Abstract
The Wnt family of signalling proteins is known to participate in multiple developmental decisions during embryogenesis. We misexpressed Wnt1 in medaka embryos and observed anterior truncations, similar to those described for ectopic activation of canonical Wnt signalling in other species. Interestingly, when we induced a heat-shock Wnt1 transgenic line exactly at 30% epiboly, we observed multiple ectopic otic vesicles in the truncated embryos. The vesicles then fused, forming a single large ear structure. These “cyclopic ears” filled the complete anterior region of the embryos. The ectopic induction of otic development can be explained by the juxtaposition of hindbrain tissue with anterior ectoderm. Fibroblast growth factor (Fgf) ligands are thought to mediate the otic-inducing properties of the hindbrain. However, signals different from Fgf3 and Fgf8 are necessary to explain the formation of the ectopic ear structures, suggesting that Wnt signalling is involved in the otic induction process in medaka.
Electronic supplementary material
The online version of this article (doi:10.1007/s00427-009-0302-z) contains supplementary material, which is available to authorized users.
Keywords: Wnt1, Otic induction, Cyclopic ear, Medaka
Introduction
In teleost fish, the inner ear originates from an ectodermal placode located at the level of rhombomere 4 (r4) of the hindbrain, and the otic placode then cavitates to form the otic vesicle (reviewed in Whitfield et al. 2002). Classical transplantation experiments demonstrated a prominent role of the hindbrain in the otic induction process. In zebrafish, grafting hindbrain tissue to the ventral side of the embryo induces ectopic otic vesicles (Woo and Fraser 1998). Members of the fibroblast growth factor (Fgf) family of secreted ligands are the best candidates for otic-inducing factors (reviewed in Riley and Phillips 2003; Whitfield et al. 2002), but the role of other modulating factors such as Wnt signalling in this process is not clear.
Experiments with lithium chloride treatment by Gutknecht and Fritzsch (1990) in Xenopus embryos led to multiple expanded otic vesicles. Since lithium is a potent inhibitor of GSK3; these experiments can be interpreted as a first indication of otic-inducing effects by canonical Wnt signalling. A model for induction of the chick inner ear was proposed in which Fgf19 cooperates with Wnt8c to induce ectopic otic placodes (Ladher et al. 2000). Neither Fgf19 nor Wnt8c was able to induce the expression of all otic markers in explants of uncommitted ectoderm, whereas a combination of both genes strongly induced the full range of markers genes. Loss of Wnt8 function experiments in zebrafish suggest that otic placode induction occurs normally in the absence of Wnt8 expression (Phillips et al. 2004). In that study, morpholino knockdown of the Wnt8 gene and misexpression of the Wnt antagonist dickkopf 1 failed to block otic induction. Phillips and colleagues proposed that Wnt serves to regulate the temporal expression of Fgf3 and Fgf8 in the hindbrain, hence playing an indirect role in otic induction (Phillips et al. 2004). Furthermore, Wnt signalling was proposed to affect the preplacodal state in chicks (Bailey and Streit 2006), and in mice, it was suggested to be required after Fgf-dependent induction to maintain otic fate (Ohyama et al. 2006). Here, we address the question whether Wnt signalling is involved in otic induction in medaka fish. We demonstrate that ectopic activation of this pathway affects otic development at the time of induction by juxtaposing regions normally separated in the embryo. This is in good agreement with hindbrain tissue as a source for otic-inducing signals. However our experiments suggest that signals in addition to Fgf3 and Fgf8 induce the formation of ectopic ear structures in medaka.
Materials and methods
Fish strain and transgenic lines
Embryos of the medaka Cab inbred strain were used for all experiments. Stages were determined according to Iwamatsu (2004). All studies on heat-inducible Wnt1-transgenic embryos were carried out using three independent lines described previously (Bajoghli et al. 2007).
Embryo injections
Medaka embryos were microinjected into single blastomeres at the one- to two-cell stage. mRNA of mouse Wnt1 was injected at 200 ng/μl. For transient experiments, a heat-inducible Wnt1 construct (Bajoghli et al. 2007) was injected at 10 ng/μl together with I-SceI meganuclease.
