Abstract
Carrageenans are sulfated galactans found in the cell walls of numerous red seaweeds (Rhodophyta). They are classified according to the number and the position of sulfate ester groups and the occurrence of 3,6-anhydro-galactose. Although the carrageenan biosynthesis pathway is not fully understood, it is usually accepted that the last step consists of the formation of a 3,6-anhydro ring found in κ- and ι-carrageenans through the enzymatic conversion of d-galactose-6-sulfate or d-galactose-2,6-disulfate occurring in μ- and ν-carrageenan, respectively. We purified two enzymes, sulfurylase I (65 kD) and sulfurylase II (32 kD), that are able to catalyze the conversion of ν- into ι-carrageenan. We compared their sulfate release rates (i.e. arising from the formation of the anhydro ring) with the viscosity of the solution and demonstrated two distinct modes of action. In addition, we found that some mixtures of sulfurylase I and II lead to the formation of carrageenan solutions with unexpectedly low viscosities. We discuss the implication of these findings for the assembly of a densely aggregated matrix in red algal cell walls.
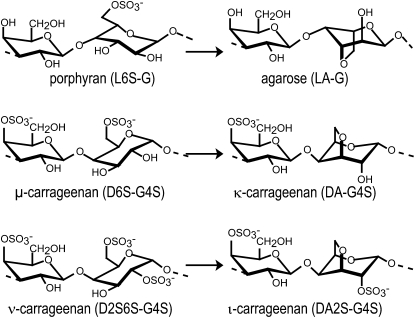
Agars and carrageenans are the most abundant components of the cell walls in numerous red algae (Rhodophyta) and can represent up to 50% of algal dry weight. These sulfated galactans are densely packed in the cell wall in a three-dimensional solid network of pseudocrystalline fibers, which assemble during the deposition of cell wall macromolecules (Craigie, 1990). Sulfated galactans constitute a large family of hydrocolloids that are made up of linear chains of Gal with alternating α(1→3) and β(1→4) linkages. In agarose, the α-linked Gal units are in the l configuration (l unit), while in carrageenans, they are in the d configuration (d unit; Rees, 1969). Agarose refers to an unmodified neutral backbone of agarobioses (LA-G) of which up to 20% may carry methyl groups or sulfate ester groups (Fig. 1). Carrageenans are classified according to the number and position of sulfated esters (S) and by the occurrence of a 3,6-anhydro ring in the α-linked residues (DA unit) found in gelling κ-carrageenan (DA-G4S) and ι-carrageenan (DA2S-G4S; Rees, 1969; Knutsen et al., 1994; Usov, 1998). However, carrageenans have very heterogeneous chemical structures, depending on algal source, life stage, and extraction procedure (Craigie, 1990; Usov, 1998). This structural complexity is attributed to the occurrence of a mixture of carrageenans in extracts as well as to the presence of hybrid or copolymer chains that arise when ideal carrabiose motifs co-occur in purified carrageenans (Greer and Yaphe, 1984; Craigie, 1990; Bixler, 1996; Guibet et al., 2008). The most well-known carrageenan copolymers are those found in native or unprocessed κ- and ι-carrageenan chains. They usually contain fractions of their biosynthetic precursors named μ-carrageenan (D6S-G4S) and ν-carrageenan (D2S6S-G4S), respectively (Fig. 1; Bellion et al., 1983; van de Velde et al., 2002b).
Figure 1.
Formation of agarose, κ-, and ι-carrageenan repeating disaccharide moieties generated enzymatically from their respective biosynthetic precursors, porphyran, μ-, and ν-carrageenan.
The biosynthetic pathways of agars and carrageenans are currently only hypothetical. However, based on the structures of agarobiose and carrabiose moieties that co-occur in polysaccharide chains, the proposed sequences of catalytic events accounting for the chemical structures of agars and carrageenans usually assume that three classes of enzymes are involved: galactosyl transferases, sulfotransferases, and Gal-6-sulfurylases (in agarose; Rees, 1961a, 1961b), referred to as “sulfohydrolases” in carrageenans (Wong and Craigie, 1978). The α- and β-galactosyl transferases catalyze the polymerization of the Gal backbone. Sulfotransferases decorate the neutral Gal chain with sulfate side groups. These reactions have been shown to take place in the Golgi apparatus, but none of these enzymes have been isolated in vitro (Tveter-Gallagher et al., 1984; Gretz et al., 1990). Only the final step of agarose biosynthesis has been unambiguously demonstrated with the characterization of the enzymatic activity of Gal-6-sulfurylases, which catalyze the formation of the 3,6-anhydro rings in agarose (Rees, 1961a, 1961b). This reaction was also shown to occur in vivo (Hemmingson et al., 1996a, 1996b). The formation of the κ- and ι-carrageenan anhydro rings was also observed after incubation of μ- and ν-carrageenans, respectively, with red algal protein extracts that have been named sulfohydrolases (Wong and Craigie, 1978; Zinoun et al., 1997). These observations confirm that the formation of anhydro rings is catalyzed at the final step of biosynthesis.
The anhydro ring is a chemical structure widely used in carbohydrate chemistry to selectively modify sugar residues (Varma et al., 2004; Hou and Lowary, 2009; Tanaka et al., 2009). In contrast to synthetic moieties, there are very few examples of sugar residues with an anhydro ring in vivo. To our knowledge, in addition to the 3,6-anhydro-Gal residues encountered in agars and carrageenans, the only other documented natural anhydro ring is the 1,6-anhydro N-acetyl-muramic acid located at the glucan chain end of a peptidoglycan (murein; Hölfte et al., 1975; Heidrich and Wollmer, 2002). This 1,6-anhydro ring is an intraresidue glycosidic bond and occurs when a glycosidic bond is cleaved via a transglycosylation mechanism. The enzymatic mechanism leading to the 3,6-anhydro ring in agars and carrageenans is, to date, unknown. Nevertheless, the anhydro ring is probably the result of a nucleophilic substitution where the ester sulfate located at position 6 is replaced with the hydroxyl group at position 3, similar to the reaction that occurs when carrageenans are treated with alkalis (Cianca et al., 1997; Viana et al., 2004). The conversion of the α-d-Gal-6-sulfate (μ-carrabiose) and 2,6-disulfate (ν-carrabiose) into their corresponding 3,6-anhydro derivatives greatly reduces the hydrophilic nature of the Gal residues, inverts the chair conformation of the pyranose rings from 1C4 to 4C1, and, as a consequence, allows the carrageenans to adopt a helical conformation required for sol/gel transition (Lawson and Rees, 1970; van de Velde et al., 2002b).
