants were selected on Murashige and Skoog medium (Gibco BRL Life Technologies) containing 35 μg mL−1 kanamycin or 50 μg mL−1 glufosinate ammonium (Crescent Chemical). Kanamycin- or BASTA-resistant plants were transferred to soil, and the presence of the transgene was confirmed by PCR.
Confocal images of the AtSMU1-GFP plants were obtained as described above. Bright-field and epifluorescence images were obtained using a Zeiss Axiophot compound epifluorescence microscope (Carl Zeiss) equipped with a GFP band-pass filter (exciter, 450–490; dichroic, 495; emitter, 500–550 [Chroma Technology]), a fluorescein isothiocyanate long-pass filter (exciter, 450–490; dichroic, 510; emitter, 515 [Carl Zeiss]), a DAPI long-pass filter (exciter, 365; dichroic, 395; emitter, 420 [Carl Zeiss]), and an Optronics MicroFire CCD camera.
Analysis of RNA and Protein
Arabidopsis plants were grown under a 16/8-h light/dark cycle at 20°C. RNA samples were prepared from whole Arabidopsis seedlings grown on solid medium at 14 DAG and roots, leaves, and flowers of Arabidopsis plants grown on soil at 30 DAG.
RNA was isolated using Trizol according to the manufacturer's instructions (Invitrogen). Total RNA was used in a reverse transcription reaction with SuperScript II (Invitrogen) and oligo(dT) primers to generate first-strand cDNA, which was subsequently used to amplify specific cDNAs using standard techniques. Primers for PCR were as follows: for AtACT8, AtACT8_F (5′-TAAACTAAAGAGACATCGTTTCCA-3′) and AtACT8_R (5′-TTTTTATCCGAGTTTGAAGAGGCT-3′); for the 5′ coding sequence of AtSMU2, AtSMU2_1 (5′-ATGAAACCTTCAAAATCGCATCACAAG-3′) and AtSMU2_2 (5′-AGTCTTCCCACCATCACCATC-3′); for the middle region of the AtSMU2 coding sequence, AtSMU2_3 (5′-CCAGTGGATTGTTAAGCCTCAGAC-3′) and AtSMU2_4 (5′-CACAGAAGATCCATTCTCAAT-3′); for the 3′ coding sequence of AtSMU2, AtSMU2_5 (5′-GAGAAAGATAGGGGTTTGGGA-3′) and AtSMU2_6 (5′-TCAATGCTTGGATCTCTTAGGAGTT-3′); for AtSRp30, AtSRp30_F1 (5′-ATGAGTAGCCGATGGAATCGTAC-3′) and AtSRp30_R1 (5′-CAGTTTTCATTTTCAACCAGATATCAC-3′); for AtTRA2, AtTRA2_F2 (5′-ATGTCTTACTCAAGAAGGTCAAGA-3′) and AtTRA2_R3 (5′-CGGGGAGAGTAGCTAGGAGACTTA-3′); and for AtSMU1, either AtSMU1_1 (5′-AGAGTTTCTCATCCGGTAATAGGGAAG-3′) and AtSMU1_2 (5′-TCAGGGCTTCCATAACTTCATAGTG-3′) or AtSMU1_3 (5′-ACTGAGTACCTCCTTTGACCAAACTGC-3′) and AtSMU1_4 (5′-ACGGAGGTGGCGGCTTGAATGTTTGTA-3′).
For protein extraction, tissues were homogenized in a 2× Laemmli buffer. The extract was centrifuged, and the supernatant was analyzed by SDS-PAGE. Western-blot analysis was conducted as described by Chung et al. (2007).