Isolation of medaka EphA4 and Hoxb1a probes
The primers used for the RT-PCR were as follows: for EphA4, 5- GAAAAGAACATCCCCATTCG-3 and 5-GAGTTGCGTTCCTCATATCCT-3; for Hoxb1a, 5-ATGGAAAACATGAACTCCTTTG-3 and 5-GTGCGGGACCGTTAGGTA-3. The DNA fragments were cloned into the pGEM-Teasy vector (Promega).
Whole-mount in situ hybridisation
Whole-mount in situ hybridisation was performed as described previously using DIG- or FITC-labelled probes (Aghaallaei et al. 2005).
Results
To elucidate the function of Wnt signalling during medaka otic induction, we decided to use gain-of-function analysis. Wnt1, Wnt8 and Wnt3a proteins define a functional class of Wnt ligands (Torres et al. 1996), which preferentially stimulate the canonical Wnt pathway (reviewed by Logan and Nusse 2004). We selected Wnt1 for our misexpression experiments. Injection of Wnt1 mRNA into medaka embryos resulted in a posteriorised phenotype characterised by severe truncations of anterior structures (Fig. 1b). Similar phenotypes have been observed upon ectopic activation of canonical Wnt signalling in various vertebrate species (Kelly et al. 1995; Stachel et al. 1993; van de Water et al. 2001; Yamaguchi and Shinagawa 1989). Since Wnt signalling has multiple functions during early embryonic development depending on the time window (Liu et al. 2005), we switched to induced expression using a heat-shock-inducible system (Bajoghli et al. 2004). Activation of the injected heat-shock Wnt1 construct at early gastrulation resulted in the same posteriorisation phenotype seen for mRNA. However, 18% of the embryos (13 of 70) showed enlarged otic vesicles (data not shown), and in addition, 7% of the embryos (five of 70) exhibited small ectopic otic vesicles located anteriorly to the endogenous otic vesicle (Fig 1c, arrow). When we repeated these experiments with an expression construct for the zebrafish Wnt8 gene, we also observed ectopic otic vesicles in 20% of the embryos (11 of 56; data not shown). These experiments are consistent with data for injection of a Wnt8 expression construct in zebrafish (Phillips et al. 2004). In order to overcome the mosaic nature of the injection technique, we repeated the experiments with a heat-shock Wnt1 transgenic medaka line (Bajoghli et al. 2007). In agreement with transient misexpression, uniform activation of Wnt1 in the whole embryo at early gastrulation led to posteriorisation. However, instead of the isolated ectopic vesicles observed before, one large vesicle appeared in the Wnt1-transgenic embryos, covering the complete anterior region starting from the position of the endogenous otic vesicles (Fig 1d). At later stages, the vesicle forms inner ear structures and contains multiple otoliths (Supplementary Fig. S1). We confirmed the identity of this ectopic otic structure by marker gene expression. The expression patterns of Starmaker (Fig. 1f), Eya1 and Six1 identified the structures as otic vesicles (Supplementary Fig. S1). Due its similarity with cyclopic eyes, we named this phenotype “cyclopic ear”.
Fig. 1.