The Gal-6-sulfurylases found in agarophytes and the analogous sulfohydrolase activities extracted from carrageenophytes represent a novel class of enzymes owing to the chemical reactions they catalyze as well as to their involvement in building the matrix of the cell wall. In addition, these enzymes have only been found in the red algal lineage. As a consequence, they represent one of the evolutionary innovations associated with the emergence of red seaweeds. In this context, we purified and biochemically characterized the enzymes that catalyze the formation of anhydro rings in carrageenans. We found that at least two enzymes convert ν- into ι-carrageenan in Chondrus crispus gametophytes and that these enzymes differ in their Mr and in their mode of action. In addition, we prepared a viscous carrageenan solution whose properties were directly related to the mode of action of these enzymes. The implications of our findings are discussed in the context of cell wall biosynthesis. More specifically, given that carrageenans are reputed to give high-strength gels at low concentrations, we suggest that sulfurylases allow carrageenans to be densely packed in algal cell walls.
RESULTS
Purification of Enzymes That Modify ν-Carrageenans
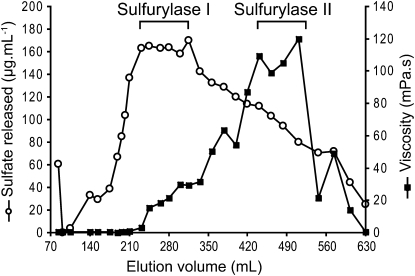
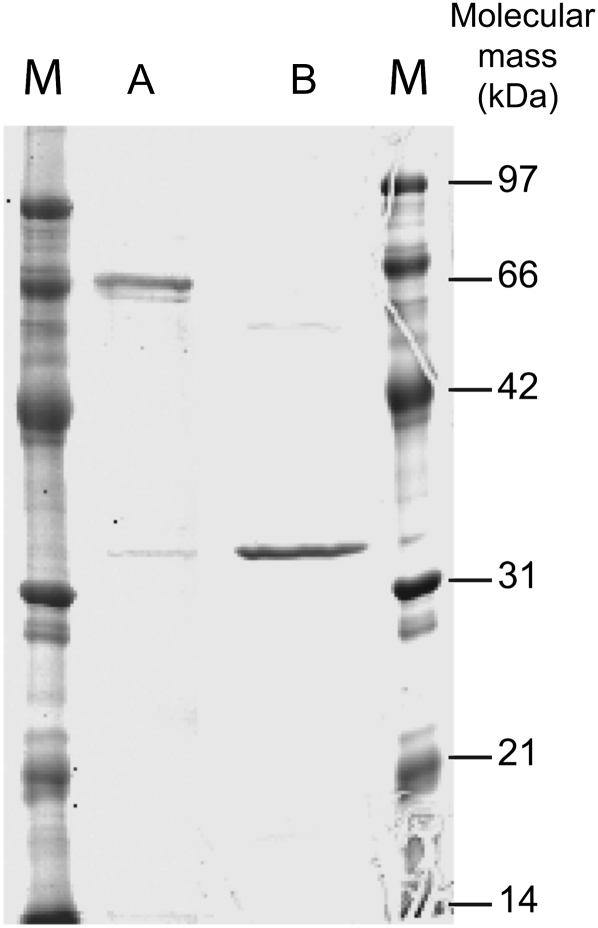
A combination of different chromatographic methods were used to isolate two enzymes from C. crispus protein extracts, namely sulfurylase I and II, which converted ν- into ι-carrageenan. At each stage of purification, specific enzyme activity was determined and combined with SDS-PAGE and Coomassie Brilliant Blue staining of the protein fractions. Results from a typical sulfurylase purification experiment are presented in Table I. The first chromatographic step of purification using hydrophobic interaction chromatography on phenyl-Sepharose essentially removed the red phycoerythrin pigment. The subsequent DEAE-Sepharose chromatography highlighted two distinct sulfurylase activities. The first fraction, which led to a significant release of sulfate groups without substantially modifying the viscosity of the medium, was observed at elution volumes of about 200 to 350 mL (i.e. about 300–580 mm NaCl) and was named sulfurylase I (Fig. 2). The second enzyme, sulfurylase II, eluted at about 400 to 500 mL (i.e. about 650–800 mm NaCl) and induced a strong increase in viscosity that was associated with a moderate release of sulfate (Fig. 2). At this stage, sulfurylase II was pure and its mass was estimated by SDS-PAGE at about 32 kD (Fig. 3). An additional step of semiaffinity chromatography on a HiTrap Heparin column was required to obtain pure sulfurylase I, which migrated as a 65-kD single band by SDS-PAGE (Fig. 3).
Table I.
Purification yields of sulfurylases
| Purification Step | Protein | Yield | Total SO42− | Specific Activity |
|---|---|---|---|---|
| mg | % | μg | μm mg−1min−1 | |
| Crude extract | 104.9 | 100.0 | 581,460 | 56.3 |
| (NH4)2SO4 fraction | 84.1 | 80.2 | 457,753 | 55.3 |
| Phenyl-Sepharose | 24.8 | 23.6 | 356,559 | 145.9 |
| DEAE-Sepharose | ||||
| Sulfurylase I | 4.7a | 4.5 | 166,215 | 359.0 |
| Sulfurylase II | 0.1 | 0.1 | 40,215 | 4,082.9 |
| HiTrap Heparin HP | ||||
| Sulfurylase I |
0.1 |
0.1 |
39,331 |
3,993.1 |
At this stage, sulfurylase I was still contaminated and was further purified on a HiTrap Heparin column, as described in “Materials and Methods.”
