Abstract
Background
Neuroimaging studies of attention-deficit/hyperactivity disorder (ADHD) have revealed structural abnormalities in the brains of affected individuals. One of the most replicated alterations is a significantly smaller corpus callosum (CC), for which conflicting reports exist with respect to the affected callosal segments.
Methods
We applied novel surface-based geometrical modeling methods to establish the presence, direction, and exact location of callosal alterations in ADHD at high spatial resolution. For this purpose, we calculated the thickness of the CC at 100 equidistant midsagittal points in an age-matched male sample of 19 individuals with ADHD and 19 typically developing control subjects.
Results
In close agreement with many prior observations, the CC was shown to be significantly thinner in ADHD subjects in anterior and, particularly, posterior callosal sections. Covarying for intelligence did not significantly alter the observed ADHD effects. However, group differences were no longer present in anterior sections when covarying for brain volume and after excluding ADHD subjects comorbid for oppositional defiant disorder.
Conclusions
Decreased callosal thickness may be associated with fewer fibers or a decrease in the myelination of fibers connecting the parietal and prefrontal cortices. This might affect interhemispheric communication channels that are necessary to sustain attention or motor control, thus contributing to symptoms of hyperactivity and impulsivity, or inattention, observed in ADHD. Future studies are necessary to determine whether callosal abnormalities reflect maturational delays or persist into adulthood.
Keywords: Corpus callosum, isthmus, MRI, ODD, splenium
Attention-deficit/hyperactivity disorder (ADHD) is a highly heritable developmental and behavioral disorder with an estimated incidence of approximately 5%. Affected individuals show symptoms of hyperactivity and impulsivity or inattention, or a combination of these symptoms (1). Converging evidence suggests a neurobiological basis for ADHD, but its precise etiology remains unclear (2). Numerous neuroimaging studies have revealed structural abnormalities at the gross anatomic level involving cerebellar, cortical, and subcortical regions (2–5). One of the most replicated alterations in ADHD individuals is a significantly smaller midsagittal area of the corpus callosum (CC) (3,4). Because the CC is the largest cerebral commissure known to influence cerebral specialization and interhemispheric information transfer, callosal abnormalities in affected individuals are consistent with data suggesting abnormal asymmetry patterns and differential hemispheric effects associated with ADHD (2,5). A recent meta-analysis revealed that the callosal splenium in particular is significantly decreased in ADHD individuals (3). Even so, other studies have not detected splenial abnormalities (6,7) or they have found altered callosal morphology in additional sections, such as the callosal rostrum (6), rostral body (6,8), genu (9), isthmus (10), and the total CC (11). Discrepancies regarding the location of ADHD-specific effects might be attributable to heterogeneity in sample characteristics (e.g., exposure to stimulant medication, age, gender, etc.) but also to differences in methodologic approaches for measuring the CC. More specifically, most prior examinations of the CC in ADHD have employed gross parcellation schemes to define functionally distinct callosal regions (12,13), a method that has generated some controversy (14,15). Moreover, studies differ in the degree to which they control for the effect of decreased overall brain volumes and lower intelligence, which are frequently reported in ADHD (16,17).
To address the limitations of some prior research, the current study was designed to compare callosal morphology between age-matched ADHD subjects and normally developing control subjects, with and without removing the variance associated with brain volume and intelligence. We hypothesized a reduced thickness of the CC in ADHD individuals and used novel image analysis methods to establish the presence and exact location of callosal thickness aberrations in ADHD at high spatial resolution without relying on parcellation schemes.
Methods and Materials
Subjects
We analyzed a sample of 19 children and adolescents with ADHD (mean age ± SD: 11.8 ± 2.7 years) and 19 age-matched normally developing control subjects (mean age ± SD: 11.7 ± 2.6 years), ranging from 7.2 to 16.2 years. The maximum allowed age difference within a matched pair was 6 months (for further demographic and clinical details, see Supplements 1 and 2). Only male subjects were studied because of the greater prevalence of ADHD among boys (18), as well as to minimize variance due to sex-dependent rates of myelination during neurodevelopment (19,20) and controversially discussed effects of sex on callosal morphology (21). The ADHD and control subjects were recruited from local clinics, schools, and health organizations. Additional control subjects were recruited from ongoing studies of normal development at the University of California—Los Angeles (UCLA). After complete description of the study, written informed assent from the subject and consent from their guardian was obtained. Experimental protocols were approved by the Institutional Review Board of UCLA.