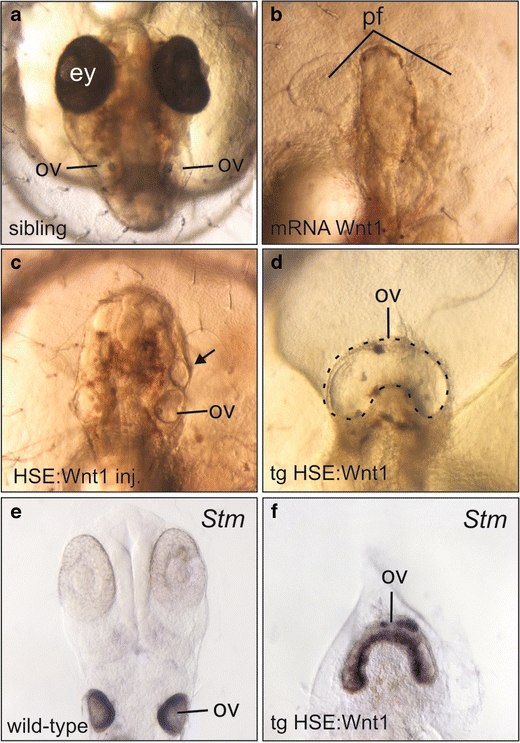
Activation of the canonical Wnt pathway in medaka embryos. Dorsal views of embryos at 2dpf (a, c, d) and 5dpf (b). A non-transgenic sibling (a) is compared to embryos with transient injections of Wnt1 mRNA (b), a heat-inducible Wnt1 construct (c) and Wnt1-transgenic lines (d). Starmaker is strongly expressed in normal otic vesicles (e) and in cyclopic ear structures (f). An ectopic otic vesicle is indicated by an arrow. The heat-shock treatment was performed for 2 h at 39°C for c, d and f and 3 h at 30% for a. ey eye, ov otic vesicle, pf pectoral fin
In order to learn more about the effect of timing, we performed a time-course experiment by systematically varying the time of heat-shock activation in Wnt1 transgenic embryos from 30% epiboly to the three-somite stage (Fig. 2a). The experiments indicate a strict time dependence on the extent of the anterior truncations. Early activation of Wnt signalling leads to a severe truncation of the body axis at the level of the hindbrain, whereas posterior parts of the embryos are not affected. The extent of the axis truncations does not differ dramatically between the one cell stage (mRNA injection, see Fig 1b) and early gastrulation (Fig. 2a). However between early and late gastrulation, the truncations are gradually reduced, with midbrain structures appearing at heat shocks at 60% epiboly and forebrain structures at 80% epiboly. Ectopic Wnt activation at neurula stages only affects eye development, whereas embryos heat-shocked during somitogenesis appear normal. These phenotypes would correlate with the timing of neural plate induction by the migrating mesoderm, which might be blocked at different time points by the ectopic Wnt signals. The gradual loss of anterior structures can also be observed with hindbrain marker gene expression (see below).
Fig. 2.
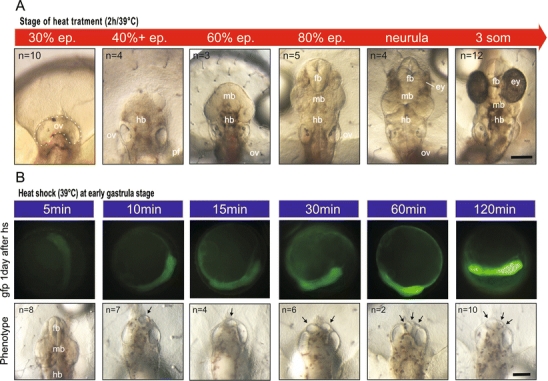
Temporal and dosage effects of Wnt1 activation during early development. Dorsal views of Wnt1-transgenic embryos. a The heat shock was performed at different stages for 2 h at 39°C. b Wnt1-transgenic embryos were heat-shocked at early gastrulation for different durations. The Gfp activity 1 day after heat shock was used as a marker. Ectopic otic vesicles are indicated by arrows. n the number of examined embryos, ep. epiboly, ey eye, fb forebrain, md midbrain, hb hindbrain, ov otic vesicle
In the time-course experiment, the cyclopic ear phenotype was only observed when Wnt signalling was induced at 30% epiboly. Slightly later activation of the pathway at 40% epiboly generates embryos with normal otic vesicle morphology. In previous work, we identified early gastrulation as a critical phase for otic induction in medaka (Aghaallaei et al. 2007). The ectopic Wnt activity therefore affects otic development during the induction phase.
To examine whether the effect of Wnt signalling is dosage dependent, we varied the time of heat treatment in embryos at early gastrulation from 5 min to 2 h. We used the Gfp signals originating from the bidirectional heat-shock promoter as a marker for the expression level (Fig. 2b). Interestingly, all embryos heat-treated for more than 10 min showed the same phenotype; the only difference was the number of ectopic otic vesicles, which increased upon longer heat treatment (Fig. 2b, arrows).
Next, we performed a time-lapse analysis. The experiments revealed that the cyclopic ear phenotype is an “end-product” of the fusion of the endogenous otic vesicles with several small ectopic structures into a single otic vesicle (Fig. 3, yellow arrows). The fusion of the vesicles started immediately after their formation (1-day-old embryos) and ended when the embryos where 3 days old and further development was arrested. Our observations show that the fusion process is dynamic and does not occur in a reproducible manner. However, the number of ectopic otic vesicles at the beginning is Wnt1 dosage dependent.
