Abstract
We applied a new method to visualize the three-dimensional profile of sex differences in brain structure based on MRI scans of 100 young adults. We compared 50 men with 50 women, matched for age and other relevant demographics. As predicted, left hemisphere auditory and language-related regions were proportionally expanded in women versus men, suggesting a possible structural basis for the widely replicated sex differences in language processing. In men, primary visual, and visuo-spatial association areas of the parietal lobes were proportionally expanded, in line with prior reports of relative strengths in visuo-spatial processing in men. We relate these three-dimensional patterns to prior functional and structural studies, and to theoretical predictions based on nonlinear scaling of brain morphometry.
Keywords: brain, sex differences, size, tensor-based morphometry
Introduction
Sex differences in human brain structure and function have been extensively studied, using methods ranging from cognitive testing to computational anatomy. Consistent sex differences are found across the human lifespan from early childhood [1] to young adulthood [2] and old age [3]. Males are consistently found to have larger total brain volumes (TBVs) and global gray and white matter volumes [4], whereas women tend to have proportionally greater gray matter volumes, partly because substructures do not scale linearly with TBVAQ2.
Cognitive tests reveal sex differences in average performance in language acquisition and processing [4], visuo-spatial ability, and other tasks [5]. Understanding sex differences in these skills is of interest, as many disorders affecting language, for example, such as autism, dyslexia, or schizophrenia are expressed differently in men versus women. Functional MRI (fMRI) studies have also found sex differences in brain activation during both verbal and spatial tasks [6]. However, most fMRI results have not been consistently replicated, perhaps because of small sample sizes, age differences across studies [4], or methodological differences [7].
Other researchers have used structural MRI and diffusion tensor imaging to understand sex differences in neuroanatomy. In the study by Schlaepfer et al. [8], greater gray matter volumes were found in the dorsolateral prefrontal cortex and the superior temporal gyrus in females.AQ3 In a cortical mapping study [9], women had a higher complexity of sulcal and gyral convolutions in the superior-frontal and parietal lobes, and greater gray matter proportions in all the lobes, with greatest effect sizes in precentral and postcentral gyri, and the left temporal and occipital regions [2]. Furthermore, in a study of callosal thickness [10], the authors found that that the corpus callosum was thicker in men, but the sex difference was no longer found after accounting for overall brain volume. Using diffusion tensor imaging to study white matter microstructure [11], a higher fractional anisotropy was found in the left frontal lobes of women, as well as a leftward asymmetry of fractional anisotropy that did not appear in men, and that correlated with better verbal comprehension and memory. More recently, computational mapping methods, such as voxel-based morphometry (VBM) (http://pni.med.jhu.edu/methods/vbm.htm), have enabled the mapping of structural differences between two populations throughout the brain, without needing to specify regions of interest in advance.
Two VBM studies [12,13] found sex differences in the language areas (Broca's and Wernicke's), but no overlap was found between the two studies except for a higher proportion of gray matter in women in the anterior cingulate region. Although many of the above studies point to differences between males and females, particularly in the language areas, results have not been consistent enough to draw any definitive conclusions. Here, we examined 100 MRI scans (from 50 men and 50 women) using tensor-based morphometry, a technique that has gained popularity in recent years, as it reveals the three-dimensional (3D) pattern of regional volume excesses and deficits between two groups, throughout the brain. This voxel-based morphometric technique differs from the standard VBM method as it visualizes the profile of local volume differences between two groups rather than local gray matter and white matter density differences. To our knowledge, this is the first study to plot volumetric differences between men and women as 3D maps of relative volume excesses or deficits. The goal was to test whether there is evidence for disproportionately larger structures in women in candidate regions (subserving language and affective processing), beyond what would be expected from how structures scale regardless of sex. We also aimed to map these differences throughout the brain, with automated measures that do not require prior manual specification of regions of interest.
All scans are first scaled to adjust for the influence of TBV, and then linearly and fluidly registered to a target image. Volumetric differences are inferred from the deformations required to match each participant's image to the common brain template.AQ4 We surveyed differences throughout the entire brain, but we hypothesized that we would find proportionally larger temporal lobe volumes in women, particularly in classical left hemisphere language areas.
