Abstract
The postembryonic development of lateral roots and nodules is a highly regulated process. Recent studies suggest the existence of cross talk and interdependency in the growth of these two organs. Although plant hormones, including auxin and cytokinin, appear to be key players in coordinating this cross talk, very few genes that cross-regulate root and nodule development have been uncovered so far. This study reports that a homolog of CELL DIVISION CYCLE16 (CDC16), a core component of the Anaphase Promoting Complex, is one of the key mediators in controlling the overall number of lateral roots and nodules. A partial suppression of this gene in Medicago truncatula leads to a decrease in number of lateral roots and a 4-fold increase in number of nodules. The roots showing lowered expression of MtCDC16 also show reduced sensitivity to phytohormone auxin, thus providing a potential function of CDC16 in auxin signaling.
As in all eukaryotic organisms, cell division in plants is strictly controlled by a concerted action of several key regulators, such as cyclin-dependent kinases and cyclins (De Veylder et al., 2007). The progression of the cell cycle from one phase to another requires the targeted degradation of selected cyclin molecules mediated by two ubiquitin-mediated proteolytic pathways. The SKP1-Cullin/F-Box protein (SCF) pathway acts in the G1-to-S phase transition by degrading the D-type cyclins and other substrate proteins (Yanagawa and Kimura, 2005). The second pathway, mediated by Anaphase Promoting Complex/Cyclosome (APC/C), regulates the sequential destruction of A- and B-type cyclins in a D-box or a KEN-box-dependent manner, resulting in chromosome segregation and exit from mitosis (Genschik et al., 1998; Pfleger and Kirschner, 2000). Evidence of the role of the APC/C in plant development comes from studies of the Arabidopsis (Arabidopsis thaliana) hobbit (hbt) mutant that shows defects in root growth. The HBT gene is required for both cell division and cell differentiation in root meristems and encodes CDC27, a core subunit of APC/C (Blilou et al., 2002; Perez-Perez et al., 2008). Cebolla et al. (1999) used the root nodule system of the model legume Medicago truncatula to study the function of an APC/C activator, CCS52, which is homologous to the yeast APC/C activator CDH1. A nodule-specific homolog of CCS52, CCS52A, was found to be required to initiate endoreduplication in the dividing cells, and its down-regulation affected nodule development, resulting in lower ploidy, reduced cell size, and inefficient rhizobial invasion and nodule maturation (Vinardell et al., 2003; Kondorosi et al., 2005). T-DNA insertions in the Arabidopsis CELL DIVISION CYCLE16 (CDC16) and APC2 genes result in gametophytic lethality due to the failure to degrade mitotic cyclins (Capron et al., 2003b; Kwee and Sundaresan, 2003). Although the completed Arabidopsis genome has allowed the identification of homologs of almost all vertebrate APC/C subunits in plants (Capron et al., 2003a), the functions of most of these subunits still remains largely unexplored.
Direct links between root growth and auxin signaling have been well documented. Several Arabidopsis mutants with decreased auxin sensitivity often exhibit an overall defect in both primary and lateral root development (Hellmann and Estelle, 2002; Casimiro et al., 2003; Hellmann et al., 2003; Vanneste et al., 2005). A number of these auxin-resistant mutants belong to the SCF proteolysis pathway, supporting a role for the SCF pathway in auxin signaling (Teale et al., 2006; Benjamins and Scheres, 2008). Auxin appears to control lateral root development by promoting G1-to-S transition in selected xylem pericycle cells, perhaps by targeting KRP2, a cyclin-dependent kinase inhibitor, and E2F, an S phase inhibitor to SCF-mediated proteolysis (del Pozo et al., 2002; Himanen et al., 2002). Unlike SCF, the role of APC/C in auxin-mediated plant development is not clear. The only report that has so far integrated APC/C with auxin signaling pertains to the hbt mutant, which shows an increased resistance to exogenous auxin due to accumulation of Aux/IAAs in the roots (Blilou et al., 2002).
As in lateral roots, auxin is an important player in the development of nodules on the roots of leguminous plants (Beveridge et al., 2007). Studies with auxin-responsive reporter gene constructs have shown auxin's participation in cortical cell reactivation and initiation of nodule primordia (Mathesius et al., 1998). The exogenous application of Nod factor results in a transient inhibition of auxin transport capacity in roots of Vicia sativa (Boot et al., 1999) and Trifolium repens (Mathesius et al., 1998). Consistent with this, localized application of synthetic auxin transport inhibitors on alfalfa (Medicago sativa) roots induces pseudonodules (Hirsch et al., 1989). Complementing these findings, a more recent study in M. truncatula has demonstrated that increased auxin transport, caused by silencing the flavonoid pathway, results in reduced nodule formation in response to rhizobia (Wasson et al., 2006). Finally, hypernodulating mutants like sunn and skl show defective long-distance transport of auxin, further suggesting the importance of polar auxin transport, not only in regulating nodule induction but also in controlling nodule numbers (Prayitno et al., 2006; van Noorden et al., 2006).
In this report, we investigated the role of the APC/C component CDC16 in root and nodule development in M. truncatula. CDC16 was identified via microarray analysis as a gene that was significantly induced in roots of M. truncatula following inoculation by Sinorhizobium meliloti and in nodules relative to uninoculated roots (Kuppusamy, 2005), thus encouraging further functional analysis of this gene. To overcome the problem of the gametophytic lethality resulting from CDC16 knockout, as seen from analysis of an insertional mutation in Arabidopsis (Kwee and Sundaresan, 2003) we undertook an RNA interference (RNAi) approach to partially suppress the expression of CDC16 gene in Agrobacterium rhizogenes-transformed roots of M. truncatula. We report that roots transformed with the CDC16 RNAi construct (hereafter called Mtcdc16i roots) displayed a decreased sensitivity to auxin, defective primary root growth, and fewer lateral roots. Interestingly, in response to S. meliloti, the Mtcdc16i roots showed almost 4-fold increase in number of nodules, suggesting that decreased sensitivity to auxin leads to a hypernodulation phenotype. Thus, this work highlights the importance of CDC16 in root and nodule development and indicates a possible role for this gene in auxin signaling.