RNA and protein analyses using maize (Zea mays) endosperm extracts were performed as described (Chung et al., 2007). Primers for PCR were as follows: for rpl15, ZmrpL15_F1 (5′-AGCCGCATCCGCTATGGGTGCGTA-3′) and ZmrpL15_R2 (5′-CTACCTTTAGGCACAGGCCTCTTCC-3′); for rpl7a, ZmrpL7a_F2 (5′-ACGGCCTTAACCATGTGACTTACC-3′) and ZmrpL7a_R3 (5′-CTTGATAGCCTCCAGGATTTTGCT-3′); for eEf-1α, ZmEF1a_F2 (5′-TCTCCCCCTACAGGATGTGTACAA-3′) and ZmEF1a_R3 (5′-GCAAACACAGAACCAGCCAGACAC-3′); for O2 SV1 (major transcript), O2_F1 (5′-ATGGAGCACGTCATCTCAATGGAGG-3′) and O2_R3 (5′-TGGATTCCTTTCTTTTCCTCACTC-3′); for O2 SV2, O2_F1 and O2_R2a2 (5′-TCCGCTGCTACCCTCCACAT-3′); for tra2, ZmTRA2_F1 (5′-ATGTCTTACTCAAGGGGCTCAAGTGA-3′), ZmTRA2_R3 (5′-GATACTTCCCAGGTGTTGGGGTTC-3′), and ZmTRA2_R2a (5′-CAACCCTTTGGCTGAGGTTGGCAT-3′); for rsp31b, ZmRSp31B_F2 (5′-AACCCTAGGTCGCCGCCTCTTTTC-3′), ZmRSp31B_R3 (5′-ATGGAAGGAGTTGAAGAGCCCTGG-3′), and ZmRSp31B_R5 (5′-CATCACGTCTACCAGCTTGCTCAC-3′); and for otux, ZmOTUX1_F2 (5′-CTATGATGGATACGTTCCCATGGC-3′) and ZmOTUX1_R1 (5′-AAGAGAGCTGAACCCGCCATCAAT-3′).
All RT-PCR and immunoblot analyses were performed with at least three biological replicates. Representative gel images are shown in the figures.
Detection of ZmSMU2-Interacting Proteins with the Y2H System
A full-length ZmSmu2 cDNA bait sequence for the Y2H screen was prepared by PCR using primers E1 (5′-GGAATTCATGTCATCGAAGAAGAACTACTATAAG-3′) and S1 (5′-TCCCCCGGGGGATCAGCCACGCTGTTTCTTCGAGCT-3′) for insertion between the EcoRI and SmaI sites of the pBD-GAL4 Cam phagemid vector (Stratagene). Because this construct self-activated in the Y2H system, a series of deletion clones encoding portions of the ZmSMU2 protein were made as follows: the ZmSMU2(I) construct (nucleotides 148–1,698 from the ATG) was created by PCR with primers 38YCONST-I (5′-GGAATTCATGTCGTTTCATGCAGTGGCA-3′) and S1, and the amplified DNA fragment was cloned into pBD-GAL4 between the EcoRI and SmaI sites; the ZmSMU2(II) construct was created by PCR using primers 38YCONST-II (5′-GGAATTCATGAAAGAAGATCAGGCAGTC-3′) and S1, and the amplified DNA was cloned into pBD-GAL4, as with ZmSMU2(I); the ZmSMU2(III) construct was created by PCR with primers 38YCONST-III (5′-GGAATTCATGCCACCACCACCGGCTCCA-3′) and S1, and the amplified DNA was cloned into pBD-GAL4, as with ZmSMU2(I); the ZmSMU2(IV) construct was created by PCR with primers 38YCONST-IV (5′-GGAATTCATGGGTTATCCAGAACAGTAT-3′) and S1, and the amplified DNA was cloned into pBD-GAL4, as with ZmSMU2(I); the ZmSMU2(V) construct was created by PCR with primers E1 and R4 (5′-CCTGAGTCATGAACCTCGGATC-3′), and the amplified DNA was digested with EcoRI and EcoRV and inserted into pBD-GAL4, as with ZmSMU2(I). Each construct was verified using DNA sequencing. The pBD-GAL4-ZmSMU2(IV) construct produced a detectable 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside product in 2.5 h, and we monitored the yeast colonies for a color reaction that developed within 30 min. On the other hand, deletion of the COOH-terminal 207 amino acids of the ZmSMU2 protein dramatically reduced self-activation in the Y2H system. Consequently, we used the ZmSMU2(V) bait clone to screen maize and Arabidopsis target libraries. Developing maize endosperm (10–14 d after pollination) and immature ear libraries (HybriZAP-2.1 Two-Hybrid Predigested Vector Kit; Stratagene) were obtained from Dr. Bob Schmidt (Department of Biology, University of California San Diego). The Arabidopsis library was from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/abrc/libraries.jsp; stock no. CD4-22). Yeast transformation and screening procedures were performed according to the manufacturer's instructions. To identify the interacting proteins encoded by Arabidopsis target DNAs, colony PCRs were carried out with primers pACTForward (5′-CTATCTATTCGATGATGAAG-3′) and pACTReverse (5′-ACAGTTGAAGTGAACTTGCG-3′); for maize target genes, the pACTForward and PADT7 (5′-TAATACGACTCACTATAGGG-3′) primers were used. The amplified PCR product was inserted into the pCR4-TOPO vector (Invitrogen) for DNA sequencing.