Methods
Participants and image acquisition
3D T1-weighted images were acquired from 100 healthy young adults (50 men, age: 24.2±4.2 years, and 50 women, age: 25.1±4.5 years) using a 1.5λT GE Signa MRI scanner (rapid gradient echo sequence with repetition timeλ=λ24λms; echo timeλ=λ8λms; flip angleλ=λ15°; field of viewλ=λ250 × 250λmm2; matrix sizeλ=λ256 × 256 × 124; voxel sizeλ=λ0.98 × 0.98 × 1.5λmm3).AQ5 All participants were right-handed Caucasian volunteers, who were matched for sex and age. Handedness was determined based on self-reported hand preference. All participants gave informed consent according to institutional guidelines.
Preprocessing
Extracerebral (nonbrain) tissues were manually deleted from the MRI scans using the Display software program (Montreal Neurological Institute, McGill University, Canada). All scans were then aligned to the standard ICBM-53 template using a nine-parameter registration method (i.e. translational and rotational alignment, allowing scaling in three independent directions) found in the FMRIB's Linear Image Registration Toolbox, FLIRT (http://www.fmrib.ox.ac.uk/fsl/fsl/macosx.html). Lobar regions of interest were also delineated on the mean deformation template used as a target (see next paragraph) using the BrainSuite software package (http://www.loni.ucla.edu/Software/BrainSuite2) according to the anatomical criteria used to define lobar boundaries in the ICBM-53 atlas (http://www.loni.ucla.edu/ICBM/).
Fluid registration
Each volunteer's image was registered (warped), using a fluid registration approach [14], to match a specially constructed average target image to obtain a 3D deformation field. As the target image, we created a mean deformation template, by transforming one of the images of volunteers using the average of the deformation fields from its registration to all other volunteers' images.
Statistical analysis
At each point in the brain, the relative volumetric differences between each volunteer and the target image were computed from the local compressions or expansions required to align each image to the target. The map of relative volumes, also known as a Jacobian map J, was statistically analyzed using a univariate Student's t-test at each voxel. To avoid assuming that our measures of relative volumes were normally distributed at each point, we used voxel-wise permutation tests to establish a null distribution at each voxel [15], giving a map of uncorrected significance values. As is standard in brain imaging, we then thresholded the map at a fixed a priori threshold and computed the suprathreshold volume. This volume is a single number (e.g. 234 voxels), the null distribution of which is established through permutation, and then a significance value is assigned to the overall suprathreshold volume found in the experiment. In the case of this map-based statistic, multiple comparisons are not involved, as this is a summary statistic from the overall map, confirming the overall significance of the observed pattern of statistical effects. The probability that measures the overall significance is called pcorrected (see Results). It determines how likely it would be to find a pattern by chance with a still greater number of significant voxels than was observed in the true experiment. These pcorrected values were reported in the whole brain, each hemisphere and each lobe; total lobar volumes of all volunteers were also computed from the deformation fields and the lobe segmentations of the template image (see Preprocessing).AQ6 Bonferroni corrections were applied in the lobes, where no prior hypothesis was made.
Results
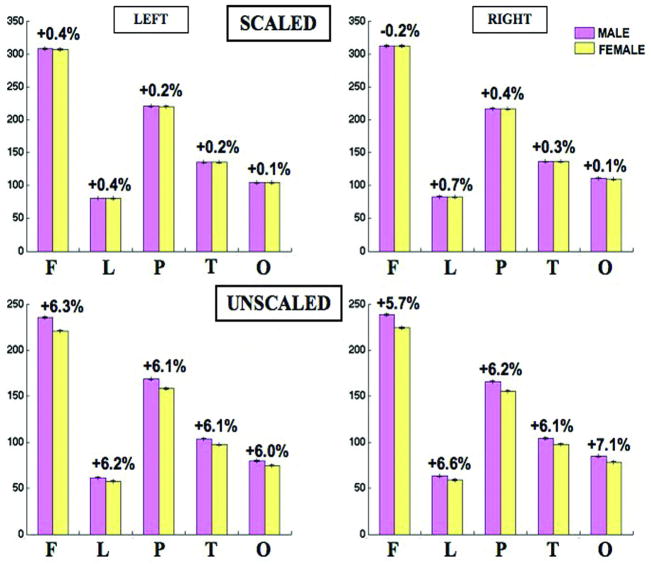
All lobar volumes were larger in men than in women Fig. 1, but no significant sex differences remained after linearly scaling the brains to adjust for individual differences in brain size. Nevertheless, the voxel-based maps detected significant sex differences at a more local level.