RESULTS
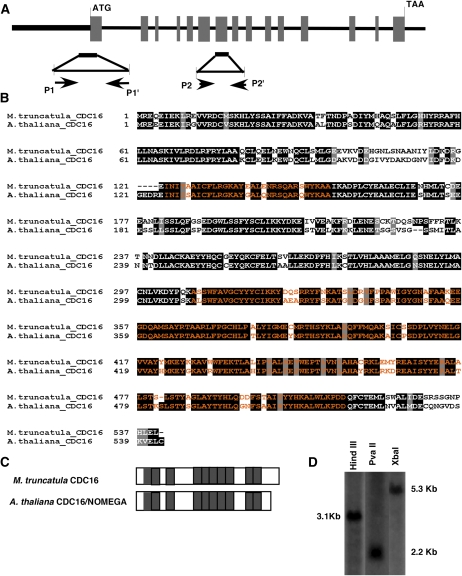
MtCDC16 Gene Structure Organization
The MtCDC16 genomic and cDNA sequences were obtained from the M. truncatula BAC library (http://www.medicago.org/genome/blast.php) and the Medicago Gene Index (MtGI) version 8.0 (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=medicago) sequence databases, respectively. Alignment of the predicted MtCDC16 genomic region (approximately 12 kb) with the full-length cDNA revealed that MtCDC16 is composed of 15 exons and 14 introns (Fig. 1A). As depicted in Figure 1B, the MtCDC16 gene was predicted to encode a 540-amino-acid sequence that exhibits approximately 70% identity to the Arabidopsis CDC16 (Kwee and Sundaresan, 2003). Homologs of CDC16 in yeast and mammals contain 8 to 10 copies of 34-amino-acid tetra-tricopeptide repeat (TPR) motifs that form superhelical structures that function in protein-protein interactions (D'Andrea and Regan, 2003; Passmore, 2004). The predicted MtCDC16 amino acid sequence contained a similar number and distribution of TPR motifs as seen in AtCDC16 (Fig. 1C). Southern-blot analysis (Fig. 1D) and searches of the MtGI (version 8.0) and the partially completed genomic sequence provided no evidence of additional MtCDC16 genes in M. truncatula genome.
Figure 1.
Structural analysis of the M. truncatula CDC16 gene. A, Gene structure of the MtCDC16 gene. Translated regions of exons are shown as gray boxes. The boldface line before the translational start site represents the 5′-untranslated and the putative promoter regions. The 500-bp fragment indicated below, which includes portions of exon 1 and the 5′-untranslated region, marks the area that was used for constructing the RNAi vector. The location of the two primer sets (P1-P1' and P2-P2') used for RT-PCR analysis of MtCDC16 transcripts is indicated. B, Alignment of the predicted M. truncatula CDC16 protein with the Arabidopsis CDC16 protein. Blocks of amino acid sequences marked in orange indicate the TPR motifs. C, Graphical representation of the distribution of TPR motifs within M. truncatula CDC16 and Arabidopsis CDC16 proteins. Gray boxes indicate the TPR motifs. D, Southern-blot analysis to estimate the copy number of MtCDC16 in M. truncatula genome.
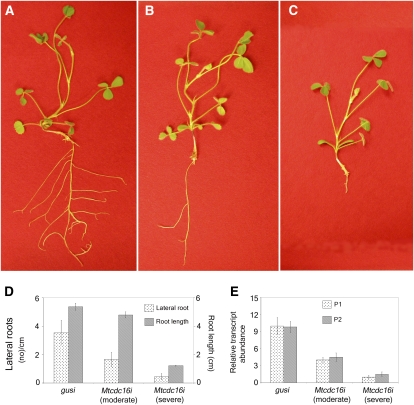
Knockdown of MtCDC16 Leads to Reduction in Lateral Root Number and Root Length Elongation
To silence MtCDC16 gene expression, the RNAi-inducing construct (pRNAi-CDC16) was designed to target the first two TPR units encoded in exon 1, along with part of the 5′-untranslated region (Fig. 1A) of the transcript. In each of three independently constructed populations of Mtcdc16i roots (n = 131 total roots examined), the root phenotype was found to vary in severity. To facilitate further analysis, we divided roots into two classes, using root length and lateral root numbers as the phenotypic criteria. The first class, designated as Mtcdc16i (moderate) roots comprised on average 30% of the transformed roots. This class of roots showed up to a 50% reduction in the number of lateral roots, as compared to the control roots in which the GUS gene was silenced (henceforth called the gusi or control roots). However, the root length of Mtcdc16i (moderate) plants (Fig. 2, B and D) was similar to that of the control plants (Fig. 2, A and D). The second class of Mtcdc16i roots comprised 60% of the transformed roots (n = 131 roots) and showed almost 80% reduction in the number of lateral roots and an average of a 70% reduction in primary root length (Fig. 2, C and D). Due to a more severe phenotype, this class was designated as Mtcdc16i (severe).
Figure 2.
MtCDC16-suppressed (Mtcdc16i) roots show decreased lateral root number and root length. A. rhizogenes carrying the pRNAi-GUS or pRNAi-CDC16 construct was used to transform L416 seedlings (see “Materials and Methods”). A, Control root (gusi), showing normal primary and lateral root growth. B, MtCDC16-suppressed root with a moderate phenotype [Mtcdc16i (moderate)], showing reduced lateral root development. C, MtCDC16-suppressed root with a severe phenotype [Mtcdc16i (severe)], showing extreme reduction in root length and lateral roots. D, Root length and lateral root numbers from three independent sets of control (gusi) and Mtcdc16i roots. Error bars represent sd from the mean. E, Quantitative RT-PCR analysis of MtCDC16 expression in Mtcdc16i roots. P1 indicates the primers designed within region used for the RNAi construct, and P2 primers were designed downstream of the region used for the RNAi construct.
Quantitative reverse transcription (RT)-PCR analysis was performed on RNA isolated from three independent pools of control, Mtcdc16i (severe), and Mtcdc16i (moderate) roots (30 roots in each pool). This analysis revealed an average of 3-fold decrease in CDC16 transcripts in Mtcdc16i (moderate) roots and an 8- to 9-fold decrease in the Mtcdc16i (severe) roots, consistent with the severity in the phenotype (Fig. 2E). To ensure that the decrease in transcript abundance was not limited to the region that was silenced, RT-PCR was carried out with a second pair of primers (Primer P2-P2') spanning a region downstream of the targeted region in addition to the primers targeting the silenced region (Primer P1-P1'). The two analyses showed a similar reduction in transcript accumulation of MtCDC16 (Fig. 2E).
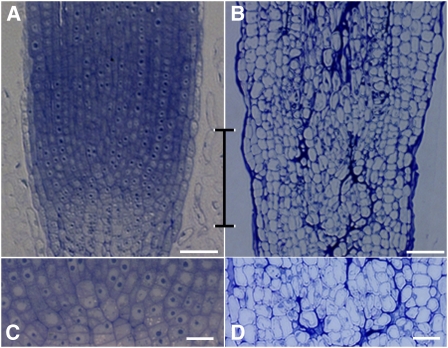
MtCDC16-Suppressed Roots Display Aberrant Cell Morphology
The Mtcdc16i plants with a severe phenotype often showed pronounced root tip defects. Microscopy analysis revealed that Mtcdc16i (severe) roots had cells that were irregular in shape and size at the distal end, with no clear meristem (Fig. 3, B and D). This was in striking contrast to the regularity of the cell shape and tissue organization seen in the control (gusi) roots (Fig. 3, A and C). This result indicates that a severe reduction in CDC16 expression leads to a dramatic disruption in the root meristem organization. Such root tip defects were not seen in Mtcdc16i (moderate) roots (Supplemental Fig. S1); hence, detailed microscopy analysis was not carried out with these roots. Examination of the Mtcdc16i (severe) root elongation zone by light microscopy showed that the cortical cells in the elongation zone were comparable in length to that of the control roots (Fig. 3A). This suggested that the stunted root growth of the Mtcdc16i (severe) roots was likely due to the defect in the root meristem caused by aberrant cell division rather than to a defect in cell elongation.