Figure 2.
Chromatographic purification of sulfurylases I and II on a DEAE-Sepharose fast-flow column (2 × 22 cm). Elution of proteins was performed using an increasing gradient of NaCl. The concentration of released sulfate (white circles) and the viscosity (black squares) of the resulting polymer obtained after incubation with ν-/ι-carrageenan are presented for the different fractions.
Figure 3.
SDS-PAGE of purified sulfurylase I (lane A) and II (lane B) stained with Coomassie Brilliant Blue R-250. Lanes M, Size markers.
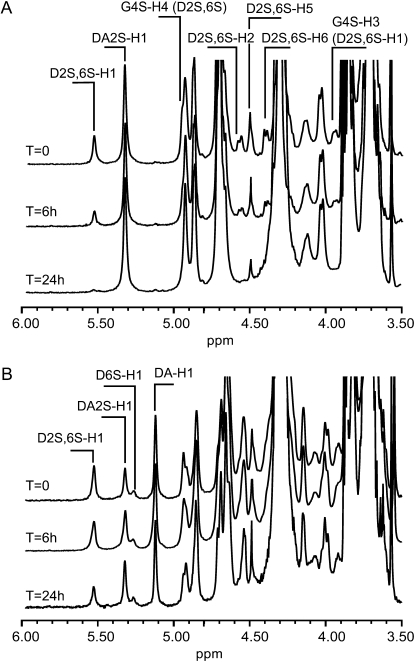
During the preliminary purification experiments, the cyclization of the 3,6-anhydro ring was determined chemically according to Jol et al. (1999), and the release of sulfate was correlated with an increase of 3,6-anhydro-Gal (data not shown). Consequently, the purification protocol of the sulfurylases was mainly based on their ability to catalyze the release of sulfate, which was assayed by high-performance anion-exchange chromatography (HPAEC). The sulfurylases' capacity to increase viscosity was also measured using a viscosimeter. This increase in viscosity excluded, for example, the possibility that carrageenan-degrading enzymes (i.e. carrageenases) co-occurred in the extract. If they had been present, polysaccharide molecular mass would have been reduced, resulting in a lower viscosity of the carrageenan solutions. These analytical methods were chosen because they require only minute amounts of proteins to demonstrate enzyme activity. However, neither the release of sulfate nor the increase in viscosity definitely proves the formation of the 3,6-anhydro ring. Nonetheless, sulfurylase activities were unambiguously characterized by 1H NMR experiments performed with the two purified proteins (Fig. 4A). The ν- and ι-carrabiose moieties were characterized by the signal of the anomeric proton of the α-linked Gal (D2S,6S-H1 and DA2S) resonating at 5.5 and 5.3 ppm, respectively (van de Velde et al., 2002a). Before incubating the ν-/ι-carrageenan, the ν-carrabiose to ι-carrabiose ratio was estimated at about 19.5% by integrating the anomeric signals. During incubation with sulfurylase I, the signal of the anomeric proton (D2S,6S-H1) as well as signals ascribed to other protons of the ν-carrabiose moieties (D2S,6S-H2, -H3, and -H6) strongly decreased. At the end of the enzymatic reaction, only the proton signal corresponding to ι-carrabiose moieties occurred without any new signals. This suggests that ν-carrabiose units were converted into ι-carrabiose units. Similar results were obtained with sulfurylase II (data not shown).
Figure 4.
1H NMR spectra of the conversion kinetics of ν- to ι-carrabiose using ν-/ι-carrageenan (A) and hybrid ν-/ι-/κ-/μ-carrageenan (B) as substrates. These substrates were incubated with sulfurylase I (52 ng) at 40°C for up to 24 h. The anomeric protons of the various carrabiose units (ν-, D2S,6S-H1; ι-, DA2S-H1; μ-, D6S-H1; κ-, DA-H1) as well as the protons corresponding to ν-carrabiose moieties are indicated on the spectra.
Similar experiments were conducted on hybrid κ-/μ-/ι-/ν-carrageenan. The 1H NMR spectrum of this carrageenan was more complex (Fig. 4B), but signals corresponding to the anomeric protons were straightforwardly ascribed (van de Velde et al., 2002a) to D2S,6S-H1 (ν-; 5.5 ppm), DA2S-H1 (ι-; 5.32 ppm), D6S-H1 (μ-; 5.27 ppm), and DA-H1 (κ-; 5.11 ppm). By integrating the anomeric signal, we estimated the composition in ν-, ι-, μ-, and κ-carrabiose moieties at about 23.5%, 21.6%, 8.9%, and 45.9%, respectively. After incubation with sulfurylase, the signals corresponding to ν-carrabiose decreased dramatically, as previously observed with ν-/ι-carrageenan. Using the κ-carrabiose moieties as an internal reference, the decrease in intensity of the D2S,6S-H1 signal was equal to the increase in the DA2S-H1 signal, thus demonstrating that ν-carrabiose moieties were converted into ι-carrabiose moieties.
It should be noted (Fig. 4B) that the intensity of anomeric signal of μ-carrabiose (5.27 ppm) remained constant throughout the incubation experiments, highlighting the specificity of sulfurylases I and II for ν-carrabiose moieties. In addition, we observed that the enzymes were ineffective on λ-carrageenan, which occurs in C. crispus sporophytes. The NMR spectra also lacked any signal corresponding to anomeric protons localized at the reducing end. This result confirms that the cyclization of the anhydro ring was not associated with detectable carrageenan depolymerization.
