Abstract
Objective
To determine whether microRNAs are differentially expressed in human leiomyoma versus matched myometrial tissue.
Design
Microarray with real-time PCR validation.
Setting
Academic medical center
Patients
Premenopausal subjects (n=13), who were undergoing hysterectomies for leiomyoma-related symptoms.
Interventions
none
Main Outcome Measure
Statistically differential expression of microRNAs in leiomyoma versus myometrium.
Results
Forty-six miRNA species were differentially expressed in leiomyoma versus normal myometrium with p-values <0.01. Of these, 19 were overexpressed whereas 27 were downregulated in leiomyomas. The fold changes ranged from 1.2 to 11.8. These findings were confirmed using real time RT-PCR for selected miRNAs (miRNAs 21, 34a, 125b, 139 and 323).
Conclusions
Our findings indicate that miRNAs are differentially expressed between human leiomyoma and matched myometrium. Given this differential expression, miRNAs may play a role in the pathogenesis of uterine leiomyoma and may serve as future therapeutic targets for the treatment of these tumors.
Keywords: miRNA, microarray, uterine leiomyoma, myometrium
Introduction
Uterine leiomyomas, or fibroids, are benign smooth muscle tumors of the uterus that have been shown to be exquisitely sensitive to estrogen and progesterone. They are present in approximately 30–50% of reproductive-age women and have an overall cumulative incidence in women of 70–75% by the age of fifty (1). Furthermore, leiomyomas disproportionately affect African-American women with epidemiological studies showing a cumulative incidence of about 80% by the age of fifty (1). Signs and symptoms associated with fibroids include irregular uterine bleeding which can lead to clinically significant anemia; pelvic pain and pressure; infertility; and recurrent pregnancy loss. The morbidity associated with the spectrum of symptoms caused by leiomyomas explains why they remain the leading cause of hysterectomy in the United States (2). Conservative analyses estimate the cost of leiomyomas to the U.S. healthcare system to be more than $2.15 billion dollars annually (3). Despite their prevalence, their etiology and pathogenesis remain unclear and we have little understanding of why these tumors develop in some women versus others, and what determines their location within the uterus or their size.
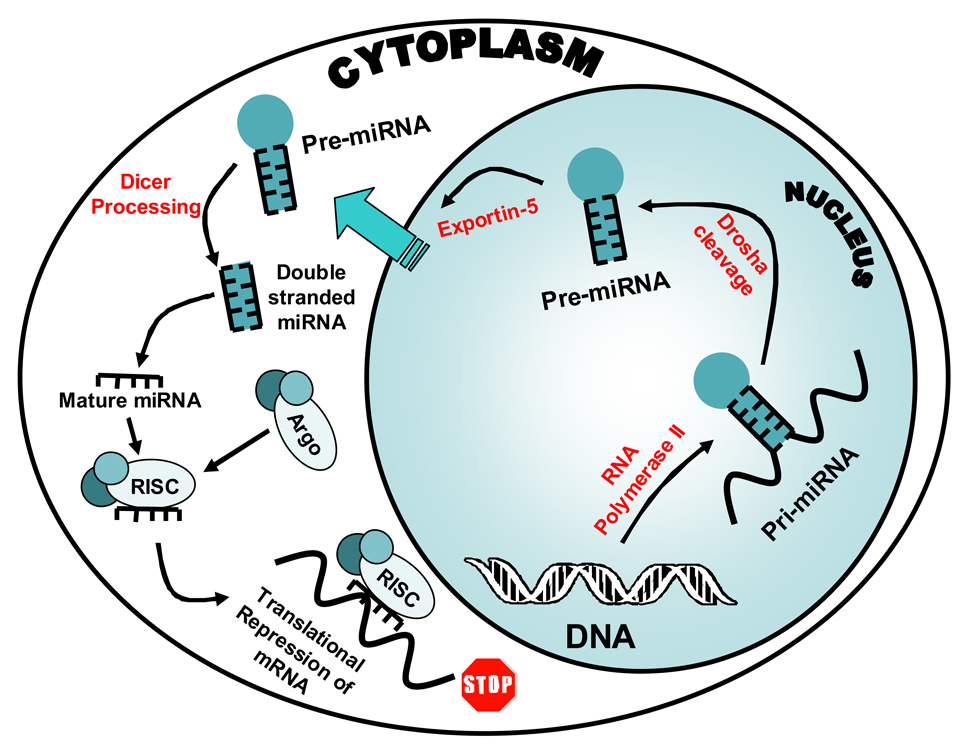
MicroRNAs (miRNAs) are a recently described, novel class of small (22–25nt) RNAs that have been shown to downregulate gene expression by blocking mRNA translation and/or degrading the messenger RNA (mRNA) transcript depending on the level of complementarity between the miRNA and its target (4). MiRNAs are versatile and ubiquitous and have been shown to have distinct expression profiles across various tissue types (5). MicroRNAs originate as genes that are transcribed by RNA polymerase II into a single strand of RNA (see Figure 1). This single strand folds into a hair-pin like structure secondary to complementarity within the strand itself. This folded single strand of RNA then becomes the double stranded primary miRNA or “Pri-miRNA”. The hairpin like structure is subsequently cleaved in the nucleus by an RNase III-like protein called “Drosha”, forming the pre-miRNA (6). The pre-miRNA hairpin structure then enters the cytoplasm via the protein Exportin-5, where it is cleaved into a small piece of double stranded RNA by the enzyme “Dicer”(7). The double stranded small RNA then separates into single strands with one strand being degraded and the other strand forming a complex with Argonaute proteins (8). This newly formed protein/nucleic acid complex is called a RNA induced silencing complex or RISC. These RISC complexes then either bind mRNA with exact complementarity leading to cleavage of the mRNA by the RISC or they bind with partial complementarity leading to repression of translation.