Inclusion criteria for ADHD subjects involved meeting DSM-IV criteria for ADHD by parental interview using the National Institute of Mental Health (NIMH) Diagnostic Interview Schedule for Children, Version IV (NIMH DISC-IV) (22). Further criteria included obtaining a score over 1.5 SD from the mean on the parent-rated and/or teacher-rated Inattentive and Hyperactive-Impulsive subscales of the SNAP-IV (23). The ultimate diagnosis, however, was established by clinical interview and subsequent case consensus with the senior clinicians on the project that included a clinical psychologist, a neuropsychologist, and a psychiatrist. The ADHD sample (n = 19) included both subjects diagnosed as the inattentive type (n = 6) and subjects diagnosed as the combined type (n = 12), showing both inattentive and hyperactive-impulsive symptoms. For one subject, ADHD subtype was not specified. 53% (n = 10) of all ADHD subjects had oppositional defiant disorder (ODD). Subjects with the co-occurrence of a known genetic syndrome associated with ADHD including fragile X, tuberous sclerosis, generalized resistance to thyroid hormone, and those taking nonstimulant psychotropic medication were excluded from the study. 37% (n = 7) of all ADHD subjects were medicated. More specifically, four subjects were receiving methylphenidates (Ritalin, Novartis Pharmaceuticals Corporation, East Hanover, New Jersey; Concerta, ALZA Corporation, Mountain View, California), two subjects were taking dextroamphetamines (Dexedrine, GlaxoSmithKline, Middlesex, England; Addalrel, Barr Laboratories, Montvale, New Jersey), and one subject was on atomoxetines (Strattera, Eli Lilly and Company, Indianapolis, Indiana). These subjects withheld medication for at least 24 hours before scanning.
Control subjects were required to be free from any current or lifetime history of major Axis I mental disorder as assessed by DISC interview (22). Additional exclusion criteria for control subjects included the presence of a serious medical or neurological illness, a history of closed head trauma or other neurological disorders, and a first-degree relative with a history of any disruptive behavior disorder (including ADHD), antisocial personality disorder, schizophrenia, or bipolar disorder. Exclusion criteria for both the ADHD and control group included weight or height smaller than the fifth or larger than the 95th percentile.
Individual intelligence quotients (IQ) in ADHD and control subjects were estimated by using the Block Design and Vocabulary subtests of the Wechsler Intelligence Scale for Children, 3rd edition (WISC-III) (24). The variance associated with IQ was later considered when conducting statistical comparisons between groups. In addition, the presence of learning disorders was examined and defined in two ways: either by obtaining standard scores less than 85 on academic achievement subtests of the Wide Range Achievement Test-3 (WRAT3) (25) and the Woodcock-Johnson Tests of Cognitive Abilities (WJ-III) (26) or based on discrepancy of greater than 22 points between any of the subtest standard scores and IQ (with IQ being greater than academic achievement). Given that only three ADHD subjects suffered from learning disorders, we abstained from covarying for learning disorders.
Image Acquisition and Preprocessing
Brain images were acquired on a Siemens Sonata 1.5-Tesla magnetic resonance imaging (MRI) scanner using a high-resolution three-dimensional T1-weighted spoiled gradient echo (SPGR) sequence. A sagittal plane image acquisition protocol was used to acquire two SPGR scans with the following parameters: repetition time (TR): 24 msec; echo time (TE): 12.6 msec; flip angle: 22°; one excitation; acquisition matrix 256 × 196; and field of view: 240 × 240 mm2. The image voxel size was 1.3 × .9 × 1.2 mm3. The two scans were averaged together and corrected for head tilt and alignment by reorienting each volume into the standard position of the International Consortium for Brain Mapping—305 average brain (27) using rigid-body transformations (28).
Measurement of Total Brain Volume and Callosal Thickness
Brain tissue was classified using a partial volume method validated on real and phantom data, as described elsewhere (29). Briefly, this method first removes nonbrain tissue from the whole-head MRI using a sequence of anisotropic diffusion filtering, Marr-Hildreth edge detection, and mathematical morphology. It then eliminates intensity drifts due to magnetic field inhomogeneities (bias correction). After the image has been bias-corrected, each voxel is classified according to tissue type (white matter [WM], grey matter [GM], cerebrospinal fluid [CSF], and partial volume mixtures) by combining the partial volume tissue measurements with a Gibbs spatial prior that models the contiguous nature of brain tissue. Total brain volume (TBV) was determined in centimeters cubed as the sum of voxels representing GM, WM, and CSF (including ventricular CSF).