Biochemical Characterization of Gal-2,6-Sulfurylases
The formation of 3,6-anhydro rings was monitored by following the concentration of sulfate released in the medium. The conversion of ν-carrabiose moieties into ι-carrabiose moieties by pure sulfurylase I and II was a slow phenomenon. Conversion kinetics exhibited a linear rate of sulfate release for incubations of up to 20 h. In an attempt to reduce these conversion times, we assayed other incubation conditions by varying enzyme and substrate concentrations. We observed that increased enzyme concentrations (>0.5 μg mL−1) did not accelerate the kinetic reactions. Moreover, higher amounts of carrageenan (>0.7%, w/v) dramatically increased the viscosity of the medium and, as a consequence, affected enzyme efficiency. Therefore, biochemical and kinetic parameters were determined using the initial velocity corresponding to the slope of the linear increase observed between 0 and 15 h of incubation.
Pure ν-carrageenan cannot be directly obtained from a biological source, and chemical synthesis of this polysaccharide has never been reported. Consequently, biochemical and kinetic parameters of the enzymes were determined using a hybrid ν-/ι-carrageenan (Table II). Under these conditions, we cannot exclude the possibility that the sulfurylases are inhibited by the ι-carrabiose moieties. Nevertheless, we observed that sulfurylases I and II seemed to have a similar temperature optimum of 48°C. For temperatures higher than 60°C, we observed a rapid loss of activity, probably due to enzyme denaturation. The pH optima were also similar and measured at about pH 8 to 9 and pH 7 to 8 for sulfurylase I and II, respectively. Wong and Craigie (1978) reported a pH optimum of 6.5 for the C. crispus sulfohydrolase that converts μ- into κ-carrageenan. Biochemical parameters—apparent Km, Vmax, and kcat of the enzymes reported in Table II—were also very similar between the two enzymes.
Table II.
Biochemical and kinetic parameters for sulfurylases I and II
| Parameter | Sulfurylase I | Sulfurylase II |
|---|---|---|
| Molecular mass (kD) | 65 | 32 |
| Temperature optimum (°C) | 48 | 48 |
| pH optimum | 8–9 | 7–8 |
| Km (μm) | 3.3 | 4.8 |
| Vmax (mm min−1) | 0.24 | 0.28 |
|
kcat (min−1) |
6.5 |
4.7 |
Comparison of the Mode of Action of the Two Gal-2,6-Sulfurylases
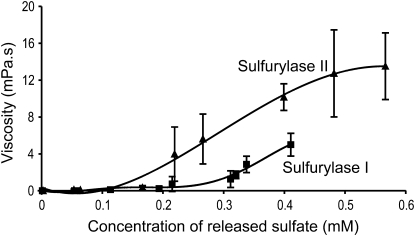
Conversion kinetic series were performed to simultaneously measure the concentration of sulfate released (i.e. the number of 3,6-anhydro rings formed) and the viscosity of the medium. Figure 5 shows that, for both enzymes, desulfation of the substrate was associated with an increase in viscosity, eventually leading, when incubated for long time periods, to the gelation of ι-carrageenan. However, at an equal concentration of released sulfate, the viscosities were not the same when the conversion was catalyzed by sulfurylase I or II. This is clearly illustrated in Figure 5, which shows that sulfurylase II induced greater viscosity at lower concentrations of sulfate than sulfurylase I. For example, a release of only 0.2 mm sulfate was sufficient to increase the viscosity of the medium to 4 mPa with sulfurylase II, while twice as many catalytic events were required to reach a viscosity of the same order with sulfurylase I.
Figure 5.
Variation in the viscosity of the incubation medium determined after treatment of ι-/ν-carrageenan with sulfurylase I and II as a function of the concentration of released sulfate. Error bars correspond to the sd determined from three independent experiments.
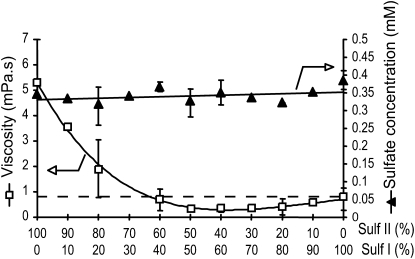
We assayed the conversion of ν- into ι-carrageenan by incubating the substrate with a range of sulfurylase I-to-II ratios. Figure 6 shows the concentration of sulfate released as well as the viscosity of the medium for each sulfurylase I-to-II ratio. Incubations were conducted for 12 h, which, for both enzymes, corresponded to a release of about 0.35 μm sulfate. We observed that the concentration of sulfate released by the various mixtures of sulfurylase was almost constant, suggesting that the sulfate released was in fact the sum of sulfate released independently by each enzyme. As demonstrated in Figure 5, the viscosity of the medium was higher when incubation was conducted with sulfurylase II alone. Indeed, the viscosity of the medium decreased dramatically when low amounts (10%–20%) of sulfurylase I were added to sulfurylase II. The viscosity of the medium was lowest when sulfurylase I-to-II ratios were between 50:50 and 80:20, and viscosity was lower than when sulfurylase I was used alone.
Figure 6.
Viscosity (white squares) and concentration of released sulfate (black triangles) after 12 h of incubation of ι-/ν-carrageenan with increasing sulfurylase I (Sulf I)-to-sulfurylase II (Sulf II) ratios. Error bars correspond to the sd determined from three independent experiments.