Figure 1.
MicroRNA Cellular Pathway. The gene for the miRNA is transcribed by RNA polymerase II into a single strand of RNA. This single strand folds into a hair-pin like structure and becomes the double stranded primary miRNA or “Pri-miRNA”. The hairpin like structure is subsequently cleaved in the nucleus by Drosha forming the pre-miRNA The pre-miRNA then enters the cytoplasm via the protein channel Exportin, where it is cleaved into a small piece of double stranded RNA by the enzyme Dicer. The double stranded small RNA then separates into single strands with one strand being degraded and the other strand forming a complex with Argonaute proteins. This newly formed protein/nucleic acid complex is called a RNA induced silencing complex or RISC. These complexes then either bind mRNA with exact complementarity leading to cleavage of the mRNA by the RISC or they bind with partial complementarity leading to repression of translation.
While the specific functions of most of the miRNAs remain unknown, as a class they induce gene silencing. This suppression of gene expression has been shown to play roles in major cellular functions including differentiation, gene regulation, apoptosis, and malignant transformation (9). Much of our understanding of miRNAs in humans comes from studies determining their expression levels in tissues in diseased versus normal states. These studies have shown that miRNAs were indeed differentially expressed in malignancies of the colon, breast, prostate, thyroid, and lung (10). To date, the differential expression of miRNAs have not been studied in benign tumors such as uterine leiomyomas.
Leiomyomas are extremely common and clearly estrogen/progesterone-dependent benign tumors with a polygenic inheritance pattern (11, 12). Gene microarray studies have shown that there are hundreds of genes that are differentially expressed in leiomyoma versus normal myometrium (13, 14). Given the complex endocrine, paracrine and autocrine regulation of these genes by estrogen, progesterone and cytokines, we envision that posttranscriptional mechanisms such as miRNA-targeted degradation of messenger RNAs may also contribute to steady-state levels of specific messenger RNAs arising from these genes. In this study, we seek to identify miRNAs with differential expression in human leiomyoma versus myometrium.
Materials and Methods
Study Subjects
Uterine leiomyoma and matched myometrial tissue was collected from subjects (n=15) who were undergoing hysterectomy for symptomatic leiomyomata. The subjects were all premenopausal women, 39–53 years old (45.8 ± 1.12 years; mean ± SEM), who were on no hormonal medications and who were nonsmokers. Five of the subjects were African-American, one was Asian-American and nine were Caucasian (see Table I). All of the subjects gave written informed consent for the study. The study protocol was approved by the IRB of Northwestern University and all surgeries were performed at Northwestern Memorial Hospital.
Table 1.
Descriptive Characteristics of Subjects
| Sub# | Age | Race | Cycle Phase |
Microarray | PCR Validation |
|---|---|---|---|---|---|
| 1 | 47 | As | Follicular | ✓ | |
| 2 | 48 | AA | Follicular | ✓ | |
| 3 | 50 | C | Follicular | ✓ | |
| 4 | 39 | C | Luteal | ✓ | |
| 5 | 45 | AA | Follicular | ✓ | |
| 6 | 39 | C | Luteal | ✓ | ✓ |
| 7 | 40 | C | Follicular | ✓ | ✓ |
| 8 | 44 | AA | Follicular | ✓ | ✓ |
| 9 | 42 | C | Luteal | ✓ | ✓ |
| 10 | 53 | C | Follicular | ✓ | ✓ |
| 11 | 51 | C | Follicular | ✓ | |
| 12 | 45 | AA | Follicular | ✓ | |
| 13 | 47 | AA | Luteal | ✓ | |
| 14 | 48 | C | Follicular | ✓ | |
| 15 | 49 | C | Luteal | ✓ |
AA=African American; As=Asian; C=Caucasian
Tissue Specimens
The tissue samples were collected from pathologists within an hour of being removed from the subjects. The size of the tumors ranged from 3.5 to 12 cm in diameter. The samples were routinely obtained at 1 cm from the outercapsule of the leiomyoma. 67% of the tumors were removed during the follicular phase of the menstrual cycle, whereas 33% of the subjects were in the luteal phase. Portions of subserosal and intramural leiomyomas were collected from the specimens and adjacent (within 2 cm) myometrium was collected. The tissues were rinsed in cold PBS three times and then cut into 4mm3 pieces and placed in vials containing RNALater (Ambion; Austin, TX) for nucleic acid preservation. The vials of tissue were kept at 4° for 24 hours to allow penetration of the RNALater. The vials were then stored at −80°C until RNA isolation.