Figure 3.
Severe suppression of MtCDC16 results in aberrant cell morphology in the root meristem. A and B, Median longitudinal, toluidine blue-stained sections of the root tips of a control root (A) and an Mtcdc16i (severe) root (B). The double-sided bracket marks the root apical meristem of the control root and the corresponding region of the Mtcdc16i root. C and D, Higher-magnification image of the bracketed regions in A and B. C shows regularity in cell morphology at the root apical meristem in the control roots. D shows irregularity in cell morphology in comparable zone of the Mtcdc16i (severe) root. Bars = 50 μm.
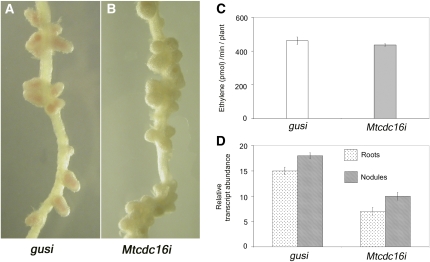
A Controlled Suppression of MtCDC16 Results in an Increase in Nodule Number
In order to elucidate the role of MtCDC16 in nodule development, we carried out nodulation assays using Mtcdc16i (moderate) roots. Mtcdc16i (severe) roots do not survive in the nitrate-free medium used for nodulation assays and hence were not analyzed. In the presence of S. meliloti, 54 out of 60 Mtcdc16i (moderate) roots developed approximately 4 times more nodules than the control roots (n = 60). While the control roots developed 8 to 10 nodules per seedling (Fig. 4A), Mtcdc16i (moderate) roots typically developed 35 to 40 nodules (Fig. 4B). Most of the nodules on Mtcdc16i (moderate) roots were restricted to the primary nodulation zone and were more densely packed (Fig. 4B) than nodules on control roots. The primary nodulation zone of Mtcdc16i (moderate) roots was also longer than that seen in the control roots (10 ± 0.8 cm in Mtcdc16i [moderate] roots and 6 ± 1.2 cm in control roots; n = 30 roots examined). To characterize the Mtcdc16i symbiotic phenotype at the cellular level, we examined nodule sections at 12 d after inoculation from control and Mtcdc16i nodules (n = 22 Mtcdc16i nodules examined from 12 plants; n = 11 control nodules examined from seven gusi plants). The organization of the tissue layers in Mtcdc16i nodules was very similar to the control nodules and contained a clear meristem, an infection zone containing infection threads, and bacteroid-containing cells in the nitrogen-fixing zone (Fig. 5). However, the length of the infection zone in the Mtcdc16i nodules (0.14 μm ± 0.006) was significantly longer than the control nodules (0.08 μm ± 0.009; P < 0.01; Fig. 5A). Also, the infection zone of the Mtcdc16i nodules occupied an average of 21% of total nodule length in contrast to the infection zone of control nodules that occupied an average of 13% total nodule length. There was no significant difference in the size of the nitrogen-fixing zone between Mtcdc16i and control nodules. To further verify the status of symbiotic development in the Mtcdc16i nodules, nitrogenase activity was assessed in nodulating Mtcdc16i and control roots using two independent pools of samples (n = 10 ± 2 roots examined in each pool). On a per root basis, Mtcdc16i and control plants possessed similar nitrogenase activity, as measured by acetylene reduction to ethylene (Fig. 4C), suggesting that on a per nodule basis the amount of nitrogen that was fixed was comparatively lower in Mtcdc16i roots than in control roots. Similar negative correlation between nodule number and nitrogenase activity/nodule has been described in hypernodulating mutants in M. truncatula (Penmetsa and Cook, 1997; Penmetsa et al., 2003) and other legumes (Wopereis et al., 2000). To ensure that the hypernodulating phenotype seen in the Mtcdc16i (moderate) roots is due to the suppression of MtCDC16 expression, a comparison of MtCDC16 transcript abundance was carried out in nodulating and non-nodulating parts of Mtcdc16i and control root systems in three independent experiments (n = 60 roots examined per pool). A significant decrease in MtCDC16 expression was noted in both nodulating and non-nodulating parts of Mtcdc16i roots as compared to the corresponding control root systems (Fig. 4D). Moreover, in both control and Mtcdc16i plants, the nodulating parts of the roots showed a relatively higher CDC16 transcript level than the non-nodulating parts of the same pool of roots.
Figure 4.
Partial suppression of MtCDC16 results in hypernodulation phenotype. A and B, Control roots (A) and Mtcdc16i roots (B) inoculated with S. meliloti and grown in an aeroponic chamber for 4 weeks. C, Acetylene reduction assay. Nitrogenase activity of intact nodules on control roots and Mtcdc16i roots was measured on a whole-root basis. The error bars indicate sd from the mean from three independent experiments. D, Quantitative RT-PCR analysis shows significant suppression of MtCDC16 in Mtcdc16i roots and nodules. Each point represents the mean of three replicates with error bars representing the sd.
Figure 5.
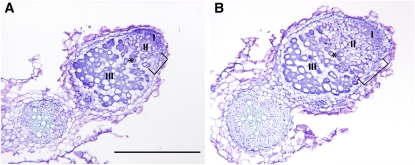
Longitudinal sections of root nodules from Mtcdc16i and control roots. Longitudinal section of a control nodule (A) and Mtcdc16i (moderate) nodule (B). The length of the infection zones is designated by brackets. Nodule zones are labeled according to Vasse et al. (1990): meristem (I), infection zone (II), interzone (asterisk), nitrogen-fixing zone (III). Bar = 0.5 mm.
MtCDC16 Expression Is Activated in the Zone of Cell Division of Growing Roots and Nodules
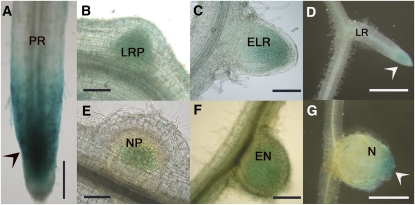
To examine the temporal and spatial expression pattern of MtCDC16, its putative promoter region (Fig. 1A) was fused to a GUS reporter gene. The transcriptional activation of the reporter gene was monitored with and without rhizobial inoculation in the transgenic hairy roots of M. truncatula generated in the wild-type ecotype A17. In the uninoculated roots, strong GUS expression was observed at the root tip (Fig. 6A, arrowhead). Blue staining was also visible at the site of lateral root primordia and emerging lateral roots (Fig. 6, B and C, respectively), and it subsequently became confined to the tip as the lateral roots fully emerged out of the primary root (Fig. 6D, arrowhead). In response to rhizobia, GUS expression was noted in the nodule primordia and emerging nodules (Fig. 6, E and F, respectively) and then became restricted to the meristematic zone of fully developed nodules (Fig. 6G, arrowhead).
Figure 6.