Fig. 3.
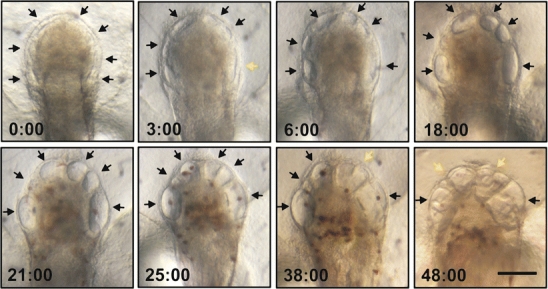
Time-lapse analysis of the cyclopic ear phenotype. The otic vesicle morphology was analysed in Wnt1-transgenic embryos from stage 20 to stage 30. Heat shock was performed at 30% epiboly for 2 h at 39°C. Ectopic otic vesicles are indicated by black arrows. The fusion of vesicles is indicated by yellow arrows
To understand the molecular events responsible for the cyclopic ear phenotype, we analysed the expression pattern of some hindbrain and otic marker genes (for schematic presentation, see supplementary Fig. 2S online). For this purpose, one group of Wnt1-transgenic embryos (group A) was induced at 30% epiboly. For comparison, another group of Wnt1-transgenic embryos (group B) was induced at 50% epiboly, which did not develop ectopic otic vesicles. The embryos were fixed at a preplacodal stage (two-somite stage). For analysis of the hindbrain structures, we used probes directed against EphA4 and Hoxb1a, expressed in r3/r5 and r4, respectively (see “Materials and methods”; Fig 4a). The expression patterns of the hindbrain marker genes confirmed the truncations of the anteroposterior axis in the Wnt1 transgenic embryos. In group B embryos, r3 and all structures posterior to this rhombomere appeared normal (Fig. 4c, f, i, r), whereas in group A embryos, the last distinguishable rhombomere was r5 (Fig. 4b, e, h). Interestingly, the expression patterns of the marker genes suggest that the remaining anterior structures of the embryos are of one uniform fate. In group A embryos, this region anterior to r5 expressed Hox1a, Fgf8 and Fgf3 (Fig. 4b, e, h), which would be consistent with an r4 fate. In group B embryos, the region anterior to r3 expressed Fgf8 (Fig. 4f), indicating an r2 fate. However, we also found expression of Fgf3 and Engrailed 2 (En2; Fig. 4i, r), normally not expressed in r2. In wild-type embryos, these two genes are expressed in the more anteriorly positioned midbrain–hindbrain boundary (mhb). The fate of the region anterior to r3 in group B embryos therefore remains unclear.
Fig. 4.
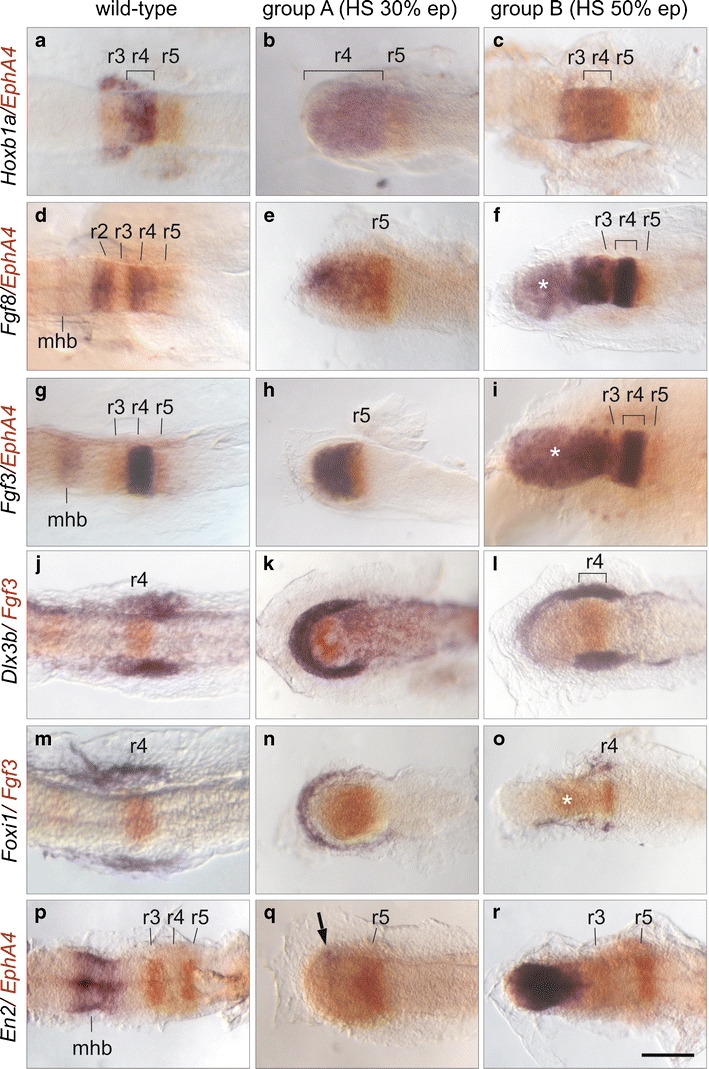
Analysis of hindbrain and otic marker genes in the Wnt1-transgenic lines. Dorsal views of embryos at two somites (a–r). Anterior is to the left. The positions of rhombomeres (r) and the mid-hindbrain boundary (mhb) are indicated. The heat shock of Wnt1-transgenic embryos was performed at 30% epiboly in group A and 50% epiboly in group B, respectively. The asterisks indicate anterior expression of Fgf3 and Fgf8, respectively, in group B embryos, despite the fact that they lack ectopic ear structures. The arrow indicates weak En2 expression. mhb. mid-hindbrain boundary