Fig. 1.
Lobar volumes in men and women before scaling (bottom) and after scaling (top). Means and standard deviations are shown for the two hemispheres. In the unscaled data, all lobes were significantly bigger in men, whereas in the scaled data, there was no evidence for an overall excess or reduction in male or female in any region; maps revealed some subregional differences within each lobe.AQ11
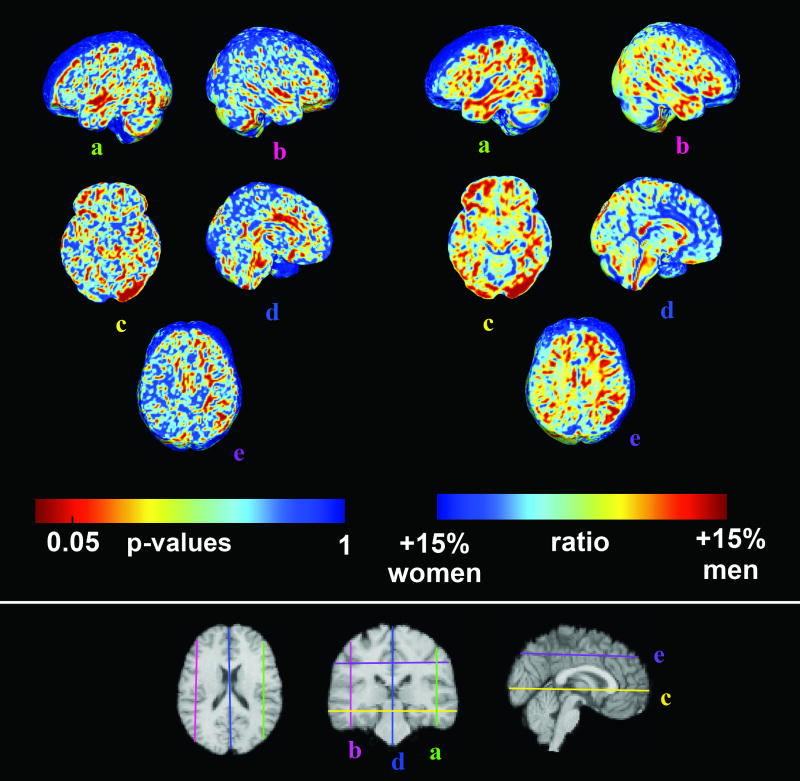
Figure 2 shows voxel-wise significance maps of volumetric differences between men and women, after images were scaled to adjust for TBV. Ratio maps indicating the mean male volume divided by the mean female volume are also shown at the corresponding slices.
Fig. 2.
Three-dimensional maps of sex differences in cerebral volumes. The top right panel shows P values corresponding to local volume expansion or shrinkage. Red indicates significance at the voxel level. To assess the direction of changes, we also mapped the relative volume (right panel), computed as the ratio of the mean volumes obtained from both groups. Here, red indicates 15% volume excess in male and blue indicates 15% volume excess in female. Sex differences were detected in the left temporal lobe (a), right superior temporal gyrus (b), left occipital lobe (c), cingulate (d), and the left superior lateral fasciculus II (e).
In agreement with our prior hypothesis (that women have larger temporal lobe volumes, particularly in the left hemisphere language areas – see Introduction), in scaled images, women had proportionally larger left temporal lobe regions (pcorrectedλ=λ0.029), left parietal, and left occipital lobes (pcorrectedλ=λ0.038 and pcorrectedλ=λ0.016, respectively). Applying a strict Bonferroni correction to adjust for the multiple statistical tests performed, the left temporal lobe sex differences should be considered significant, with trends in the left parietal and left occipital lobes (see Table 1 for results in each lobe). Proportionally larger regions were found in women in the left but not the right hemisphere (pcorrectedλ=λ0.044, left; pcorrectedλ=λ0.12, right).