Promoter-GUS fusion analysis of MtCDC16 promoter activity in transgenic hairy roots of M. truncatula. The expression of GUS is indicated by blue staining. A to D, GUS activity in the uninoculated plant roots. GUS expression observed at the root tip (arrowhead) of primary root (PR; A), lateral root primordium (LRP; B), emerging lateral root (ELR; C), and at the tip (arrowhead) of fully emerged lateral root (LR; D). E to G, Induction of GUS during nodule development. Blue staining observed in the nodule primordium (NP; E), emerging nodule (EN; F), and at the meristematic zone (arrowhead) of a mature nodule (N; G). Bars = 50 μm.
MtCDC16 Gene Expression Is Auxin Regulated
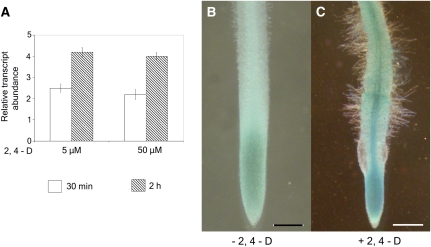
Plant mutants that show defects in different subunits of the SCF ubiquitin ligase exhibit a decreased sensitivity to the plant hormone auxin (Hellmann et al., 2003; Risseeuw et al., 2003; Quint et al., 2005). Since CDC16 functions as a component of APC/C (Passmore, 2004), another important member of the family of ubiquitin ligases, we hypothesized that MtCDC16 is regulated by auxin. Previous reports have demonstrated that TGTCTC motifs in auxin response elements confer auxin responsiveness to some auxin-regulated genes (Ulmasov et al., 1995, 1997). Sequence analysis of the promoter region (−1 to −1,000 bp upstream of the putative transcription initiation site) of MtCDC16 revealed two such motifs at −488 and −581 bp upstream of the putative transcription initiation site, providing initial clues that MtCDC16 could be auxin regulated. To investigate whether the expression of MtCDC16 is indeed auxin mediated, the effect of exogenous auxin on its transcriptional activity was investigated. The roots of the wild-type seedlings depleted of endogenous auxin were incubated in 5 or 50 μm concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) for 30 min and 2 h. Figure 7A presents the transcript abundance of MtCDC16 in response to exogenous auxin, relative to that seen in comparable samples without application of exogenous auxin. The transcript level increased about 2-fold by 30 min after exposure to auxin and remained at elevated levels at 2 h of auxin treatment. In general, the fold induction was similar for the two concentrations of auxin used. Consistent with this finding, transgenic hairy roots from three independent replicates (n = 30) carrying the CDC16:GUS reporter construct displayed strong GUS staining in the vascular bundle and the root tip when exposed to 0.5 μm of synthetic auxin, 2,4-D, for 2 d (Fig. 7C). In contrast, in the absence of applied auxin, the transgenic roots (n = 30) showed GUS expression only at the root tip, but no expression was discernible in the vascular bundle (Fig. 7B).
Figure 7.
Auxin response phenotype of MtCDC16. A, Quantitative RT-PCR analysis to estimate the transcript abundance of MtCDC16 in wild-type roots in the presence and absence of 2,4-D. Each point represents the mean of three replicates with error bars representing the sd. B and C, Histochemical GUS staining patterns of CDC16:GUS in transgenic hairy roots in the absence (B) and presence (C) of 0.5 μm 2,4-D. Bars = 50 μm.
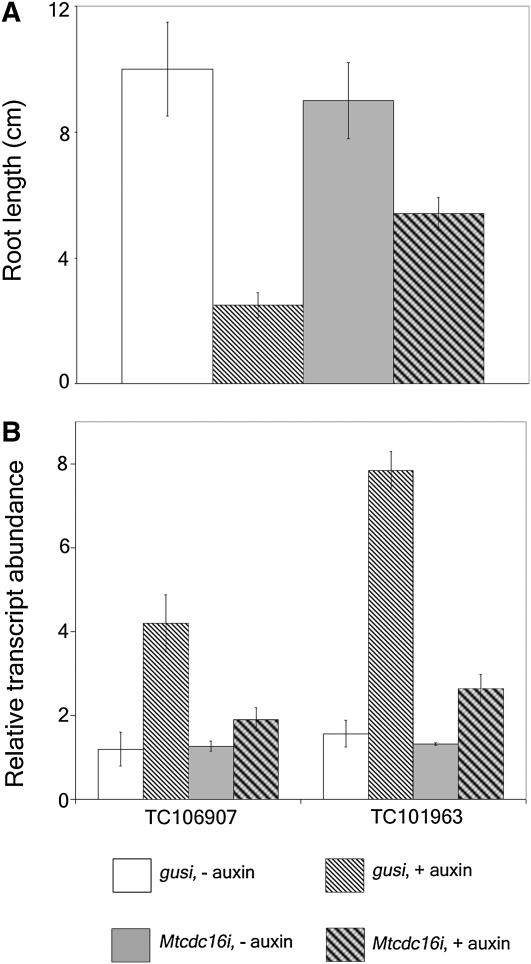
To determine whether the Mtcdc16i roots show resistance to applied auxin in comparison to control roots, we performed a dose-response assay measuring auxin inhibition of root elongation. The root growth assay revealed that growth of control root was inhibited at 0.3 μm 2,4-D and above (data not shown). At 0.5 μm 2,4-D, the control roots from three independent experiments showed almost an 80% reduction in root length (Fig. 8A). The average length of the control roots with no auxin treatment was approximately 10.1 cm. In contrast, the average root length in plants exposed to 0.5 μm 2,4-D auxin was around 2.2 cm. This concentration of 2,4-D was chosen to evaluate the root growth inhibition of Mtcdc16i (moderate) roots. We examined 15 Mtcdc16i (moderate) roots from three independent experiments with and without 2,4-D (n = 15 ± 2 roots examined per pool). This analysis revealed that Mtcdc16i (moderate) roots displayed <40% inhibition in root growth at this concentration of 2,4-D, suggesting that they were much less sensitive to exogenous auxin than were the control roots (Fig. 8A). To further investigate the auxin responsiveness of Mtcdc16i roots, the expression of two transcripts corresponding to TC106907 and TC101963 (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb= medicago) that encode proteins similar to auxin-responsive GH3 and an auxin-induced protein, respectively, was evaluated in two independent experiments. As seen in Figure 8B, their transcript levels were similar in the control roots and in Mtcdc16i roots in the absence of 2,4-D treatment, when the constitutively expressed gene TC105607 was used to normalize values. By contrast, after exposure to 5.0 μm 2,4-D for 30 min, the transcript abundance of TC106907 and TC101963 increased about 3- to 5-fold in control roots but showed only slight increase in Mtcdc16i roots (n = 20 ± 2 roots examined in each pool).
Figure 8.
Auxin resistance phenotype of MtCDC16. A, Root growth assay for auxin resistance. Inhibition of root growth in the presence of 0.5 μm 2,4-D was assayed in control (gusi) and Mtcdc16i roots. B, Quantitative RT-PCR analysis to estimate the transcript abundance of TC106907 and TC101963 in gusi and Mtcdc16i (moderate) roots in the presence and absence of 5 μm 2,4-D. In A and B, each point represents the mean of three replicates with error bars representing the sd.