DISCUSSION
d-Gal-2,6-Sulfurylases Make up a New Class of Enzymes
In 1961, Rees (1961a) unambiguously demonstrated the occurrence of an enzyme extracted from Porphyra umbilicalis that catalyzes the formation of 3,6-anhydro rings in agarose, which occurs when the sulfate ester group is eliminated from l-Gal-6-sulfate. The enzyme was then named Gal-6-sulfurylase (systematic name: l-Gal-6-sulfate:alkyltransferase [cyclizing]; EC 2.5.1.5). The conversion of μ-carrageenan into κ-carrageenan by protein fractions from C. crispus (Wong and Craigie, 1978) and Gigartina stellata (Lawson and Rees, 1970), and the conversion of ν-carrageenan into ι-carrageenan with Calliblepharis jubata extracts (Zinoun et al., 1997), have also been reported. Although the purification of these carrageenan-modifying enzymes was only partial, the sulfohydrolase extracts were thought to have enzyme activity with a similar mechanism as the l-Gal-6-sulfurylase that acts on agarose precursors. Here, to our knowledge for the first time, we purified, to near electrophoretic homogeneity, two enzymes named sulfurylase I and II that are able to catalyze the desulfation and the cyclization of α-d-Gal-2,6-disulfate (ν-carrabiose unit) to form α-d-(3,6)-anhydro-Gal-2-sulfate (ι-carrabiose unit). SDS-PAGE analysis indicated that these enzymes are monomers in solution. The purified enzymes were specific to ν-carrageenan and were inactive on μ-carrageenan. Our investigations unambiguously demonstrate that the formation of anhydro rings is an enzymatic reaction that is carried out by single proteins. Consequently, this observation is compatible with a reaction mechanism involving a nucleophilic substitution, as proposed for the cyclization of anhydro rings in alkaline solutions. At the molecular level, the catalysis of the cyclization by sulfurylases may occur via a multistep mechanism, as usually encountered with polysaccharide-modifying enzymes. However, the cyclization of the anhydro ring and the release of the sulfate ester group is probably a concerted mechanism.
The sulfurylases belong to transferase-type enzymes (and not to hydrolase-type enzymes), as already suggested by Lawson and Rees (1970). Therefore, the term sulfohydrolase used to describe the active fraction containing an enzyme that catalyzes anhydro ring formation in carrageenans now appears inappropriate. Consequently, in analogy with agarose-acting enzymes, the enzymes forming anhydro rings in κ- and ι-carrageenans should be named d-Gal-6-sulfurylase and d-Gal-2,6-sulfurylase, respectively.
Sulfurylases I and II Induce Different Viscous Carrageenan Solutions in Vitro
As expected, we detected d-Gal-2,6-sulfurylases in other carrageenophyte seaweeds, such as Mastocarpus stellatus and Kappaphycus alvarezii, suggesting that these enzymes are common in carrageenophyte algae. Sulfurylases I and II also had very similar biochemical parameters, which may indicate that they have similar localizations in algal cells. As already noted by Wong and Craigie (1978), C. crispus Gal-6-sulfurylases are very stable, which means that they can be stored at 4°C for several weeks without any reduction in activity and enzyme kinetics can be completed within 1 d. The main difference between the purified sulfurylases I and II we isolated here was their molecular masses (65 and 32 kD, respectively) and their modes of action. As shown in Figure 5, the increase of viscosity of carrageenan solutions was not induced in the same way by the two sulfurylases. We found that for the same amount of sulfate molecules released and leading to the same ν- to ι-carrabiose ratio, the viscosities of the carrageenan solutions were very different. These viscous solutions obtained through the action of enzymes involved in biosynthesis may have some parallels with in vivo carrageenan assembly.
The differences in viscosity between carrageenan solutions composed of equal quantities of ν- and ι-carrabiose moieties suggest that the properties of the biocatalyzed ν-/ι-carrageenan are probably caused by differences in the distribution of carrabioses along the chain. The conversion of ν- into ι-carrageenan may occur in a sequence of catalytic events leading to various distributions of modified carrageenans according to whether the reaction is catalyzed by sulfurylase I or II. As already observed for hybrid κ-/ι-carrageenans, carrabiose distribution is an important parameter controlling the functional properties of carrageenans (Guibet et al., 2008). For example, ν- to ι-carrageenan conversion may proceed via random conversion of d-Gal-2,6-sulfate or by processive conversion of a stretch of ν-carrageenans. Unfortunately, no pure ν-carrageenan precursors have ever been isolated, limiting the possibility of enzymological studies. In addition, the distribution of the ν- and ι-carrageenan moieties along the hybrid ν-/ι-carrageenan substrates we used was unknown. Consequently, it is difficult to determine the mode of action of sulfurylases. However, as observed for alginate-C5-epimerase, another enzyme that catalyzes the gelation of an anionic algal polysaccharide, the mode of action as well as the mechanism of recognition were found to be the key parameters that determine the polysaccharide's properties (Campa et al., 2004; Steigedal et al., 2008).
Role of Sulfurylases in Algal Cell Wall Assembly
As in terrestrial plants, red algal cell walls are a composite material made most often of cellulose microfibrils embedded in a matrix composed of polysaccharides and, to a lesser extent, proteins and aromatic substances. In contrast to those found in terrestrial plants, matrix polysaccharides in agarophytes and carrageenophytes represent the main constituent of the cell wall. As a consequence, the physical properties of the cell wall are directly related to agar or carrageenan assembly.
The gelling properties of agarose and carrageenans have been the subject of many studies, which are usually carried out with polysaccharides having almost ideal structure and at concentrations ranging from 0.1% to 1% (w/v). These experimental conditions are very unlike those encountered in the cell wall. Indeed, agarophyte and carrageenophyte cell walls contain about 10% (w/v) polysaccharides, with hybrid—not ideal—structures. It is widely accepted that the hybridity of carrageenans, as they are found in vivo, strongly affects their gelation properties (Bixler et al., 2001; van de Velde, 2008). Consequently, given this biological reality, the cell wall is probably a very dense arrangement of carrageenan molecules. This type of compact arrangement is possible due to the low chain stiffness, low viscosity, and low gelling properties of the polysaccharides.
Under the speculation that the biosynthesis of carrageenan contributes to making a material of low viscosity but high density, the low viscosity of carrageenan solutions obtained after incubation with sulfurylase mixtures may be seen as a synergistic mode of action that developed in carrageenophytes. Indeed, the combination of both sulfurylases has made it possible to make less viscous solutions than those obtained with individual enzymes. There is no evidence that the sulfurylases are colocalized in algae; furthermore, they may arise at different steps in cell wall biosynthesis and/or in the regulation of cell wall properties. However, the ability of these enzyme mixtures to make low-viscosity solutions of ι-carrageenan in vitro remains intriguing.