A combination of local competence factors together with signals from a distance is thought to induce otic structures in vertebrates (Riley and Phillips 2003; Whitfield et al. 2002). Fgf ligands are thought to mediate the signals originating from r4 (Maves et al. 2002; Phillips et al. 2001), and Dlx3b and Foxi1 are the best candidates for the competence factors (Esterberg and Fritz 2009; Lee et al. 2003; Solomon et al. 2004). Dlx3b is a preplacodal marker gene (Liu et al. 2003) initially expressed in a horseshoe-shape surrounding the entire neural plate (Supplementary Fig. S3; Hochmann et al. 2007; Kaji and Artinger 2004). Due to the anterior truncations, the neural plate was shortened in the Wnt1 embryos, but Dlx3b expression in the adjacent ectoderm remained in both groups (Fig. 4k, l and Supplementary Fig. S3). In group A embryos, the expression was upregulated in the anterior region (Fig. 4k), anticipating the development of the ectopic otic structures. In group B, strong Dlx3b expression was restricted to regions flanking r4, coinciding with the normal position of otic structures (Fig. 4l). The forkhead-domain containing transcription factor Foxi1 is expressed in two domains positioned laterally to the neural plate (Hochmann et al. 2007), which we also found in group B embryos (Fig. 4o). Interestingly we detected strong Foxi1 expression in group A embryos at the position where the ectopic otic structures develop (Fig. 4n), contrary to group B embryos, which do not show any Foxi1 expression in this region (Fig. 4o).
Sox3 is a marker for both the epibranchial and the otic preplacodal region, whereas Pax2 marks otic tissue specifically (Nikaido et al. 2007). In order to see whether the ectopic placodes of Wnt1 expressing embryos are restricted to otic development, we analysed group A embryos for expression of the two marker genes (Supplementary Fig. S4). We found both genes activated in overlapping anterior regions, indicating a clear bias towards ear development. This is in agreement with data from chick and mouse experiments, where Wnt signalling was found to partition the progenitor region, positively into inner ear and negatively into epibranchial placodes (Freter et al. 2008; Ohyama et al. 2006). Group B embryos did not show expression of either marker gene in the anterior region (data not shown).
Discussion
Ectopic expression of Wnt1 during early development of vertebrates is known to result in anterior truncations (Christian and Moon 1993). The heat-shock transgenic line allowed us to perform highly specific time-course experiments for ectopic Wnt1 activity in medaka fish. By systematically varying the time of activation, we observed a strict dependence of timing on the extent of the anterior truncations (Fig. 2a). These phenotypes can be interpreted by a gradient of Wnt signalling being responsible for patterning the anteroposterior axis (Kiecker and Niehrs 2001). The critical influence of timing in our experiments is in good agreement with lithium exposure experiments (Yamaguchi and Shinagawa 1989).