DISCUSSION
The continuous mitotic activity in the root apical meristem results in the indeterminate growth of primary roots. In primary roots, the activation of pericycle cells leads to de novo meristem and lateral root formation (Jiang and Feldman, 2005). Similarly, in leguminous plants, the presence of rhizobia elicits mitotic reactivation of root cortical cells and formation of meristems that develop into nitrogen-fixing symbiotic nodules (Gage, 2004). Previous studies have identified several core cell cycle-regulating genes that function in both root and nodule formation (Foucher and Kondorosi, 2000; Himanen et al., 2002). The expression and functional analysis of a number of these genes provides a molecular basis that supports a possible integration in the development of these two organs, possibly via auxin signaling (Aloni et al., 2006).
In this study, we took a reverse genetic approach to demonstrate that MtCDC16, a homolog of a core component of APC/C, plays a key role in the developmental pathway of both roots and nodules. The MtCDC16 silenced root system revealed impaired meristem maintenance. This finding correlates well with the study by Vanstraelen et al. (2009) that showed that APC/CCCS52A complexes control meristem maintenance in Arabidopsis roots. Interestingly, down-regulation of MtCDC16 resulted in mature nodules that appeared similar to control nodules in terms of tissue organization except for an enlarged infection zone. Such discrepancies in the zonal length have been observed in nodules that exhibit defects in endoreduplication or in bacteroid differentiation (Vinardell et al., 2003; Vernie et al., 2008). Based on our results and the previous reports, we postulate that in Mtcdc16i nodules, the timing of endoreduplication is impaired, resulting in a delay in the conversion from uninfected type cells with infection threads (an apoplastic compartment) to infected-type cells containing bacteroids in intracellular compartments (symbiosomes). The delay of endoreduplication may impair the release of the rhizobia from the infection threads and/or impair conversion of type 2 to type 3 bacteroids, thus slowing down the differentiation process and perhaps reducing the number of nitrogen-fixing bacteroids in the nodules. Although further experiments will be needed to test these hypotheses, it is consistent with our data that on a per nodule basis the Mtcdc16i roots fixed a relatively lower amount of nitrogen compared to the control roots.
Since the development and structure of legume nodules resemble in some respects that of lateral roots, it has been proposed that their ontogeny may require the same molecular signals (Nutman, 1948; Ferguson and Mathesius, 2003). Supporting this proposition, nts mutants in soybean (Glycine max) and har1 mutants in Lotus japonicus exhibit an excessive number of both nodules and lateral roots (Wopereis et al., 2000). The sequence of the HAR1/NARK protein is very similar to the Arabidopsis CLAVATA1-like receptor kinase that mediates cell-cell signaling during shoot meristem development (Nishimura et al., 2002). Interestingly, suppression of MtCDC16 in M. truncatula roots leads to a decrease in the number of lateral roots but a simultaneous increase in the number of nodules. Such an inverse relationship was observed even at the stage of primordial development (data not shown), thus suggesting that the Mtcdc16i root and nodule phenotype is not due to an impaired outgrowth but is most likely due to abnormal initiation of the two organs. Existence of such inverse relationship between nodule and lateral root development was also noticed in roots silenced for a homolog of CYTOKININ RESPONSE ELEMENT1 (MtCRE1) in M. truncatula. MtCRE1-silenced roots showed significant increase in lateral root development with a simultaneous reduction in nodulation (Gonzalez-Rizzo et al., 2006).
Several studies have suggested that one of the common regulatory signals controlling the development of roots and nodules could be the plant hormone auxin. Using an auxin-responsive GH3 promoter, Mathesius et al. (1998) showed that the two organs share similar auxin requirements during both initiation and emergence. Supporting this idea, de Billy et al. (2001) demonstrated that the MtLAX genes, members of the AUX1 gene family in M. truncatula, are expressed at two common stages during lateral root and nodule development: primordium formation and vasculature differentiation. Independent from this, it has also been found that rhizobia are able to hijack the cells that are activated during lateral root development to form nodules (Mathesius et al., 2000).
Detailed genetic and developmental studies have clearly implicated auxin as the major molecular signal in promoting lateral root development. A larger number of lateral roots occur in plants treated with auxin or those containing a higher level of this hormone (Boerjan et al., 1995; Fukaki et al., 2005), whereas auxin response mutants such as slr-1 (Fukaki et al., 2002, Vanneste et al., 2005), aux1, axr1, and axr4 (Hobbie and Estelle, 1994) produce fewer laterals. The physiological studies with auxin transport inhibitors like naphthylphthalamic acid (NPA) suggest the importance of polar auxin transport in lateral root development. Reed et al. (1998) showed that application of NPA at the root-shoot junction causes a block in the acropetal transfer of auxin in the root, thus inhibiting the lateral root formation. The importance of auxin in root development has been further emphasized by its role in SCF-mediated proteolysis. Mutations in the components of the SCF complex often confer auxin response defects (Dharmasiri and Estelle, 2004; Parry and Estelle, 2006). Such studies with respect to APC subunits have been fairly limited. Our study has provided strong evidence that MtCDC16 is an auxin-responsive gene and that the suppression of MtCDC16 results in auxin insensitivity. This indicates a possibility that auxin could be a key regulatory signal for APC-mediated proteolysis during cell division. Conversely, it is also possible that the defects seen in Mtcdc16i roots are not primarily caused by altered auxin response but in fact are a direct consequence of hampered cell division either due to partial knockdown of MtCDC16 or alternatively due to the disassembly of the APC complex as a result of the knockdown of MtCDC16. Such a possibility was tested with the hbt mutant (encoding CDC27) by Blilou et al. (2002), who found that although HBT influences the stability of some auxin response regulators, the defect seen in the mutant was not due to perturbed auxin perception (Serralbo et al., 2006). Future studies on MtCDC16 will be directed toward investigating these possibilities.
Numerous studies so far have suggested the involvement of polar auxin transport in nodulation. Application of auxin transport inhibitors like NPA to roots results in nodule-like structures on the roots of alfalfa (Hirsch et al., 1989). Also, application of the rhizobial Nod factor was found to cause a transient reduction in auxin transport in vetch roots (Boot et al., 1999). In parallel to this, Mathesius et al. (1998) demonstrated that in white clover roots, spot inoculation with rhizobia leads to an acropetal decrease in transport of auxin followed by its basipetal accumulation resulting in nodule primordium formation. In general, it has been suggested that rhizobial Nod factors cause a localized induction of flavonoids in the roots that in turn may perturb the polar auxin transport by acting as endogenous auxin transport inhibitors (Hirsch, 1992). The disturbance in the mobilization of auxin results in its localized accumulation that further activates cortical cell division and nodule formation. Consistent with this idea, it has been recently shown that flavonoid-deficient roots generated by silencing the flavonoid biosynthetic pathway are much less competent in forming nodules and also show altered auxin transport (Wasson et al., 2006).
After inoculation with rhizobia, the Mtcdc16i roots form 3- to 4-fold more nodules as compared to control roots. Based on this result, it is tempting to speculate that auxin insensitivity might lead to an increase in nodule number in Mtcdc16i roots. Alternatively, reduction in numbers of lateral root meristems as a result of defective cell cycle progression could trigger initiation of more nodule meristems. This alternative hypothesis opens up a new possibility of an as yet undefined mechanism wherein the plant imposes a regulatory control that balances out the reduction in lateral root meristems with a greater number of nodule meristems in the Mtcdc16i root. Further study is needed to distinguish between these possibilities.