Like other plant and algal matrix polysaccharides, including pectins, hemicelluloses, alginates, and agars, carrageenans are synthesized in the Golgi apparatus and exported to the wall in secretory vesicles (Tveter-Gallagher et al., 1984). Assembly of these polysaccharides in the wall involves enzymes (i.e. pectin methyl esterase, alginate-C5-epimerase, and now d-/l-Gal-6-sulfurylases) that confer the ability to make gels or viscous solutions in vitro. Consequently, although the chemical structures of these polysaccharides and their associated biosynthetic enzymes are very different, in all cases the distribution of the repetition moieties and, thus, the rheological properties of the polysaccharides are fine-tuned in vivo by enzymes.
MATERIALS AND METHODS
Unmodified ι-carrageenan extracted from Eucheuma denticulatum was provided by CP Kelco (Copenhagen). The polysaccharide was composed of about 19.5% ν-carrageenan moieties according to 1H NMR analyses. The present hybrid carrageenan, referred to here as ι-/ν-carrageenan, was used to screen and study d-Gal-2,6-sulfurylase activities. All solutions and buffers containing NaN3 (about 0.01%, w/v) were filtered at 0.22 μm prior to use to prevent bacterial contamination.
Purification of Chondrus crispus d-Gal-2,6-Sulfurylases
C. crispus gametophytes were collected on the shore at Roscoff, Brittany, France. Specimens were frozen in liquid nitrogen and stored at −80°C. Successive purification experiments were performed with several batches of seaweed randomly chosen from collections acquired between September 1997 and October 2006.
Frozen C. crispus (approximately 650 g) was ground in liquid nitrogen and allowed to thaw in 1.5 L of cold extracting buffer (50 mm Tris-HCl, pH 9.5, 500 mm KCl, and 10 mm 2-β-mercaptoethanol). The suspension was maintained at 4°C, and all of the following fractionation and purification steps were performed at this temperature. The suspension was gently stirred overnight prior to centrifugation at 10,000g for 75 min. The supernatant was brought to 30% (NH4)2SO4 saturation [16.4 g (NH4)2SO4 per 100 mL of extract] by slowly adding the (NH4)2SO4 salt and allowing it to dissolve. The precipitate was discarded after centrifugation at 24,700g for 60 min. The supernatant was loaded on a phenyl-Sepharose 6 fast-flow (low-sub) column (2.0 × 24 cm; GE Healthcare) previously equilibrated in buffer A [50 mm Tris-HCl buffer, 30% (NH4)2SO4, 500 mm KCl, and 10 mm 2-β-mercaptoethanol, pH 8.7]. The gel was washed with buffer A at a flow rate of 1.5 mL min−1 until effluent A280 was negligible. Elution of the bound proteins was achieved by applying a linearly decreasing gradient from 30% to 0% (NH4)2SO4 [buffer A to buffer A without (NH4)2SO4, respectively] for 720 min (flow rate of 1.5 mL min−1). Extraction, fractionation, and hydrophobic interaction chromatography were repeated once in order to have enough material for the following step. All of the active fractions (approximately 970 mL) were pooled and dialyzed for 36 h against 5 × 10 L of buffer B (50 mm Tris-HCl, pH 7.1, and 10 mm 2-β-mercaptoethanol).
The desalted sample was loaded on a DEAE-Sepharose column (2.0 × 22 cm; GE Healthcare) previously equilibrated with buffer B. The column was washed with buffer B to remove the unbound proteins and eluted at a flow rate of 1 mL min−1 with an increasing step gradient of NaCl in buffer B (0 min, 0 mm NaCl; 390 min, 650 mm NaCl; 450 min, 650 mm NaCl; 630 min, 1 m NaCl). Active fractions eluting between 300 and 600 mm NaCl (sulfurylase I) and 650 and 800 mm NaCl (sulfurylase II) were collected and dialyzed about 6 h against buffer B.
Active fractions corresponding to sulfurylase I were then loaded at a flow rate of 1 mL min−1 on a HiTrap Heparin HP prepacked column (1 mL; GE Healthcare) previously equilibrated in buffer B. The column was washed with buffer B to remove unbound proteins. Elution of sulfurylase I was performed at a flow rate of 1 mL min−1 with an increasing step gradient of NaCl in buffer B (step 1, linear increase of NaCl concentration from 0 min, 0 mm NaCl to 10 min, 350 mm NaCl; step 2, the 350 mm NaCl concentration was kept constant until 15 min; step 3, linear increase of NaCl concentration from 15 min, 350 mm NaCl to 25 min, 800 mm NaCl; step 4, the 800 mm NaCl concentration was kept constant until 30 min; step 5, linear increase of NaCl concentration from 30 min, 800 mm NaCl to 35 min, 1,200 mm NaCl; the 1,200 mm NaCl concentration was maintained until the elution ended). The active fractions were collected when the NaCl concentration was kept for 10 min at 800 mm.
At the different steps of purification, the active fractions were analyzed by SDS-PAGE (Laemmli and Favre, 1973) using a 12% polyacrylamide gel, and protein quantification was performed according to Bradford (1976) using the Bio-Rad protein assay. Bovine serum albumin was used as a standard. In addition, the sulfurylase activity was detected by measuring the concentration of sulfate released after incubating the seaweed extracts with ι-/ν-carrageenan. The standard reaction mixture contained 100 μL of each protein fraction in 50 mm Tris-HCl, pH 7.1, and 100 μL of ι-/ν-carrageenan (1.4%, w/v) in MilliQ (Millipore) water. The reaction was conducted at 40°C for 6 to 15 h. Carrageenans were discarded from the reaction mixture by filtering the reaction mixture previously diluted 2-fold through a Microcon-10 (Amicon; cutoff of 10 kD) unit subjected to centrifugation (3,320g, 20 min, 30°C). The fractions were compared with similar enzyme extracts boiled for 10 min used as a reference. The release of sulfate was measured by HPAEC (see below).