Regional callosal thickness was estimated in a three-step approach as detailed elsewhere (30–32). Briefly, one rater (EL) manually outlined upper and lower callosal boundaries (top and bottom) in the midsagittal section of each inhomogeneity-corrected and spatially aligned brain volume (Step I). A new midline segment was then automatically created by calculating the spatial average from 100 equidistant surface points representing the top and bottom traces (Step II). Subsequently, the distances between 100 surface points of the midline segment and the 100 corresponding surface points of the callosal top/bottom segments were automatically quantified (Step III). These regional distances indicate callosal thickness with a high spatial resolution (i.e., at 100 locations distributed evenly over the callosal surface).
Statistical Analysis
Using independent sample Student's t tests, we tested for group differences in callosal thickness and generated color-coded statistical maps illustrating where ADHD subjects differed significantly from normally developing control subjects. Permutation testing, with 10,000 permutations computed, was employed to control for multiple comparisons, testing for the proportion of the surface area of the CC with suprathreshold statistics when statistical maps were thresholded at p = .05. ADHD and control subjects in the study differed significantly with respect to IQ and TBV (see Results). We thus conducted follow-up analyses of covariance (ANCOVAs) controlling for IQ or TBV, respectively, when examining main effects of group status on callosal thickness. Finally, to confirm ADHD effects independent of ODD occurrence, we performed a post hoc analysis and compared a subsample of ADHD subjects (n = 9) that were not comorbid for ODD to age-matched control subjects (n = 9) using independent sample Student's t tests.
Supplemental Analysis
To examine possible developmental effects on callosal morphology, we performed additional linear regression analyses mapping the relationships between age and callosal distance measures at 100 equidistant points for the combined sample (n = 38). We also tested whether age effects on callosal thickness were significantly different between ADHD subjects and control subjects.
Results
ADHD Effects
Recruitment procedures attempted to match control to ADHD participants for overall intelligence, but ADHD individuals still had significantly lower IQ scores than normally developing age-matched control subjects (mean IQ ± SD: ADHD group = 92.11 ± 13.75; control group = 104.37 ± 9.95; p ≤ .03). In addition, ADHD individuals had significantly smaller total brain volumes than normally developing age-matched control subjects (mean TBV ± SD: ADHD group = 1430 cm3 ± .01; control group = 1530 cm3 ± .12; p ≤ .017).
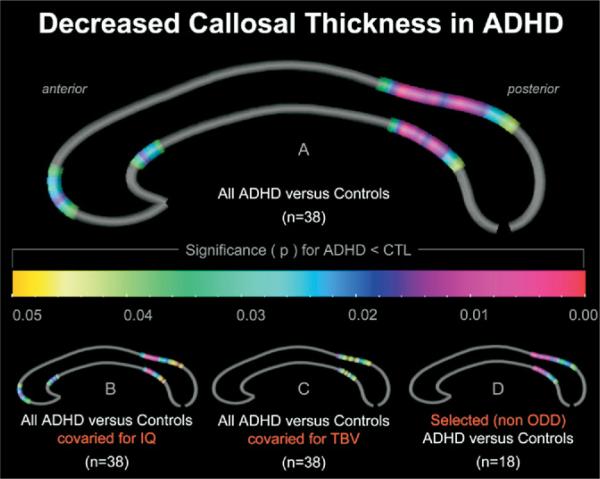
As shown in Figure 1 (Panel A), the CC was significantly thinner in ADHD individuals than in healthy control subjects (permutation corrected p = .04). More specifically, ADHD was associated with a decreased callosal thickness in regions corresponding to the anterior third (mainly genu and rostral body), isthmus, and splenium (mainly anterior splenial section). Control subjects did not show significantly reduced callosal thickness relative to ADHD subjects in any area of the CC. Significant p values are depicted in Figure 1, and the respective t values are provided together with the p values in Figure 1 in Supplement 3.
Figure 1.