Two hypernodulating mutants of M. truncatula, sunn (Schnabel et al., 2005) and skl (Penmetsa and Cook, 1997), show an increased transport of auxin from shoot to root compared to the wild-type plants (Prayitno et al., 2006; van Noorden et al., 2006). The wild-type plants show an increased flow of auxin from shoot to the root immediately after inoculation with rhizobia, but this is followed by a significant decrease 24 h after inoculation. In contrast, both sunn and skl maintain increased auxin transport for a longer period, even a day after inoculation. SUNN, a homolog of HAR1, controls nodule numbers through the autoregulation of nodulation pathway, whereas SKL is known to regulate nodule numbers through the ethylene pathway (Oka-Kira and Kawaguchi, 2006; Penmetsa et al., 2008). In spite of the two genes being regulated by two different pathways, it is very striking that the corresponding mutants show a similar auxin transport phenotype, suggesting that auxin is a key regulator of nodule numbers. It will be interesting to determine whether Mtcdc16i roots are also altered in auxin transport. Whether due to auxin insensitivity or altered auxin transport, our data provide the possibility of a third gene, MtCDC16, that controls nodule number in an auxin-dependent manner.
MATERIALS AND METHODS
Plasmid Construction
To create an RNAi construct of MtCDC16, a region corresponding to −143 to 354 nucleotides (relative to the ATG start codon) was amplified from a corresponding cDNA clone (pKVKC-6A3; GenBank accession no. BQ165117). The amplified fragment was introduced into the RNAi-inducing pHellsgate 8 vector (Wesley et al., 2001) using the GATEWAY system (Invitrogen). The pHellsgate 8 vector carrying a fragment of the GUS gene was used as a control. The resulting recombinant constructs were introduced into Agrobacterium rhizogenes ARqua1 and used for plant transformation as described (Ivashuta et al., 2005).
Plant Growth Conditions
Medicago truncatula L416 seeds (A17 containing a PROENOD11-GUS construct; Journet et al., 2001) were used for transformation and generation of transgenic roots on Fahraeus-agar medium supplemented with 22.5 to 27 mg/L of kanamycin. The transgenic roots thus generated were used for scoring visible root phenotypes using a Nikon SMZ1500 zoom stereomicroscope (DIAPHOT 200) between 7 and 10 d after transformation. For nodulation experiments, plants showing a visible root phenotype were transferred to either aeroponic chambers or to Turface (Profile Products) and inoculated with Sinorhizobium meliloti ABS7M as described previously (Kuppusamy et al., 2004). Plants transformed with different constructs were grown independently in different aeroponic systems. Nodules were scored using a Nikon SMZ1500 zoom stereomicroscope (DIAPHOT 200) between 10 and 15 d after inoculation in plants grown in aeroponics and at 20 d after inoculation in those grown in Turface.
Gene Structure and Promoter-Reporter Fusion Analysis of MtCDC16
The cDNA clone, pKVKC-6A3 (GenBank accession no. BQ165117), the sequence of which is similar to Arabidopsis (Arabidopsis thaliana) CDC16, was identified at MtGI, version 8.0 (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb= medicago). The clone was sequenced in its entirety from the 3′ end. A search of the M. truncatula BAC library database (http://www.medicago.org/genome/blast.php) using the full-length cDNA sequence identified the MtCDC16 gene on an 11.9-kb region of a BAC clone, mth2-4n3 (GenBank accession no. AC121241). Using the GENSCAN program (http://genes.mit.edu/GEN SCAN.html; Burge and Karlin, 1997) and the PileUP multiple sequence alignment algorithm from GCG (Wisconsin Package Version 10.3; Accelrys), alignment of the putative MtCDC16 gene sequence with the cDNA was carried out, and the gene structure of MtCDC16 was predicted.
For generating a promoter-reporter construct, a PCR-amplified HindIII-HindIII fragment containing an approximately 2.3-kb region upstream of the predicted translational start site of MtCDC16 was cloned into the HindIII site of binary plasmid vector pBI101.1 (Jefferson et al., 1987). The primers used were 5′-aagaagcttGAACGCCTGATGCATCCGC-3′ and 5′-aagaagcttCGAAGCTTCTCTATCTCTTG-3′ (the lowercase letters in each primer represent the addition of HindIII site to the amplified fragment). This construct was sequenced to confirm the presence and the orientation of the cloned fragment with respect to the GUS gene. The binary vector was transformed into A. rhizogenes strain ARquaI using standard methods. Transformed roots were created using M. truncatula A17 plants as described previously (Boisson-Dernier et al., 2001). Transgenic roots, before and after rhizobial inoculation, were stained with GUS assay buffer (Jefferson et al., 1987). Similarly, to test for auxin responsiveness of the promoter, the transgenic roots were incubated in Fahraeus-agar medium with or without 0.5 μm 2,4-D and were stained, as described above. The stained roots were cleared by incubation in 0.24 n HCl and 20% ethanol at 57°C for 15 min, 7% NaOH in 60% ethanol for 15 min at room temperature, followed by 40%, 20%, and 10% ethanol for 5 min each. The cleared roots were mounted on slides with 50% glycerol and observed under a Nikon microscope (DIAPHOT 200) using Nomarski optics. Pictures were taken with a Nikon E4500 digital camera. The expression pattern described was observed in a minimum of 15 independent transgenic roots.
Nucleic Acid Isolation and Analysis
Genomic DNA was extracted from young leaves of A17 plants using the DNeasy plant maxi kit (Qiagen) according to the manufacturer's instructions. Independent restriction digestion reactions were carried out overnight with HindIII, PvuII, and XbaI each using approximately 15 μg of the DNA. Samples of the digested DNA were electrophoresed (2.5 V/cm) overnight and transferred to Hybond N+ nylon membrane (Amersham Biosciences). The membrane was then hybridized with the labeled gene-specific probe, washed, and exposed to Hyperfilm ECL following the instructions from ECL direct nucleic acid labeling and detection systems (Amersham Biosciences).
Root samples (n = 20 ± 2) were harvested from uninoculated plants growing on Fahraeus-agar medium and nodulating plants (20 ± 2) growing in aeroponics. Samples from two independent biological replicates were pooled for RNA extraction using the RNeasy plant kits (Qiagen). DNAse treatment, cDNA synthesis, and quantitative RT-PCR analysis were carried out as described previously (Kuppusamy et al., 2004). The following sets of primers were used for RT-PCR of MtCDC16 transcripts: P1 (sense), 5′-AACAAGAGATAGAGAAGCTTCG-3′; P1' (antisense), 5′-GATCTTTATCCAAG TAGATA-3′; P2 (sense), 5′-AGAAGCCAAGTTTAGAGAG-3′; and P2' (antisense), 5′-GAGAGAAATGAATGGACA-3′. P1 primers were designed from the sequence within the region used for the RNAi construct, and P2 primers were from the region downstream of that used for the RNAi construct. To estimate the transcript abundance of TC106907 and TC101963, quantitative RT-PCR analysis was carried out with two technical replications as described previously (Kuppusamy et al., 2004). The gene-specific primers used were as follows: TC106907 (5′-TTATCACATATGAAGATCTGATT-3′; 5′-TTAGATTCATTCCAAAGCATGTAC-3′), TC101963 (5′-GTGGTTCCAATATCATACTTGA-3′; 5′-TGCATTCATCAATCTTACCTCT-3′), and TC105607 (5′-GGCAGGTCTGCCTATGGTTA-3′; 5′-GGTCAGACGCACAGATTTGA-3′).
