Abstract
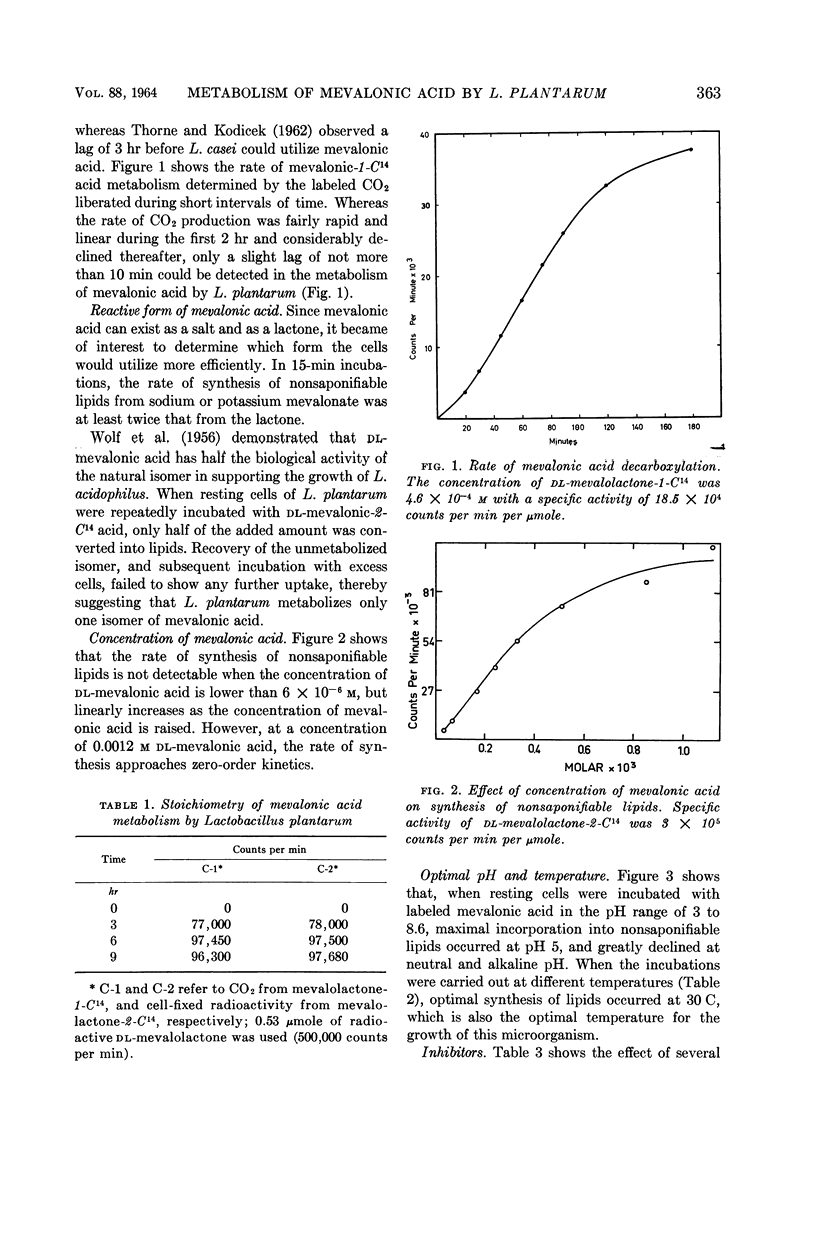
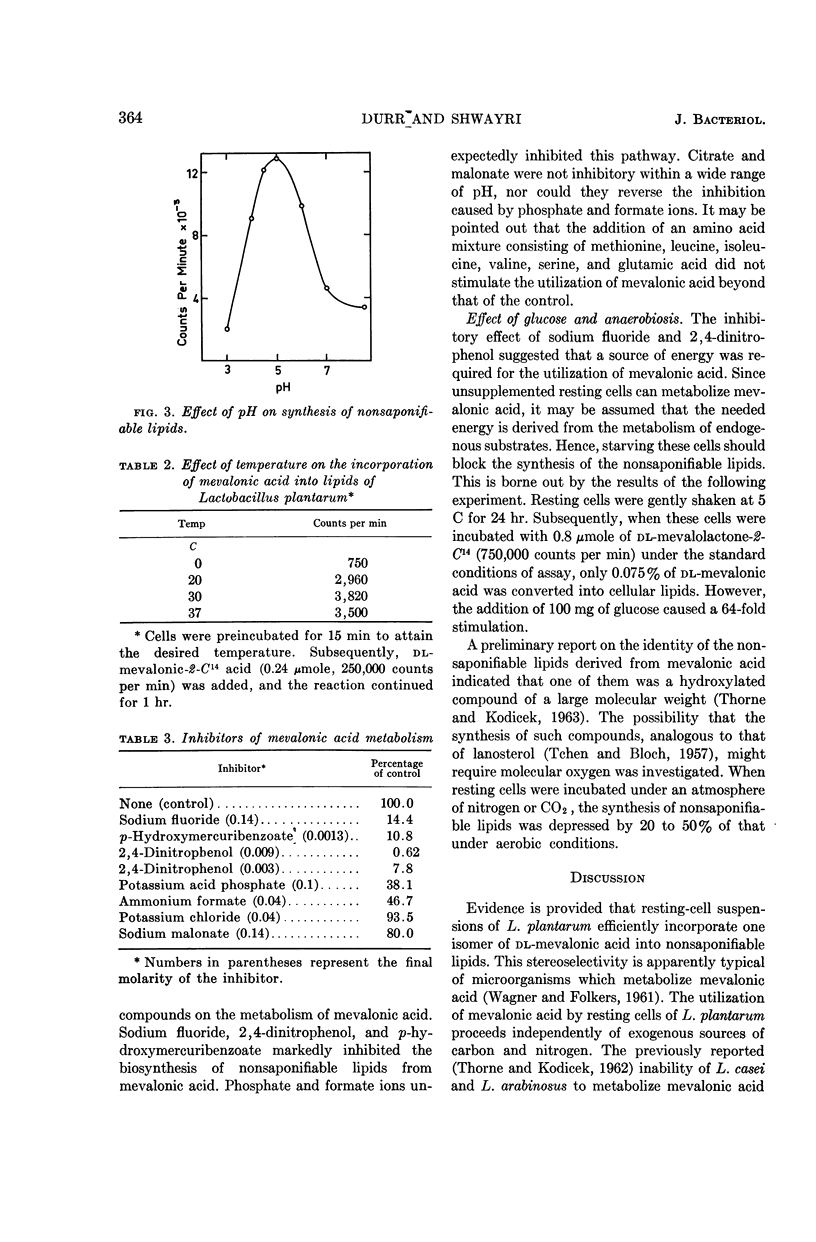
Durr, I. F. (American University of Beirut, Beirut, Lebanon), and A. N. Shwayri. Metabolism of mevalonic acid by Lactobacillus plantarum. J. Bacteriol. 88:361–366. 1964.—Lactobacillus plantarum strain 8014-H2, unlike other lactobacilli studied, does not require mevalonic acid for growth, but growing and resting cells utilize it only for the synthesis of nonsaponifiable lipids. Upon the incubation of washed cells with mevalolactone-2-C14, the label appeared in the lipids but not in CO2. On the other hand, when mevalolactone-1-C14 was used, the label appeared in CO2 but not in lipids. For every mole of CO2 liberated, 1 mole of radioactive carbon was introduced in the lipids, suggesting that terpenic polymers were synthesized. Amino acids did not stimulate the utilization of mevalonic acid. Starved cells could not synthesize nonsaponifiable lipids from mevalonic acid unless glucose was supplied. Sodium fluoride (0.14 m), 2,4-dinitrophenol (0.003 m), p-hydroxymercuribenzoate (0.0013 m), potassium phosphate (0.1 m), and ammonium formate (0.04 m) were potent inhibitors. Cells metabolized only one isomer of dl-mevalonic, and utilized the salt form at least twice as efficiently as the lactone. Optimal synthesis of lipids from mevalonic acid occurred aerobically, at pH 5 and 30 C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM B., STETTEN M. R., STETTEN D., Jr Evaluation of catabolic pathways of glucose in mammalian systems. J Biol Chem. 1953 Oct;204(2):681–694. [PubMed] [Google Scholar]