Attention-deficit/hyperactivity disorder (ADHD) effects on callosal thickness. Illustrated are regions of significantly reduced callosal thickness in ADHD subjects compared with normally developing control subjects (CTL). Results are shown for all ADHD and control subjects before covarying (Panel A), after covarying for IQ (Panel B), and after covarying for TBV (Panel C). Panel D depicts results for a selected sample of ADHD subjects without oppositional defiant disorder comorbidity (non ODD) and age-matched control subjects. The color bar encodes the p value associated with the statistical tests performed at each distance value at the upper and lower callosal boundaries.
When covarying for IQ, the earlier-described ADHD effect was evident in both anterior and posterior callosal sections (Panel B). When covarying for TBV, group differences became somewhat less pronounced in posterior sections (isthmus/anterior splenium), whereas the ADHD effect in the anterior callosal sections was no longer significant (Panel C). When analyzing the subsample of ADHD subjects who were not comorbid for ODD, the earlier-described ADHD effect remained pronounced and significant in posterior callosal regions (isthmus/anterior splenium) but also was no longer significant in anterior sections (Panel D).
Age Effects
Relationships between age and callosal thickness are included in Figure 2 in Supplement 3. When analyzing the combined sample of ADHD and control subjects, positive and negative correlations were present. However, only a small region in the callosal midbody, indicating a negative relationship between age and callosal thickness reached significance (p < .05; uncorrected). There were no differences between ADHD and control subjects with respect to the relationship between age and callosal thickness.
Discussion
In this study, we applied novel computational surface-based methods to calculate and compare callosal thickness at high spatial resolution in an age-matched sample of male ADHD and normally developing control subjects. We revealed significant ADHD effects in both anterior (genu/rostral body) and posterior sections (isthmus/anterior splenium). These findings are in agreement with previous studies that revealed a reduced callosal size in the callosal rostral body (6,8), the genu (9), the isthmus (10), and the splenium or its anterior vicinity (9,10,33), as well as the total CC (11). When analyzing the considerably smaller subsample of ODD-free ADHD subjects and their matched control subjects, anterior callosal sections no longer showed significant group effects. Insufficient statistical power may account for the lack of group differences in these anterior regions, because the reduced selected sample (n = 18) was less than half as large as the original sample (n = 38). However, it is also possible that there are no ADHD effects independently of ODD effects in the callosal anterior third. In line with this argument, it was recently suggested that anterior brain regions are associated with impulsivity in ODD (34). That is, the authors observed a hypofunction of the frontal pole in ODD children when performing an impulsive task. Moreover, ADHD effects on regional tissue volumes, as examined in an independent study (35), also appeared less pronounced and pervasive when individuals with comorbid conduct disorder (CD) and ODD were excluded. More specifically, GM volume differences, originally detected in the globus pallidus (among a number of other regions), were no longer significant when comparing the CD/ODD-free ADHD sample against control subjects. Notwithstanding, significant WM volume deficits in ADHD subjects, including reductions “in the vicinity of corpus callosal radiation fibres” appeared to be unaffected by excluding individuals with CD/ODD comorbidities (35). Unfortunately, more detailed outcomes for callosal regions are not available and future studies in larger ODD-free ADHD samples are clearly necessary to elucidate whether the detected group differences in anterior callosal sections are driven solely by ODD-related variance.
Functional Relevance with Respect to Attentional Processes in ADHD
Regardless of whether we 1) did not covary at all, 2) covaried for IQ or TBV, or 3) excluded subjects comorbid for ODD, callosal aberrations were most pronounced in posterior callosal sections within the isthmus—a callosal region suggested to contain fibers mainly projecting to parietal regions (12,14,36). The parietal cortex is thought to be a component of the neural network underlying voluntary attentional control (37,38), and previous reports have shown structural abnormalities (i.e., reduced volumes) in the parietal lobe in ADHD subjects (2,4,5). Similarly, the prefrontal cortex has been proposed to be involved in attentional regulation (38), and our observation of reduced thickness across the anterior callosal surface coincides well with previously reported structural and functional alterations of the prefrontal cortex in ADHD patients (2–5). Decreased callosal thickness might be associated with fewer fibers or less myelination of fibers (or both) connecting the parietal and prefrontal cortices. This might contribute to attentional deficits by affecting interhemispheric communication channels necessary to sustain attention (39). Alternatively, or in addition, a decreased callosal thickness might reflect abnormalities in parietal and prefrontal tissue micro- or macrostructure (e.g., a reduced number of neurons in homotopic regions) or organization (e.g., abnormal lateralization and functioning) affecting attentional processes.