Tissue Processing, Sectioning, and Staining
For morphological studies, tissues were processed using a microwave-assisted processing method (Rangell and Keller, 2000). Briefly, root samples were fixed for 40 s at approximately 240 W under vacuum in 4% glutaraldehyde in phosphate buffer, pH 7.2, using a Pelco Laboratory Microwave oven (model 3441; Ted Pella). After two buffer rinses, the tissues were dehydrated in an incremental ethanol series for 40 s each step at 240 W in the microwave. Ethanol was replaced with methacrylate resin (Kulzer) by immersion for 3 min each in 2:1 and 1:1 (v/v) mixtures of ethanol:resin in the microwave at 450 W under vacuum. This was replaced two or more times with pure resin to obtain a complete infiltration before polymerization at 60°C overnight. A series of semithin (1 μm) sections were cut using an RMC MT-7000 ultramicrotome (Boeckeler Instruments) and placed on poly-l-Lys-coated slides (Corning). Sections were stained using 0.1% toluidine blue stain and observed using bright-field optics.
Nodules were fixed in 4% paraformaldehyde and 0.25% glutaraldehyde in 50 mm sodium phosphate buffer, pH 7.2. Tissue was dehydrated through an ethanol and xylene series and embedded in Paraplast. Longitudinal sections (10 μm) of Mtcdc16i and control nodules of comparable size were stained with toluidine blue. Nodule zone lengths were measured along the medial axis of the nodule sections. The proc t test (SAS version 9.1) was used to compare zone lengths between the Mtcdc16i and control nodule sections.
Acetylene Reduction Assay
Nitrogenase activity of intact nodules on whole-root systems was estimated by the acetylene reduction assay as previously described (Vance et al., 1979). Samples consisting of a hairy root system harvested from an aeroponics chamber at 15 d after inoculation were measured for acetylene reduction in 2-mL sealed vials in the presence of 10% acetylene. After 1 h of incubation in acetylene, a sample (1 mL) was injected into a Photovac 10S Plus Gas C chromatograph (Vance et al., 1979; Photovac). Samples were compared to a standard curve generated against a 5 μL L−1 ethylene standard, and nitrogenase activity was expressed as pmoles of ethylene evolved (per minute/plant).
Auxin Response Assay
The wild-type A17 seedlings were grown in aeroponics for 6 d. Approximately 60 mg of whole-root tissue was cut into small pieces of 2 to 3 mm in length and incubated for a total of 4 h at 30°C in KPSC buffer (10 mm K2HPO4, 2% Suc, and 50 μg/mL of chloramphenicol) to deplete the endogenous auxin (Timpte et al., 1994). The auxin-depleted root tissue was distributed into three aliquots of approximately 20 mg each and incubated in three different concentrations of synthetic auxin (2,4-D): 0, 5, and 50 μm in KPSC (Timpte et al., 1994) for either 30 min or 2 h and frozen separately. Samples were pooled from two independent biological replicates for RNA extraction and quantitative RT-PCR analysis as described above. The P1 and P1' primers described earlier were used for this analysis. Relative transcript abundance was determined by monitoring the transcript abundance of selected genes in the 2,4-D-treated samples relative to the buffer-treated samples.
Root Growth Assay for Auxin Resistance
Control plants (gusi) carrying the transgenic hairy roots were transferred to Fahraeus medium containing different concentrations of 2,4-D. Root growth was recorded after 6 d and expressed as a percentage of root growth on medium without 2,4-D. The concentration of 2,4-D that showed root growth inhibition in the control plants (0.5 μm) was selected for the root growth assay of the MtCDC16i plants.
Sequence data from this article can be found in the GenBank data library under accession numbers GU075685 and GU075686.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Longitudinal section of Mtcdc16i moderate root meristem.
Supplementary Material
Acknowledgments
The authors thank Susan S. Miller for helping in the acetylene reduction assay and William Gray for providing help in the auxin response assay. The authors are also grateful for the critical input provided by the two anonymous reviewers.
This work was supported by the U.S. Department of Energy (grant no. DE–FG02–01ER15201 to K.A.V.) and by funding from the National Science Foundation (award no. DBI–0421676 to J.S.G.). K.T.K. was supported by a Bernard and Jean Phinney Fellowship and a Plant and Microbial Genetics Institute Fellowship from University of Minnesota.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kathryn A. VandenBosch (vande102@umn.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot (Lond) 97 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Scheres B (2008) Auxin: the looping star in plant development. Annu Rev Plant Biol 59 443–465 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM (2007) Common regulatory themes in meristem development and whole-plant homeostasis. Curr Opin Plant Biol 10 44–51 [DOI] [PubMed] [Google Scholar]
- Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, Eloy NB, Ferreira PC, Weisbeek P, Scheres B (2002) The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes Dev 16 2566–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Becard G, Rosenberg C, Barker DG (2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14 695–700 [DOI] [PubMed] [Google Scholar]
- Boot KJM, van Brussel AAN, Tak T, Spaink HP, Kijne JW (1999) Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativa subsp. nigra roots. Mol Plant Microbe Interact 12 839–844 [Google Scholar]
- Burge C, Karlin S (1997) Prediction of complete gene structures in human genomic DNA. J Mol Biol 268 78–94 [DOI] [PubMed] [Google Scholar]
- Capron A, Okresz L, Genschik P (2003. a) First glance at the plant APC/C, a highly conserved ubiquitin-protein ligase. Trends Plant Sci 8 83–89 [DOI] [PubMed] [Google Scholar]
- Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, Lecureuil A, Guerche P, Kondorosi E, Scheres B, et al (2003. b) The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. Plant Cell 15 2370–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8 165–171 [DOI] [PubMed] [Google Scholar]
- Cebolla A, Vinardell JM, Kiss E, Olah B, Roudier F, Kondorosi A, Kondorosi E (1999) The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J 18 4476–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea LD, Regan L (2003) TPR proteins: the versatile helix. Trends Biochem Sci 28 655–662 [DOI] [PubMed] [Google Scholar]
- de Billy F, Grosjean C, May S, Bennett M, Cullimore JV (2001) Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol Plant Microbe Interact 14 267–277 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Boniotti MB, Gutierrez C (2002) Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14 3057–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Inze D (2007) The ins and outs of the plant cell cycle. Nat Rev Mol Cell Biol 8 655–665 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Estelle M (2004) Auxin signaling and regulated protein degradation. Trends Plant Sci 9 302–308 [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mathesius U (2003) Signaling interactions during nodule development. J Plant Growth Regul 22 47–72 [Google Scholar]
- Foucher F, Kondorosi E (2000) Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol Biol 43 773–786 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M (2005) Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J 44 382–395 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29 153–168 [DOI] [PubMed] [Google Scholar]