Table 1.
P values (two-tailed) showing the overall significance of the volumetric differences in each lobe
| Frontal | Limbic | Parietal | Temporal | Occipital | |
|---|---|---|---|---|---|
| λLeft | 0.053 | 0.16 | 0.038, larger in men | 0.029, larger in women | 0.016, larger in men |
| Right | 0.081 | 0.13 | 0.31 | 0.11 | 0.28 |
Left temporal volume excesses in women were hypothesized a priori; the other results should be considered trends after multiple comparisons correction.AQ12
In the statistical maps (left panel of Fig. 2), women had proportionally greater volumes for the left (a) and the right (b) superior temporal gyri (which house the primary auditory cortex), in agreement with Table 1.
One unexpected finding was that men had proportionally larger volumes in the left occipital lobes (c), in regions housing primary and secondary visual cortices. This was not hypothesized, and is less intuitive than the other findings, as the volume difference is unlikely to be associated with a functional difference (as sex differences in primary visual function are not anticipated). This difference needs to be replicated in future studies, but it remains possible that these occipital volumetric differences may not have any obvious functional correlate.
The anterior cingulate cortex (d), which is involved in affective regulation and autonomic function, was also proportionally larger in women. Men still showed greater volumes, even after adjusting for brain scale, in the left superior lateral fasciculus (e), a white matter tract connecting the caudal inferior parietal cortex to the dorsolateral prefrontal cortex.
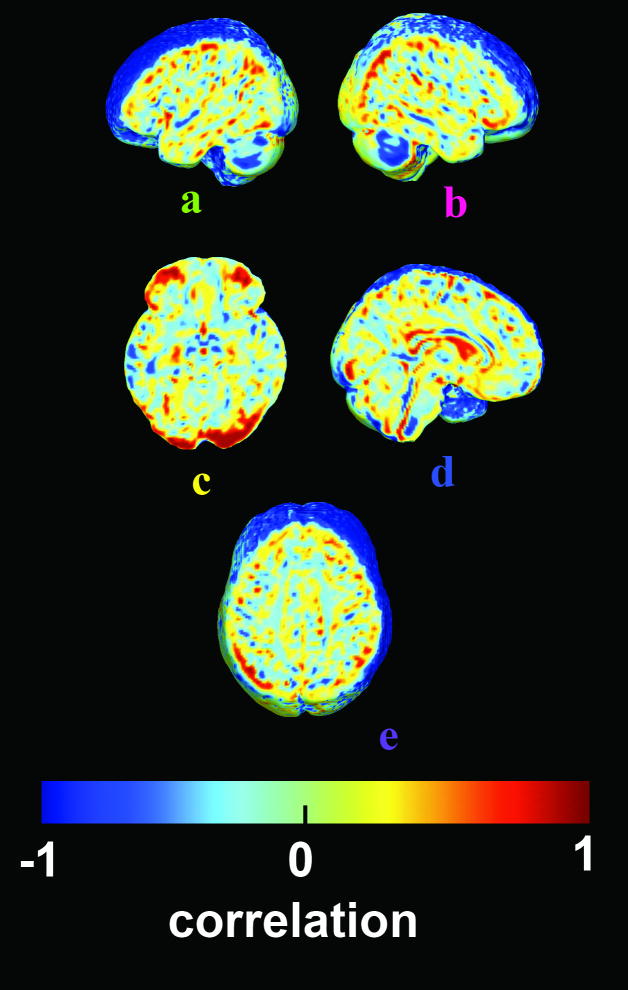
One major confound in studies of sex differences is the TBV difference between men and women. Proportional differences in brain volumes may therefore be because of nonlinear scaling effects, in which substructure volumes scale nonlinearly rather than proportionately to TBV [16]. If direct fitting of this nonlinear power law would predict sex differences in the direction found here, they may be meaningless, in the sense that selected groups of men who are matched for brain size would not be distinguishable from women.AQ7 To test this, after linear scaling of the images, we determined whether the relative volume at each voxel still correlated with the TBV. Figure 3 shows the correlation coefficient between TBV and local volume; maps are similar within each sex (not shown). Pale green (0) indicates no remaining correlation between regional volumes and the TBV, that is, the TBV effect was statistically removed by the linear one-to-one scaling, and there is no evidence for a nonlinear power law. Some regions (blue colors) are proportionally larger in volunteers with small TBV, whereas occipital regions (in red) are proportionally larger in volunteers with larger TBV. Importantly, occipital regions are shown to be proportionally larger when TBV increases, so the effects found in Fig. 2 favoring men might be because of how the brain scales, regardless of sex. However, no residual correlations were detected in the temporal and cingulate regions where women were proportionally larger (a, b, d), nor in the SLF II (e) which is larger in men, showing that these effects are likely to be mediated by biological sex operating independently of how the brain scales.