Functional Relevance with Respect to Motor Processes in ADHD
In addition to elucidating age-inappropriate symptoms of inattention, our findings might also explain symptoms of motor hyperactivity. Premotor and supplementary motor fibers are suggested to travel through the callosal anterior third (12), which, in part, was thinner in ADHD individuals. Decreased callosal thickness across the anterior callosal surface might reflect disturbances of fiber tracts that mediate transcallosal inhibition and conceivably account for a defective inhibition of motor programs in ADHD, as suggested previously (40). Giedd et al. (6) reported significant correlations between teacher and parent ratings of hyperactivity/impulsivity and midsagittal cross-sectional areas of anterior callosal sections in ADHD children, lending further support to the hypothesis that an abnormal callosal morphology is associated with impaired motor control. Notwithstanding, more recent studies (14,36) revealed (pre)motor fibers to be located more posteriorly than previously indicated, and significantly thinner anterior callosal regions, as observed in our study, therefore may not house fibers involved in motor regulation.
Possible Confounds and Implications for Future Research
We excluded individuals with fragile X syndrome, tuberous sclerosis, and generalized resistance to thyroid hormone, as well as those taking nonstimulant psychotropic medication. We have also established ADHD effects independent of ODD. Still, possible influences of other coexisting conditions and additional sources of heterogeneity (e.g., perinatal complications, family history of ADHD, age of ADHD onset) on callosal morphology cannot be ruled out. In particular, the ADHD sample's relative heterogeneity with respect to medication status might have affected outcomes because, for example, methylphenidates have been suggested to modulate callosal function (41,42) and possibly structure. Moreover, our study investigated the effects of ADHD on callosal thickness without grouping affected individuals into subtypes (i.e., hyperactive impulsive type, inattentive type, and combined type) and without systematic assessments of, for example, sustained visual and auditory attention. In addition, we only examined male children and adolescents, and the size of the ADHD sample was relatively small (n = 19), especially the size of the ODD-free ADHD sample (n = 9). Future studies including more subjects might extend the focus to female subjects or adult populations and also resolve whether different ADHD subtypes or their behavioral correlates (e.g., impaired attention) have differential effects on callosal morphology, where confounding influences are modeled.
Finally, callosal aberrations in ADHD individuals were most pronounced in the isthmus. Because callosal growth patterns in the normally developing brain were reported to reach peak values in the isthmus in children aged 6–15 years (43), any developmental delay in ADHD children and adolescents within our study (mean age ± SD: 11.8 ± 2.7 years) may result in this area appearing thinner (e.g., if the normal growth spurt did not occur or was delayed). Our preliminary age analysis did not support group-specific developmental effects on callosal morphology. However, neuroanatomic evidence for a marked delay in brain maturation in ADHD was recently provided in an investigation comparing the age of attaining peak cortical thickness in children with ADHD versus children without the disorder (44). Abnormalities in a number of other volumetric measures (e.g., volumes of the cerebrum, cerebellum, global and lobar GM and WM) have been reported to persist with age without indications of normalization over time (45). Because comparable data with respect to the CC do not exist, longitudinal studies of callosal morphology in ADHD subjects could help to determine whether the detected callosal abnormalities reflect maturational delays or whether they progress and persist into adulthood.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) through the NIH Roadmap for Medical Research, Grant No. U54 RR021813, Center for Computational Biology (CCB). Additional support was provided by the NIH/National Center for Research Resources Grant No. P41 RR013642, Dr. Strickland's Grant Nos. P50 MH073466-01 and P01 MH063357, and Dr. Narr's NIH K-award Grant No. MH073990.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Durston S. A review of the biological bases of ADHD: What have we learned from imaging studies? Ment Retard Dev Disabil Res Rev. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- 3.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamburger SD, et al. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 7.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 8.Baumgardner TL, Singer HS, Denckla MB, Rubin MA, Abrams MT, Colli MJ, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:477–482. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- 9.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: Morphometric analysis of MRI. J Learn Disabil. 1991;24:141–146. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- 10.Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: A brain magnetic resonance imaging study. Biol Psychiatry. 1996;40:1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- 11.Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 12.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 13.O'Kusky J, Strauss E, Kosaka B, Wada J, Li D, Druhan M, et al. The corpus callosum is larger with right-hemisphere cerebral speech dominance. Ann Neurol. 1988;24:379–383. doi: 10.1002/ana.410240305. [DOI] [PubMed] [Google Scholar]