- CRESSON E. L., FOLKERS K., HOFFMAN C. H., MACRAE G. D., SKEGGS H. R., WOLF D. E., WRIGHT L. D. Discovery of a new acetate-replacing factor. J Bacteriol. 1956 Oct;72(4):519–524. doi: 10.1128/jb.72.4.519-524.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. On the importance of being ionized. Arch Biochem Biophys. 1958 Dec;78(2):497–509. doi: 10.1016/0003-9861(58)90374-6. [DOI] [PubMed] [Google Scholar]
- FUMAGALLI R., GROSSI E., POGGI M., PAOLETTI P., GARATTINI S. Cholesterol synthesis in rat brain: differential incorporation of mevalonolactone-2-C14 and potassium mevalonate-2-C14. Arch Biochem Biophys. 1962 Dec;99:529–533. doi: 10.1016/0003-9861(62)90302-8. [DOI] [PubMed] [Google Scholar]
- HOFMANN K., LUCAS R. A., SAX S. M. The chemical nature of the fatty acids of Lactobacillus arabinosus. J Biol Chem. 1952 Apr;195(2):473–485. [PubMed] [Google Scholar]
- MACRAE R. M., WILKINSON J. F. Poly-beta-hyroxybutyrate metabolism in washed suspensions of Bacillus cereus and Bacillus megaterium. J Gen Microbiol. 1958 Aug;19(1):210–222. doi: 10.1099/00221287-19-1-210. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., BLOCH K. On the mechanism of enzymatic cyclization of squalene. J Biol Chem. 1957 Jun;226(2):931–939. [PubMed] [Google Scholar]
- THORNE K. J., KODICEK E. The metabolism of acetate and mevalonic acid by lactobacilli. IV. Analysis of the fatty acids by gas-liquid chromatography. Biochim Biophys Acta. 1962 May 21;59:306–312. doi: 10.1016/0006-3002(62)90178-6. [DOI] [PubMed] [Google Scholar]
- WAGNER A. F., FOLKERS K. Discovery and chemistry of mevalonic acid. Adv Enzymol Relat Subj Biochem. 1961;23:471–483. doi: 10.1002/9780470122686.ch9. [DOI] [PubMed] [Google Scholar]