Induction of canonical Wnt signalling in the transgenic embryos at 30% epiboly resulted in a novel phenotype. Extensive otic tissue developed and finally covered the complete anterior region of the embryos. Interestingly, these effects on otic induction seemed to be uncoupled from the axis truncations. Injection of both Wnt1 mRNA and DNA resulted in truncations, but only the uniform misexpression at 30% epiboly in the transgenic line produced the cyclopic ear phenotype. In addition, the number of ectopic otic vesicles was dose dependent, whereas the axis truncations appeared indistinguishable from low to high Wnt1 doses (Fig. 2b). In the zebrafish model system, activation of canonical Wnt signalling at the midblastula transition did not produce ectopic otic vesicles. This experiment was used as an argument against a role of canonical Wnt signalling in otic induction (Phillips et al. 2004). In support of these data, we also failed to induce ectopic otic tissue with constitutive active DNA constructs in medaka. However, in contrast to the latter study, we demonstrate a strict time dependence for Wnt signalling in the otic induction process, namely at 30% epiboly.
Fgf signalling plays a major role in otic induction (reviewed in Riley and Phillips 2003; Whitfield et al. 2002), and we recently could demonstrate an inducing effect of Fgf8 misexpression during early gastrulation in medaka. In these experiments, the formation of ectopic otic vesicles was further boosted by coexpression of the competence factors Dlx3b and Foxi1 (Aghaallaei et al. 2007). Here we compared groups of Wnt1-transgenic embryos induced at 30% epiboly (group A), with those induced at 50% epiboly (group B). In both groups of Wnt1-transgenic embryos, we found similar expression of Fgf8 (Fig. 4e, f); however, only group A embryos developed ectopic otic structures. Fgf3 expression was stronger in the anterior region of group A embryos (Fig. 4h, k, n) and weaker and more variable in group B (Fig. 4i, l, o). In addition, we observed Dlx3b activity in both groups (Fig. 4k, l and Supplementary Fig. S3). Therefore Fgf signalling activity and Dlx3b expression, representing two prerequisites for otic induction, were present in the anterior regions of both groups. However, in group B embryos, no Foxi1 expression was detected in this region (Fig. 4o), contrary to group A (Fig. 4n). The differential Foxi1 expression in the two groups of embryos could be critical for the induction of ectopic otic development (Fig. 4n, o). Indeed we previously found a central role for this competence factor in the preplacodal gene regulatory network in medaka (Aghaallaei et al. 2007). Loss-of-function experiments in zebrafish also confirm a critical role of Foxi1 (Lee et al. 2003; Solomon et al. 2003).
The main result of pulsed Wnt activation is the truncation of anterior structures. In order to study the role of canonical Wnt signalling on inner ear formation, it would be ideal to uncouple the effects on the otic induction process from those on axis formation. However, this is not possible with our experimental approach. Due to the axis truncations, an expanded r4 region is positioned next to the anterior expression domains of Dlx3b and Foxi1 in group A embryos. The close contact between this rhombomere and ectoderm positive for the competence factors is a hallmark of normal otic development. The ectopic activation of Foxi1 in group A embryos could be mediated by signals of this r4 region. In agreement with this, we previously described the activation of Foxi1 by Fgf8 in medaka embryos (Aghaallaei et al. 2007). However, we found equal expression of Fgf8 in both group A and group B embryos (Fig. 4e, f). Anterior Fgf3 expression was variable in group B (Fig. 4i, l, o), but since none of these embryos developed ectopic ear structures, Fgf3 expression is unlikely to be responsible for the differences. Therefore signals in addition to these Fgf ligands seem to be essential for the ectopic otic development in group A embryos. Alternatively, signals could be secreted from the hindbrain structures in group B embryos, which block the formation of otic tissue, but instead promote the midbrain–hindbrain boundary gene Engrailed 2. Interestingly, recent experiments with retinoic acid (RA) signalling in zebrafish revealed widespread ectopic otic induction upon excess activation of this pathway (Hans and Westerfield 2007). Foxi1 was ectopically activated in these embryos, whereas