Enzymological Experiments with Sulfurylases I and II
Conversion kinetics were monitored by HPAEC (see below) by measuring the number of sulfate ions released, assuming that the number of catalytic events was equal to the number of sulfate groups released. Temperature optimum was determined by measuring the initial velocity of conversion kinetics of 100 μL of ι-/ν-carrageenan in water (1.4% [w/v] ι-/ν-carrageenan is equivalent to 5.9 mm ν-carrabiose units) incubated with 50 μL of sulfurylase solution and 50 μL of buffer. The concentrations of the enzymes were 0.084 mg mL−1 and 0.0614 mg mL−1 for sulfurylases I and II, respectively. The incubation temperatures ranging from 0°C to 60°C were tested in steps of 10°C in 100 mm Tris-HCl, pH 8. To determine the pH optimum, 100 μL of ι-/ν-carrageenan in water (3% [w/v] ι-/ν-carrageenan) was incubated with 75 μL of sulfurylase solution and 125 μL of buffer at 200 mm. Sulfurylase activity was assayed at pH values of 5.5, 6.0, 6.5, 7, 7.5, 8, 8.5, and 9; the experiments were completed at 48°C. The solutions used to buffer the reaction medium were as follows: 200 mm MES +20 mm 2-β-mercaptoethanol at pH 5.5 and 6; and 200 mm Tris-HCl [(hydroxymethyl)-amino methane] +20 mm 2-β-mercaptoethanol at pH 7.0 to 9.
The Michaelis parameters were determined at the optimum conditions (pH 8 and 48°C). Kinetics experiments were conducted as above, and 100 μL of ι-/ν-carrageenan in water with concentrations ranging from 0.105% to 2.1% (w/v), corresponding to 0.44 to 8.85 mm ν-carrabiose units, was incubated with 50 μL of enzyme and 50 μL of 100 mm Tris-HCl, pH 8. The reaction was stopped by diluting the incubation medium 2-fold and by centrifuging the samples in a Microcon-10 (Millipore) to remove the ν-carrageenan. The concentration of free sulfate present in the filtrate was then determined by HPAEC.
Synergy experiments were conducted by making up solutions with varying ratios of sulfurylase I and II (0.084 mg mL−1 and 0.0614 mg mL−1, respectively). After incubation of ι-/ν-carrageenan for 15 h according to the same experimental conditions described above, viscosity and the concentration of released sulfate were determined.
HPAEC
The concentration of free sulfate present in the filtrate was then analyzed by HPAEC using a Dionex 500 chromatography system equipped with a GP40 gradient pump and an ED40 electrochemical detector. The anions were separated on an AS11 anion-exchange column (4 × 200 mm; Dionex) with accompanying AG11 guard column (4 × 50 mm; Dionex). Elution was performed with 12 mm NaOH using an isocratic flow rate of 1 mL min−1. The background conductivity originating from the anions in the eluent was reduced using the anion self-regenerating suppressor (4 mm; Dionex) with a self-regenerated suppressor current of 100 mA. After suppression, the released sulfate groups were detected with the ED40 electrochemical detector (Dionex) in the conductivity mode. According to the elution conditions, the peak of sulfate eluted separately from other ions at 3 min. The concentration of sulfate was deduced from the signal intensity and calculated from a standard sulfate solution.
Viscosity Measurements
Viscosity measurements were carried out on the reaction mixture described above except that after incubation at 48°C, the viscosity of the reaction mixture was directly measured using a Brookfield DVIII rheometer thermostated at 48°C. Unless otherwise stated, measurements were performed for 4 min using a shear rate of 120 rpm.
NMR Spectroscopy
The ratio of ι- to ν-carrageenan was determined by 1H NMR spectroscopy. One-dimensional 1H NMR spectra were recorded on a Bruker Avance DRX 500 spectrometer equipped with an indirect 5-mm gradient probehead 1H/13C/31P, at a probe temperature of 353 K. Prior to analysis, samples were exchanged twice in D2O and redissolved in 99.97 atom% D2O. Chemical shifts are expressed in ppm in reference to an external standard (trimethylsilylpropionic acid). No suppression of the HOD signal was performed.
Carrageenan Nomenclature
We used the nomenclature established by Knutsen et al. (1994) for carrageenans. The 4-linked α-d-galactopyranosyl unit is designated the d unit, and the 3-linked β-d-galactopyranosyl unit is designated the G unit. The position of the sulfate group is indicated by the letter S. For example, the disaccharide repetitive units of ν-carrageenan and ι-carrageenan are G4S-D2S,6S and DA2S-G4S, respectively.
This work was supported by a fellowship from the Brittany Regional Council (Projet de Recherche d'Intérêt Régional-Région Bretagne), the French National Center for Scientific Research, and Pierre and Marie Curie University.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: William Helbert (helbert@sb-roscoff.fr).