Expression of Recombinant Proteins in Escherichia coli and in Vitro Pull-Down Assays
The following PCR primers were used to produce target proteins fused with a 6XHis tag: for histone H4, AtHIS4_F1 (5′-ATGTCAGGAAGAGGAAAGGGAGGAA-3′) and AtHIS4_R1 (5′-TTAACCTCCGAAACCGTAGAGAGT-3′); for AtSMU1, AtSMU-1_F1 (5′-ATGGCGCTCGAAATCGAAGCTC-3′) and AtSMU-1_R1 (5′-TCAGGGCTTCCATAACTTCATAGTG-3′); and for AtMEC8, AtMEC-8_F1 (5′-ATGGCGTATCACCAACCGTACGA-3′) and AtMEC-8_R1 (5′-TTACTCTATATGCATGCCGCCTCG-3′). The RT-PCR products were cloned into pGEMT-Easy (Promega) and subsequently transferred into the EcoRI site of pRSET (Invitrogen) and used to transform the BL21(DE3) strain of E. coli. For pull-down experiments where GST fusion proteins were used as a bait protein, cell lysates containing the fused target proteins with a 6XHis tag were prepared in lysis buffer (50 mm Tris, pH 8.5, 150 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, and 0.2 mm phenylmethanesulfonyl fluoride) containing 1% (v/v) Triton X-100. Purification of GST and GST-ZmSMU2 was performed as described by Chung et al. (2007). Cell lysates containing 0.1 to 0.5 μg of target proteins were preincubated with 0.5 μg of GST and 50 μL of a 50% (v/v) glutathione-agarose slurry (Sigma) at 4°C for 1 h. The cleared cell lysates were added to 50 μL of a 50% (v/v) glutathione-agarose slurry and 10 μg of GST, GST-AtSMU2, or GST-ZmSMU2 protein. The mixtures were adjusted to 1 mL with lysis buffer and incubated at 4°C for 2 h. The beads were then washed four times with lysis buffer containing 0.1% (v/v) Triton X-100. Proteins were eluted in 1× Laemmli sample buffer containing 10 mm glutathione, separated by SDS-PAGE, and transferred onto nitrocellulose membrane. Immunoblot analysis was performed with mouse HisG antibodies (Sigma) at 1:3,000 dilution and then with goat anti-mouse IgG antibodies conjugated with horseradish peroxidase (Sigma) at 1:80,000 dilution.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Microarray data analysis of Zmsmu2-1 endosperm.
Supplemental Figure S2. Semiquantitative RT-PCR analysis of RNAs corresponding to coregulated and consistent SP classes of differentially hybridized probes (DHPs) identified in the microarray experiment.
Supplemental Table S1. List of DHP-Hs and DHP-Ls (Microsoft Excel file).
Supplemental Table S2. Correlation of random probes and DHP-L with the number of SVs in MAGI.
Supplemental Table S3. Identification of hybridization sites for random probes and DHP-L, according to the ab initio prediction-based exons and introns in MAGI.
Supplemental Table S4. Identification of hybridization sites for random probes and DHP-L, according to EST-based gene annotation in MAGI.
Supplemental Protocol S1. Results and methods for Zmsmu2-1 microarray.
Supplementary Material
Acknowledgments
We thank colleagues in the Larkins laboratory for review and editing of the manuscript.