- Gage DJ (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68 280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschik P, Criqui MC, Parmentier Y, Derevier A, Fleck J (1998) Cell cycle-dependent proteolysis in plants. Identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor mg132. Plant Cell 10 2063–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F (2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann H, Estelle M (2002) Plant development: regulation by protein degradation. Science 297 793–797 [DOI] [PubMed] [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M (2003) Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22 3314–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inze D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM (1992) Developmental biology of legume nodulation. New Phytol 122 211–237 [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bhuvaneswari TV, Torrey JG, Bisseling T (1989) Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci USA 86 1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1994) Genetic approaches to auxin action. Plant Cell Environ 17 525–540 [DOI] [PubMed] [Google Scholar]
- Ivashuta S, Liu J, Liu J, Lohar DP, Haridas S, Bucciarelli B, VandenBosch KA, Vance CP, Harrison MJ, Gantt JS (2005) RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 17 2911–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ (2005) Regulation of root apical meristem development. Annu Rev Cell Dev Biol 21 485–509 [DOI] [PubMed] [Google Scholar]
- Journet EP, El-Gachtouli N, Vernoud V, de Billy F, Pichon M, Dedieu A, Arnould C, Morandi D, Barker DG, Gianinazzi-Pearson V (2001) Medicago truncatula ENOD11: a novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. Mol Plant Microbe Interact 14 737–748 [DOI] [PubMed] [Google Scholar]
- Kondorosi E, Redondo-Nieto M, Kondorosi A (2005) Ubiquitin-mediated proteolysis. To be in the right place at the right moment during nodule development. Plant Physiol 137 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy KT (2005) Genetic and genomic approaches to dissect nodulation in the model legume Medicago truncatula. PhD thesis. University of Minnesota, St. Paul
- Kuppusamy KT, Endre G, Prabhu R, Penmetsa RV, Veereshlingam H, Cook DR, Dickstein R, VandenBosch KA (2004) LIN, a Medicago truncatula gene required for nodule differentiation and persistence of rhizobial infections. Plant Physiol 136 3682–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee HS, Sundaresan V (2003) The NOMEGA gene required for female gametophyte development encodes the putative APC6/CDC16 component of the Anaphase Promoting Complex in Arabidopsis. Plant J 36 853–866 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14 23–34 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Weinman JJ, Rolfe BG, Djordjevic MA (2000) Rhizobia can induce nodules in white clover by “hijacking” mature cortical cells activated during lateral root development. Mol Plant Microbe Interact 13 170–182 [DOI] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420 426–429 [DOI] [PubMed] [Google Scholar]
- Nutman PS (1948) Physiological studies on nodule formation - The relation between nodulation and lateral root formation in red clover. Ann Bot (Lond) 12 81–96 [Google Scholar]
- Oka-Kira E, Kawaguchi M (2006) Long-distance signaling to control root nodule number. Curr Opin Plant Biol 9 496–502 [DOI] [PubMed] [Google Scholar]
- Parry G, Estelle M (2006) Auxin receptors: a new role for F-box proteins. Curr Opin Cell Biol 18 152–156 [DOI] [PubMed] [Google Scholar]
- Passmore LA (2004) The anaphase-promoting complex (APC): the sum of its parts? Biochem Soc Trans 32 724–727 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR (1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275 527–530 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR (2003) Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol 131 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Uribe P, Anderson J, Lichenzveig J, Gish JC, Nam YW, Engstrom E, Xu K, Sckisel G, Pereira M, et al (2008) The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J 55 580–595 [DOI] [PubMed] [Google Scholar]
- Perez-Perez JM, Serralbo O, Vanstraelen M, Gonzalez C, Criqui MC, Genschik P, Kondorosi E, Scheres B (2008) Specialization of CDC27 function in the Arabidopsis thaliana anaphase-promoting complex (APC/C). Plant J 53 78–89 [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW (2000) The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 14 655–665 [PMC free article] [PubMed] [Google Scholar]
- Prayitno J, Rolfe BG, Mathesius U (2006) The ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiol 142 168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint M, Ito H, Zhang W, Gray WM (2005) Characterization of a novel temperature-sensitive allele of the CUL1/AXR6 subunit of SCF ubiquitin-ligases. Plant J 43 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangell LK, Keller GA (2000) Application of microwave technology to the processing and immunolabeling of plastic-embedded and cryosections. J Histochem Cytochem 48 1153–1159 [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL (2003) Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J 34 753–767 [DOI] [PubMed] [Google Scholar]
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J (2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58 809–822 [DOI] [PubMed] [Google Scholar]
- Serralbo O, Perez-Perez JM, Heidstra R, Scheres B (2006) Non-cell-autonomous rescue of anaphase-promoting complex function revealed by mosaic analysis of HOBBIT, an Arabidopsis CDC27 homolog. Proc Natl Acad Sci USA 103 13250–13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7 847–859 [DOI] [PubMed] [Google Scholar]
- Timpte C, Wilson AK, Estelle M (1994) The axr2-1 mutation of Arabidopsis thaliana is a gain of function mutation that disrupts an early step in auxin response. Genetics 138 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1997) ARF1, a transcription factor that binds to auxin response elements. Science 276 1865–1868 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Liu Z, Hagen G, Guilfoyle TJ (1995) Composite structure of auxin response elements. Plant Cell 7 1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Heichel GH, Barnes DK, Bryan JW, Johnson LE (1979) Nitrogen fixation, nodule development, and vegetative regrowth of alfalfa (Medicago sativa L.) following harvest. Plant Physiol 64 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, et al (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U (2006) Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol 140 1494–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen M, Baloban M, Da Ines O, Cultrone A, Lammens T, Boudolf V, Brown SC, Veylder LD, Mergaert P, Kondorosi E (2009) APC/CCCS52A complexes control meristem maintenance in the Arabidopsis root. Proc Natl Acad Sci USA 106 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J, DeBilly F, Camut S, Truchet G (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172 4295–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernie T, Moreau S, de Billy F, Plet J, Combier JP, Rogers C, Oldroyd G, Frugier F, Niebel A, Gamas P (2008) EFD is an ERF transcription factor involved in the control of nodule number and differentiation in Medicago truncatula. Plant Cell 20 2696–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinardell JM, Fedorova E, Cebolla A, Kevei Z, Horvath G, Kelemen Z, Tarayre S, Roudier F, Mergaert P, Kondorosi A, et al (2003) Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15 2093–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson AP, Pellerone FI, Mathesius U (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27 581–590 [DOI] [PubMed] [Google Scholar]
- Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, De Bruijn FJ, Stougaard J, Szczyglowski K (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23 97–114 [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Kimura S (2005) Cell cycle regulation through ubiquitin/proteasome-mediated proteolysis. J Agric Res 39 1–4 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.