- 14.Hofer S, Frahm J. Topography of the human corpus callosum revisited— comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Tomaiuolo F, Scapin M, Di PM, Le NP, Fadda L, Musicco M, et al. Gross anatomy of the corpus callosum in Alzheimer's disease: Regions of degeneration and their neuropsychological correlates. Dement Geriatr Cogn Disord. 2007;23:96–103. doi: 10.1159/000097371. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos FX, Acosta MT. [The neuroanatomy of attention deficit/hyperactivity disorder]. Rev Neurol. 2004;38(suppl 1):S131–S136. [PubMed] [Google Scholar]
- 17.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/ hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 18.Biederman J. Attention-deficit/hyperactivity disorder: A life-span perspective. J Clin Psychiatry. 1998;59(suppl 7):4–16. [PubMed] [Google Scholar]
- 19.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 20.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 21.Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: Myth or reality? Neurosci Biobehav Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Swanson J. School-Based Assessments and Interventions for ADD Students. K.C. Publishing; Irvine, CA: 1992. [Google Scholar]
- 24.Wechsler D. Wechsler Intelligence Scale for Children. 3rd edition Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 25.Wilkinson GS. Wide Range Achievement Test—3 (WRAT-3) Wide Range; Wilmington, DE: 1993. [Google Scholar]
- 26.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson Tests of Cognitive Abilities (WJ III) Riverside Publishing; Rolling Meadows, IL: 2001. [Google Scholar]
- 27.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 28.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 29.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 30.Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- 31.Luders E, Di Paola M, Tomaiuolo F, Thompson PM, Toga AW, Vicari S, et al. Callosal morphology in Williams syndrome: A new evaluation of shape and thickness. Neuroreport. 2007;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber B, Luders E, Faber J, Richter S, Quesada CM, Urbach H, et al. Distinct regional atrophy in the corpus callosum of patients with temporal lobe epilepsy. Brain. 2007;130:3149–3154. doi: 10.1093/brain/awm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semrud-Clikeman M, Filipek PA, Biederman J, Steingard R, Kennedy D, Renshaw P, et al. Attention-deficit hyperactivity disorder: Magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry. 1994;33:875–881. doi: 10.1097/00004583-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Zhu Y, Wu YZ, Su LY, Ma N, He Z, et al. [Features of functional MRI in children with oppositional defiant disorder]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:571–575. [PubMed] [Google Scholar]
- 35.McAlonan GM, Cheung V, Cheung C, Chua SE, Murphy DG, Suckling J, et al. Mapping brain structure in attention deficit-hyperactivity disorder: A voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han S, Jiang Y, Gu H, Rao H, Mao L, Cui Y, et al. The role of human parietal cortex in attention networks. Brain. 2004;127:650–659. doi: 10.1093/brain/awh071. [DOI] [PubMed] [Google Scholar]
- 38.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 39.Wong CW. Corpus callosum and cerebral laterality in a modular brain model. Med Hypotheses. 2000;55:177–182. doi: 10.1054/mehy.1999.0934. [DOI] [PubMed] [Google Scholar]
- 40.Buchmann J, Wolters A, Haessler F, Bohne S, Nordbeck R, Kunesch E. Disturbed transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD). Clin Neurophysiol. 2003;114:2036–2042. doi: 10.1016/s1388-2457(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 41.Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Wittstock M, et al. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH). Neurosci Lett. 2006;405:14–18. doi: 10.1016/j.neulet.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Hoeppner J, Wandschneider R, Neumeyer M, Gierow W, Haessler F, Herpertz SC, et al. Impaired transcallosally mediated motor inhibition in adults with attention-deficit/hyperactivity disorder is modulated by methylphenidate. J Neural Transm. 2008;115:777–785. doi: 10.1007/s00702-007-0008-1. [DOI] [PubMed] [Google Scholar]
- 43.Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 44.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.