This work was supported by the Department of Energy (grant no. DE–96ER20242 to B.A.L. and grant no. DE–FG02–03ER15438 to R.Y.), the National Science Foundation (grant no. DBI–0077676 to B.A.L. and grant no. IOB–0520008 to R.Y.), the U.S. Department of Agriculture (Cooperative State Research, Education, and Extension Service grant no. 2004–00918 to B.A.L.), and the Agricultural Plant Stress Research Center (grant no. R112001092020080, for partial salary support to C.-S.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Brian A. Larkins (larkins@ag.arizona.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen HM, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Amrein H, Gorman M, Nothiger R (1988) The sex-determining gene Tra-2 of Drosophila encodes a putative RNA-binding protein. Cell 55 1025–1035 [DOI] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20 1195–1197 [DOI] [PubMed] [Google Scholar]
- Bell LR, Horabin JI, Schedl P, Cline TW (1991) Positive autoregulation of Sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65 229–239 [DOI] [PubMed] [Google Scholar]
- Bertrand C, Benhamed M, Li YF, Ayadi M, Lemonnier G, Renou JP, Delarue M, Zhou DX (2005) Arabidopsis HAF2 gene encoding TATA-binding protein (TBP)-associated factor TAF1, is required to integrate light signals to regulate gene expression and growth. J Biol Chem 280 1465–1473 [DOI] [PubMed] [Google Scholar]
- Bessonov S, Anokhina M, Will CL, Urlaub H, Luhrmann R (2008) Isolation of an active step I spliceosome and composition of its RNP core. Nature 452 846–850 [DOI] [PubMed] [Google Scholar]
- Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DL (2003) Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem 72 291–336 [DOI] [PubMed] [Google Scholar]
- Brown JWS, Simpson CG (1998) Splice site selection in plant pre-mRNA splicing. Annu Rev Plant Physiol Plant Mol Biol 49 77–95 [DOI] [PubMed] [Google Scholar]
- Carrington JC, Freed DD, Oh CS (1990) Expression of potyviral polyproteins in transgenic plants reveals 3 proteolytic activities required for complete processing. EMBO J 9 1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Kim CS, Nguyen HN, Meeley RB, Larkins BA (2007) The maize Zmsmu2 gene encodes a putative RNA-splicing factor that affects protein synthesis and RNA processing during endosperm development. Plant Physiol 144 821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR (2000) Sorting out the complexity of SR protein functions. RNA 6 1197–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Wang BB, Stryker GA, Zanetti ME, Lal SK (2005) Two novel arginine/serine (SR) proteins in maize are differentially spliced and utilize non-canonical splice sites. Biochim Biophys Acta 1728 105–114 [DOI] [PubMed] [Google Scholar]
- Haberer G, Young S, Bharti AK, Gundlach H, Raymond C, Fuks G, Butler E, Wing RA, Rounsley S, Birren B, et al (2005) Structure and architecture of the maize genome. Plant Physiol 139 1612–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmuth K, Urlaub H, Vornlocher HP, Will CL, Gentzel M, Wilm M, Luhrmann R (2002) Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc Natl Acad Sci USA 99 16719–16724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H (2006) CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol 8 1277–1283 [DOI] [PubMed] [Google Scholar]
- Isshiki M, Tsumoto A, Shimamoto K (2006) The serine/arginine-rich protein family in rice plays important roles in constitutive and alternative splicing of pre-mRNA. Plant Cell 18 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumaa H, Nielsen PJ (1997) The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J 16 5077–5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica MS, Licklider LJ, Gygi SP, Grigorieff N, Moore MJ (2002) Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA 8 426–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica MS, Moore MJ (2003) Pre-mRNA splicing: awash in a sea of proteins. Mol Cell 12 5–14 [DOI] [PubMed] [Google Scholar]
- Kalyna M, Lopato S, Barta A (2003) Ectopic expression of atRSZ33 reveals its function in splicing and causes pleiotropic changes in development. Mol Biol Cell 14 3565–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Genet Genomics 204 383–396 [Google Scholar]
- Kumar S, Lopez AJ (2005) Negative feedback regulation among SR splicing factors encoded by Rbp1 and Rbp1-like in Drosophila. EMBO J 24 2646–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal SK, Hannah LC (1999) Maize transposable element Ds is differentially spliced from primary transcripts in endosperm and suspension cells. Biochem Biophys Res Commun 261 798–801 [DOI] [PubMed] [Google Scholar]