Fig. 3.
This map shows any remaining correlations between regional volumes and total brain volume, after images were all scaled to the same overall scale. The lack of any residual correlation in right superior temporal gyrus (b), cingulate (d), and the left superior lateral fasciculus II (e) means that the sex differences are not simply due to some nonproportional scaling of brain structures that operates regardless of sex. Red means that even after global scaling, local volumes increase more than linearly with total brain volume (TBV) (occipital lobe (c)], whereas blue indicates a negative correlation [regions are proportionally larger with smaller TBV).
DiscussionAQ8
We replicated the commonly found result that men have, on average, a larger TBV than women (12% larger in men, ±0.069 SD in female and ±0.094 SD in male, if volumes are expressed as a proportion of the mean female brain volume).
Women showed proportionally greater volumes in the left temporal lobe, and at trend level for the right temporal lobe. Some prior volumetric studies have reported sex differences in language areas, such as Wernicke's [2] and Broca's [12,13] both devoted to language production and comprehension, but our study revealed differences in the left and right superior temporal gyri, which encompass the primary auditory cortices. These results could thus be related to prior fMRI findings that showed greater activation in males' temporal lobes during language semantic tasks than during phonological tasks, whereas no difference was detected in women [17]. Women also tend to activate both temporal lobes while hearing from one side, whereas men's activation was lateralized [17]. Microstructurally, factors such as the density of cortical neurons may explain these findings. Intriguingly, Witelson et al. [18] found greater density of neurons in posterior temporal areas in women than men.
Prior fMRI studies [6] also reported differences in brain activation during spatial tasks. Here, men showed greater volumes in the left occipital lobe and in localized areas of the superior lateral fasciculus II that connects the inferior parietal lobule and the prefrontal cortex. The first region plays a major role in the perception of visual space; the SLF II exchanges information between these areas and the prefrontal cortex, which is involved in decision making and inhibition [19]. Although functional conclusions may be premature, these structural findings may relate to sex differences in cognitive processing during spatial tasks [20]. They may also relate to a more efficient visual event-categorization process in women versus men [21].
In agreement with prior structural MRI studies [13,12], we found proportionally greater volumes in the anterior cingulate regions in women. As all three studies had different mean ages and sample sizes, sex differences in this region may persist form early adulthood to old age. The region is involved in regulation emotion and affect, which are abnormal in autism for instance, a disorder that is more common in men.
Ringo [22] hypothesized in 1994 that brain size is the major factor determining morphological sex differences. This hypothesis has been supported by additional studies [23]. In a study, Leonard et al. [24] found that the only sex difference that survived adjustment for TBV was the proportion of gray matter in the brain. To address this problem, here, we presented correlation maps, which assessed any residual correlations between TBV and regional brain volumes that persisted after adjusting for brain size. The remaining cingulate and temporal lobe sex differences could not be explained by their statistical relationship with TBV. Nevertheless, studies of women and men matched for brain size could be used to further corroborate these sex differences, albeit in a sample that would not represent the general population.
Conclusion
This study used an automatic computational anatomy technique, TBM, to visualize brain volumetric differences between men and women. Even when adjusting locally for brain size, significant differences persisted in the left temporal and cingulate regions, suggesting the need to account for potential sex differences in future studies of these brain regions.