other marker genes for otic induction were not altered significantly. It is therefore possible that a genetic network of different signalling pathways (Fgf/RA/Wnt) controls otic induction and in particular Foxi1. Further experiments will be necessary to clarify the role of Wnt signalling in this process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Experimental strategies used for Fig. 4. Experimental strategy used for analysis of hindbrain and otic marker genes during preplacodal stages. Wnt1-transgenic embryos were divided into two groups. In groups A and B, the embryos were heat-shocked at 30% and 50% epiboly, respectively. All embryos were fixed at a preplacodal stage (two somites) before showing any phenotypes (GIF 202 kb)
High resolution image file (TIF 6.25 MB)
Expression of late otic marker genes in cyclopic ear embryos. Dorsal views of embryos at stage 27. Anterior is to the top. Wild-type expression is shown for Eya1 (A) and Six1 (C). The Wnt1-transgenic embryos were induced at early gastrulation for 2 h at 39°C (GIF 37 kb)
High resolution image file (TIF 4.73 MB)
Expression of Dlx3b and Fgf8 at late gastrulation in Wnt1-transgenic lines. (A, A′) the expression of Dlx3b and Fgf8 in wild-type (A, A′) and Wnt1-transgenic embryos (B) at late gastrulation. The transgenic embryos were induced at 30% epiboly for 2 h at 39°C. Scale bars for A′ and B are 250 and 100 µm, respectively (GIF 81 kb)
High resolution image file (TIF 3.79 MB)
Bias towards otic development in the anterior preplacodal region. Dorsal views of embryos at stage 19. Pax2 expression marks the region where otic placodes form (A), Sox3 expression outside the neural tissue marks the region of both the epibranchial and the otic placodes in wild-type embryos. In embryos expressing Wnt1 induced at 30% epiboly (group A), both Pax2 and Sox3 are expressed anteriorly where the ectopic ear structures form (C, D). Abbreviations: op., otic placode; ep., epibranchial placode; eop., ectopic otic placode; mhb., mid-hindbrain boundary (GIF 179 kb)
High resolution image file (TIF 5.00 MB)
Acknowledgements
We thank Jochen Wittbrodt and Mirana Ramialison for the medaka Starmaker probe, which was kindly provided before publishing. The work was supported by the Austrian Science Fund (FWF, grant 19571-B11).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Baubak Bajoghli and Narges Aghaallaei contributed equally to this work.
References
- Aghaallaei N, Bajoghli B, Walter I, Czerny T. Duplicated members of the Groucho/Tle gene family in fish. Dev Dyn. 2005;234:143–50. doi: 10.1002/dvdy.20510. [DOI] [PubMed] [Google Scholar]
- Aghaallaei N, Bajoghli B, Czerny T. Distinct roles of Fgf8, Foxi1, Dlx3b and Pax8/2 during otic vesicle induction and maintenance in medaka. Dev Biol. 2007;307:408–20. doi: 10.1016/j.ydbio.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr Top Dev Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Heimbucher T, Czerny T. An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev Biol. 2004;271:416–30. doi: 10.1016/j.ydbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Soroldoni D, Czerny T. The roles of Groucho/Tle in left-right asymmetry and Kupffer’s vesicle organogenesis. Dev Biol. 2007;303:347–61. doi: 10.1016/j.ydbio.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Esterberg R, Fritz A. dlx3b/4b are required for the formation of the preplacodal region and otic placode through local modulation of BMP activity. Dev Biol. 2009;325:189–99. doi: 10.1016/j.ydbio.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–24. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Gutknecht D, Fritzsch B. Lithium can transform ear placodes of Xenopus into multiple otic vesicles connected by tubes. Naturwissenschaften. 1990;77:235–7. doi: 10.1007/BF01138491. [DOI] [PubMed] [Google Scholar]
- Hans S, Westerfield M. Changes in retinoic acid signaling alter otic patterning. Development. 2007;134:2449–58. doi: 10.1242/dev.000448. [DOI] [PubMed] [Google Scholar]