- Lee BH, Kapoor A, Zhu JH, Zhu JK (2006) STABILIZED1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell 18 1736–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Terzaghi W, Gusmaroli G, Charron JBF, Yoon HJ, Chen HD, He YJ, Xiong Y, Deng XW (2008) Characterization of Arabidopsis and rice DWD proteins and their roles as substrate receptors for CUL4-RING E3 ubiquitin ligases. Plant Cell 20 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopato S, Forstner C, Kalyna M, Hilscher J, Langhammer U, Indrapichate K, Lorkovic ZJ, Barta A (2002) Network of interactions of a novel plant-specific Arg/Ser-rich protein, atRSZ33, with atSC35-like splicing factors. J Biol Chem 277 39989–39998 [DOI] [PubMed] [Google Scholar]
- Lopato S, Kalyna M, Dorner S, Kobayashi R, Krainer AR, Barta A (1999) atSRp30, one of two SF2/ASF-like proteins from Arabidopsis thaliana, regulates splicing of specific plant genes. Genes Dev 13 987–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist EA, Herman RK (1994) The mec-8 gene of Caenorhabditis elegans affects muscle and sensory neuron function and interacts with 3 other genes: unc-52, smu-1 and smu-2. Genetics 138 83–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist EA, Herman RK, Rogalski TM, Mullen GP, Moerman DG, Shaw JE (1996) The mec-8 gene of C. elegans encodes a protein with two RNA recognition motifs and regulates alternative splicing of unc-52 transcripts. Development 122 1601–1610 [DOI] [PubMed] [Google Scholar]
- Makarov EM, Makarova OV, Urlaub H, Gentzel M, Will CL, Wilm M, Luhrmann R (2002) Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science 298 2205–2208 [DOI] [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Koonin EV (2000) A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem Sci 25 50–52 [DOI] [PubMed] [Google Scholar]
- Maniatis T, Reed R (2002) An extensive network of coupling among gene expression machines. Nature 416 499–506 [DOI] [PubMed] [Google Scholar]
- Marillonnet S, Wessler SR (1997) Retrotransposon insertion into the maize waxy gene results in tissue-specific RNA processing. Plant Cell 9 967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N, Hisabori T, Takeyasu K, Sato MH (2004) VOZ: isolation and characterization of novel vascular plant transcription factors with a one-zinc finger from Arabidopsis thaliana. Plant Cell Physiol 45 845–854 [DOI] [PubMed] [Google Scholar]
- Mizzen CA, Yang XJ, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T, Workman J, Wang L, Berger SL, Kouzarides T, et al (1996) The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell 87 1261–1270 [DOI] [PubMed] [Google Scholar]
- Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M (1998) Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet 20 46–50 [DOI] [PubMed] [Google Scholar]
- Ortiz DF, Strommer JN (1990) The Mul Maize transposable element induces tissue-specific aberrant splicing and polyadenylation in 2 Adh1 mutants. Mol Cell Biol 10 2090–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazman C, Mayes CA, Fanto M, Haynes SR, Mlodzik M (2000) Rasputin, the Drosophila homologue of the RasGAP SH3 binding protein, functions in Ras- and Rho-mediated signaling. Development 127 1715–1725 [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Ryder U, Lamond AI, Mann M (2002) Large-scale proteomic analysis of the human spliceosome. Genome Res 12 1231–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ASN (2001) Nuclear pre-mRNA splicing in plants. Crit Rev Plant Sci 20 523–571 [Google Scholar]
- Reddy ASN (2004) Plant serine/arginine-rich proteins and their role in pre-mRNA splicing. Trends Plant Sci 9 541–547 [DOI] [PubMed] [Google Scholar]
- Smith CWJ, Valcarcel J (2000) Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem Sci 25 381–388 [DOI] [PubMed] [Google Scholar]
- Spartz AK, Herman RK, Shaw JE (2004) SMU-2 and SMU-1, Caenorhabditis elegans homologs of mammalian spliceosome-associated proteins RED and fSAP57, work together to affect splice site choice. Mol Cell Biol 24 6811–6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike CA, Shaw JE, Herman RK (2001) Analysis of smu-1, a gene that regulates the alternative splicing of unc-52 pre-mRNA in Caenorhabditis elegans. Mol Cell Biol 21 4985–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BB, Brendel V (2006) Genomewide comparative analysis of alternative splicing in plants. Proc Natl Acad Sci USA 103 7175–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Tyson MD, Jackson SS, Yadegari R (2006) Partially redundant functions of two SET-domain polycomb-group proteins in controlling initiation of seed development in Arabidopsis. Proc Natl Acad Sci USA 103 13244–13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollerton MC, Gooding C, Wagner EJ, Garcia-Blanco MA, Smith CWJ (2004) Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol Cell 13 91–100 [DOI] [PubMed] [Google Scholar]
- Xiong LM, Gong ZZ, Rock CD, Subramanian S, Guo Y, Xu WY, Galbraith D, Zhu JK (2001) Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev Cell 1 771–781 [DOI] [PubMed] [Google Scholar]
- Zhou ZL, Licklider LJ, Gygi SP, Reed R (2002) Comprehensive proteomic analysis of the human spliceosome. Nature 419 182–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.