Acknowledgments
This work was partially supported by NIH grants EB008281, EB007813, HD050735 and RR013642. The authors also thank Dr Franco Leporeé for useful discussions.
References
- 1.Berglund E, Eriksson M, Westerlund M. Communicative skills in relation to gender, birth order, childcare and socioeconomical status in 18-month-old children. Scand J Psychol. 2005;46(Suppl 6):485–491. doi: 10.1111/j.1467-9450.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 2.Luders E, Narr KL, Thompson PM, Woods RP, Rex DE, Jancke L, et al. Mapping cortical gray matter in the young adult brain: Effects of gender. Neuroimage. 2005;26:492–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28(Suppl 7):1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin M. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 2008 doi: 10.1016/j.bandl.2008.07.001. M:M–M. [DOI] [PubMed] [Google Scholar]
- 5.Kimura D. Sex and cognition. MIT press; 1999. M. AQ9. [Google Scholar]
- 6.Gur RC, Alsop D, Glahn D, Petty R, Swanson CL, Maldjian JA, et al. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain Lang. 2000;74(Suppl 2):157–172. doi: 10.1006/brln.2000.2325. [DOI] [PubMed] [Google Scholar]
- 7.Harrington GS, Faris ST. Sex differences in language processing: functional MRI methodological considerations. J Magn Reson Imaging. 2008;27(Suppl 6):1221–1228. doi: 10.1002/jmri.21374. [DOI] [PubMed] [Google Scholar]
- 8.Schlaepfer TE, Harris GT, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61:129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- 9.Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, Toga AW. Gender differences in cortical complexity. Nat Neurosci. 2004;7:799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- 10.Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006;17(Suppl 11):1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- 11.Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, et al. Sex differences in frontal lobe white matter micrsotructure: a DTI study. Neuroreport. 2003;14(Suppl 18):2469–2473. doi: 10.1097/00001756-200312190-00035. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Perminder SS, Wen W, Anstey KJ. Sex differences in regional gray matter in healthy individuals aged 44–48 years: a voxel-based morphometric study. Neuroimage. 2007;36(Suppl 3):691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometry of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 14.Brun C, Lepore N, Pennec X, Chou YY, Lee AD, Barysheva M, et al. A Tensor-based morphometry study of genetic influences on brain structure using a new fluid registration method. MICCAI. 2008 doi: 10.1007/978-3-540-85990-1_110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols TE, Holmes AP. Non parametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(Suppl 1):125. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson PM. The parallel brain. MIT Press; 2003. Mapping structural alterations of the corpus callosum during brain development and degeneration. M. [Google Scholar]
- 17.Phillips MD, Lowe MJ, Lurito JT, Dzemidzic M, Mathews VP. Temporal lobe activation demonstrates sex-based differences during passive listening. Radiology. 2001;220(Suppl 1):202–207. doi: 10.1148/radiology.220.1.r01jl34202. [DOI] [PubMed] [Google Scholar]
- 18.Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15(Suppl 5):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness V, et al. Segmentation of subcomponents within superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb cortex. 2005;15(Suppl 6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 20.Spiers MV, Sakamoto M, Elliott RJ, Baumann S. Sex differences in spatial object-location memory in a virtual grocery store. Cyberpsychol Behav. 2008;11(Suppl 4):471–473. doi: 10.1089/cpb.2007.0058. [DOI] [PubMed] [Google Scholar]
- 21.Jausovec N, Jausovec K. Do women see things differently than men do? Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.11.013. M:M–M.AQ10. [DOI] [PubMed] [Google Scholar]
- 22.Ringo JL, Doty RW, Demeter S, Simard PS. Time is of the essence: a conjecture that hemisphere specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4(Suppl 4):331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- 23.Jancke L, Staiger JF, Schlaug G, Yanxiong H, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb Cortex. 1997;7(Suppl 1):48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- 24.Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex. 2008;12(Suppl 18):2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luders E, Steinmetz H, Jancke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13:2371–2374. doi: 10.1097/01.wnr.0000049603.85580.da. [DOI] [PubMed] [Google Scholar]