- Hochmann S, Aghaallaei N, Bajoghli B, Soroldoni D, Carl M, Czerny T. Expression of marker genes during early ear development in medaka. Gene Expr Patterns. 2007;7:355–62. doi: 10.1016/j.modgep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech Dev. 2004;121:605–18. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Kaji T, Artinger KB. dlx3b and dlx4b function in the development of Rohon-Beard sensory neurons and trigeminal placode in the zebrafish neurula. Dev Biol. 2004;276:523–40. doi: 10.1016/j.ydbio.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GM, Erezyilmaz DF, Moon RT. Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mech Dev. 1995;53:261–73. doi: 10.1016/0925-4773(95)00442-4. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–7. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Lee SA, Shen EL, Fiser A, Sali A, Guo S. The zebrafish forkhead transcription factor Foxi1 specifies epibranchial placode-derived sensory neurons. Development. 2003;130:2669–79. doi: 10.1242/dev.00502. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–24. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Liu F, van den Broek O, Destree O, Hoppler S. Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development. 2005;132:5375–85. doi: 10.1242/dev.02152. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Maves L, Jackman W, Kimmel CB. FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development. 2002;129:3825–37. doi: 10.1242/dev.129.16.3825. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–71. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235:351–65. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Storch EM, Lekven AC, Riley BB. A direct role for Fgf but not Wnt in otic placode induction. Development. 2004;131:923–31. doi: 10.1242/dev.00978. [DOI] [PubMed] [Google Scholar]
- Riley BB, Phillips BT. Ringing in the new ear: resolution of cell interactions in otic development. Dev Biol. 2003;261:289–312. doi: 10.1016/S0012-1606(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–40. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kwak SJ, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev Dyn. 2004;230:419–33. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- Stachel SE, Grunwald DJ, Myers PZ. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–74. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–91. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- van de Water S, van de Wetering M, Joore J, Esseling J, Bink R, Clevers H, Zivkovic D. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development. 2001;128:3877–88. doi: 10.1242/dev.128.20.3877. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev Dyn. 2002;223:427–58. doi: 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]
- Woo K, Fraser SE. Specification of the hindbrain fate in the zebrafish. Dev Biol. 1998;197:283–96. doi: 10.1006/dbio.1998.8870. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Shinagawa A. Marked alteration at midblastula transition in the effect of lithium on formation of the larval body pattern of Xenopus laevis. Dev Growth Differ. 1989;31:531–541. doi: 10.1111/j.1440-169X.1989.00531.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental strategies used for Fig. 4. Experimental strategy used for analysis of hindbrain and otic marker genes during preplacodal stages. Wnt1-transgenic embryos were divided into two groups. In groups A and B, the embryos were heat-shocked at 30% and 50% epiboly, respectively. All embryos were fixed at a preplacodal stage (two somites) before showing any phenotypes (GIF 202 kb)
High resolution image file (TIF 6.25 MB)
Expression of late otic marker genes in cyclopic ear embryos. Dorsal views of embryos at stage 27. Anterior is to the top. Wild-type expression is shown for Eya1 (A) and Six1 (C). The Wnt1-transgenic embryos were induced at early gastrulation for 2 h at 39°C (GIF 37 kb)
High resolution image file (TIF 4.73 MB)
Expression of Dlx3b and Fgf8 at late gastrulation in Wnt1-transgenic lines. (A, A′) the expression of Dlx3b and Fgf8 in wild-type (A, A′) and Wnt1-transgenic embryos (B) at late gastrulation. The transgenic embryos were induced at 30% epiboly for 2 h at 39°C. Scale bars for A′ and B are 250 and 100 µm, respectively (GIF 81 kb)
High resolution image file (TIF 3.79 MB)
Bias towards otic development in the anterior preplacodal region. Dorsal views of embryos at stage 19. Pax2 expression marks the region where otic placodes form (A), Sox3 expression outside the neural tissue marks the region of both the epibranchial and the otic placodes in wild-type embryos. In embryos expressing Wnt1 induced at 30% epiboly (group A), both Pax2 and Sox3 are expressed anteriorly where the ectopic ear structures form (C, D). Abbreviations: op., otic placode; ep., epibranchial placode; eop., ectopic otic placode; mhb., mid-hindbrain boundary (GIF 179 kb)
High resolution image file (TIF 5.00 MB)