References
- Bellion C, Brigand G, Prome JC, Welti D, Bociek S (1983) Identification et caractérisation des précurseurs biologiques des carraghénanes par spectroscopie de R.M.N.-13C. Carbohydr Res 119 31–48 [Google Scholar]
- Bixler H (1996) Recent developments in manufacturing and marketing carrageenan. Hydrobiologia 326/327 35–37 [Google Scholar]
- Bixler H, Johndro K, Falshaw R (2001) Kappa-2 carrageenan: structure and performance of commercial extracts. II. Performance in two simulated dairy applications. Food Hydrocoll 15 619–630 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Campa C, Holtan S, Nilsen N, Bjerkan TM, Stokke BT, Skjåk-Bræk G (2004) Biochemical analysis of the processive mechanism for epimerization of alginate by mannuronan C-5 epimerase AlgE4. Biochem J 38 155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianca M, Matulewicz MC, Cerezo A (1997) Alkaline modification of carrageenan. Part III. Use of mild alkaline media and high ionic strengths. Carbohydr Polym 32 293–295 [Google Scholar]
- Craigie JS (1990) The cell wall. In KM Cole, RG Sheath, eds, Biology of the Red Algae. Cambridge University Press, Cambridge, UK, pp 221–257
- Greer CW, Yaphe W (1984) Hybrid (iota-nu-kappa) carrageenan from Eucheuma nudum (Rhodophyta, Solieriaceae), identified using iota- and kappa-carrageenases and 13C-nuclear magnetic resonance spectroscopy. Bot Mar 27 479–484 [Google Scholar]
- Gretz MR, Wu Y, Vreeland V, Scott J (1990) Iota-carrageenan biogenesis in the red alga Agardhiella subulata is Golgi mediated. J Phycol (Suppl) 26 14 [Google Scholar]
- Guibet M, Boulenguer P, Mazoyer J, Kervarec N, Antonopoulos A, Lafosse M, Helbert W (2008) Composition and distribution of carrabiose moieties in hybrid k-/ι-carrageenans using carrageenases. Biomacromolecules 9 408–415 [DOI] [PubMed] [Google Scholar]
- Heidrich C, Wollmer W (2002) Murein (peptidoglycan). In A Steinbüchel, EJ Vandamme, S De Baets, eds, Biopolymer. Wiley VCH Verlag, Weinheim, Germany, pp 431–463
- Hemmingson JA, Furneaux RH, Murray-Brown VH (1996. a) Biosynthesis of agar polysaccharides in Gracilaria chilensis Bird, McLachlan et Oliveira. Carbohydr Res 287 101–115 [Google Scholar]
- Hemmingson JA, Furneaux RH, Wong H (1996. b) In vivo conversion of 6-O-sulfo-L-galactopyranosyl residues into 3,6-anhydro-L-galactopyranosyl residues in Gracilaria chilensis Bird, McLachlan et Oliveira. Carbohydr Res 296 285–292 [Google Scholar]
- Hölfte JV, Mirelman D, Sharon N, Schwarz U (1975) Novel type of murein transglycosidase in Escherichia coli. J Bacteriol 124 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Lowary TL (2009) 2,3-Anhydrosugars in glycoside bond synthesis: application to 2,6-dideoxypyranosides. J Org Chem 74 2278–2289 [DOI] [PubMed] [Google Scholar]
- Jol CN, Neiss TG, Penninkhof B, Rudolph B, De Ruiter GA (1999) A novel high-performance anion-exchange chromatographic method for the analysis of carrageenans and agars containing 3,6-anhydrogalactose. Anal Biochem 268 213–222 [DOI] [PubMed] [Google Scholar]
- Knutsen S, Myslabodski D, Larsen B, Usov A (1994) A modified system of nomenclature for red algal galactans. Bot Mar 37 163–169 [Google Scholar]
- Laemmli UK, Favre M (1973) Links maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol 80 575–599 [DOI] [PubMed] [Google Scholar]
- Lawson CJ, Rees D (1970) An enzyme for the metabolic control of polysaccharide conformation and function. Nature 227 392–393 [DOI] [PubMed] [Google Scholar]
- Rees D (1969) Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem 24 267–332 [DOI] [PubMed] [Google Scholar]
- Rees DA (1961. a) Enzymic desulphation of porphyran. Biochem J 80 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DA (1961. b) Enzymic synthesis of 3:6-anhydro-L-galactose within porphyran from L-galactose 6-sulphate units. Biochem J 81 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigedal M, Sletta H, Moreno S, Mærk M, Christensen BE, Bjerkan T, Ellingsen TE, Espìn G, Ertesvåg H, Valla S (2008) The Azotobacter vinelandii AlgE mannuronan C-5-epimerase family is essential for the in vivo control of alginate monomer composition and for functional cyst formation. Environ Microbiol 10 1760–1770 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Huang WC, Noguchi M, Kobayashi A, Shoda SI (2009) Direct synthesis of 1,6-anhydro sugars from unprotected glycopyranoses by using 2-chloro-1,3-dimethylimidazolinium chloride. Tetrahedron Lett 50 2154–2157 [Google Scholar]
- Tveter-Gallagher E, Cogburn JN, Mathieson AC, Schiff JA (1984) Localisation of carrageenan and incorporation of 35SO42− into sulphur amino acids in the red alga Chondrus crispus. Hydrobiologia 116/117 488–492 [Google Scholar]
- Usov AI (1998) Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll 12 301–308 [Google Scholar]
- van de Velde F (2008) Structure and function of hybrid carrageenans. Food Hydrocoll 22 727–734 [Google Scholar]
- van de Velde F, Knutsen SH, Usov AI, Rollema HS, Cerezo AS (2002. a) 1H and 13C high resolution NMR spectroscopy of carrageenans: application in research and industry. Trends Food Sci Technol 13 73–92 [Google Scholar]
- van de Velde F, Rollema H, Grinberg N, Burova T, Grinberg V, Tromp R (2002. b) Coil-helix transition of ι-carrageenan as a function of chain regularity. Biopolymers 65 299–312 [DOI] [PubMed] [Google Scholar]
- Varma AJ, Kennedy JF, Galgali P (2004) Synthetic polymers functionalized by carbohydrates: a review. Carbohydr Polym 56 429–445 [Google Scholar]
- Viana AG, Noseda MD, Duarte MER, Cerezo A (2004) Alkali modification of carrageenans. Part V. The iota-nu hybrid carrageenan from Eucheuma denticulatum and its cyclization to iota-carrageenan. Carbohydr Polym 58 455–460 [Google Scholar]
- Wong KF, Craigie JS (1978) Sulfohydrolase activity and carrageenan biosynthesis in Chondrus crispus (Rhodophyceae). Plant Physiol 61 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoun M, Diouris M, Potin P, Floc'h JY, Deslandes E (1997) Evidence of sulfohydrolase activity in the red alga Calliblepharis jubata. Bot Mar 40 49–53 [Google Scholar]