Nucleic Acid Isolation
The vials containing the tissue samples were removed from the freezer and allowed to thaw. Each tissue specimen was then homogenized with a Polytron PT 2100 homogenizer (Brinkmann Instruments, Westbury, NY) in TRI-Reagent (Ambion, Austin, TX). The tissue homogenate was left at room temperature for 5minutes and then mixed with one fifth volume of chloroform. This mixture was then kept on ice for 15 minutes and then centrifuged at 14,000 rpm for 20 minutes. The resultant clear aqueous layer was transferred and mixed with an equal volume of isopropanol. The mixture was left on ice for 10 minutes and then centrifuged at 14,000 rpm for 20 minutes. The liquid was removed, and the formed pellet was resuspended in 1 mL 75% ethanol and centrifuged at 8,000 rpm for 10 minutes. The liquid was again removed and the pellet was allowed to air dry at room temperature for 5 minutes and then resuspended in diethylpyrocarbonate (DEPC)-treated sterile water. RNA purity and concentration were confirmed by spectrophotometry using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). RNA integrity was confirmed by formaldehyde gel electrophoresis.
MiRNA Microarray Analysis
MiRNA microarray analysis was performed by LC Sciences (Houston, TX) on 10 of the matched tissue pairs. Briefly, 2 to 5 µg of total RNA was size fractionated using a YM-100 Microcon centrifugal filter (Millipore, Billerica, MA) and the small RNAs (< 300 nt) isolated were 3’-extended with a poly(A) tail using poly(A) polymerase. An oligonucleotide tag was then ligated to the poly(A) tail for later fluorescent dye staining. Two different tags were used for the two RNA samples on each dual-sample chip (leiomyoma and matched myometrium). Hybridization was performed overnight on a µParaflo microfluidic chip using a micro-circulation pump (Atactic Technologies, Houston, TX). On the microfluidic chip, each detection probe consisted of a chemically modified nucleotide coding segment complementary to target microRNA (from miRBase, http://microrna.sanger.ac.uk/sequences/v8.2) or other RNA control sequences and a spacer segment of polyethylene glycol to extend the coding segment away from the substrate. The detection probes were made by in situ synthesis using photogenerated reagent chemistry. Hybridization was performed using 100µL 6xSSPE buffer (0.90 M NaCl, 60 mM Na2HPO4, 6 mM EDTA, pH 6.8) containing 25% formamide at 34 ° C. After hybridization, detection was done using fluorescence labeling using tag-specific Cy3 and Cy5 dyes. Hybridization images were collected using a GenePix 4000B laser scanner (Molecular Devices, Sunnyvale,CA) and digitized using Array-Pro image analysis software (Media Cybernetics, Silver, Spring, MD).
Statistical Analysis
Data were analyzed by first subtracting the background and then normalizing the signals using a LOWESS filter (Locally-weighted Regression) for two color experiments. The ratio of the two sets of detected signals (log2 transformed, balanced) and p-values of the t-test were calculated. Differentially detected signals were those with less than 0.05 p-values. To further validate the findings, the false discovery rate was determined for each of the miRNA species.
MicroRNA Expression Validation
The microarray findings were validated using real time RT-PCR. cDNA was made from 10 ng of total RNA from each sample with the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and miRNA specific primers (Applied Biosystems, Foster City CA) for four upregulated (miR-34a, miR-125b, miR-21 and miR-323) and one downregulated miRNA species ( miR-139). These primers and U43 as a loading control were then amplified using the TaqMan MiRNA Assay system and the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City CA). Values for each gene were normalized to the expression levels of RNU43. The real time RT-PCR was performed on matched tisuue pairs from 10 subjects: five from the microarray studies and five subjects whose tissue was not used in the microarray studies.
Results
Differential expression of miRNA in leiomyoma versus myometrium
The miRNA microarray examined expression of 454 miRNA species based on version 8.2 of the Sanger miRBase,(http://microrna.sanger.ac.uk/sequences). Eighty-one miRNAs were differentially expressed with p-value < 0.05. Forty-six miRNAs were differentially expressed with p-value < 0.01. Of those, 19 were overexpressed, and 27 were underexpressed in leiomyoma. The fold changes with p-values <0.01 ranged from 1.2 to 11.8. The miRNAs with highest fold change are listed in Table 2. False discovery rate analysis on the forty-six differentially expressed with p-values of < 0.01 revealed a mean q-value of 1.42%.
Table 2.
Twenty Most Differentially Expressed MicroRNA Species in Leiomyoma versus Myometrium Microarray
| MiRNA | Fold Difference* | P value |
|---|---|---|
| hsa-miR-542-3p | 11.78 | 0.01080 |
| hsa-miR-377 | 10.39 | 0.00225 |
| hsa-miR-582 | 6.72 | 0.00034 |
| hsa-miR-376b | 6.29 | 0.00361 |
| hsa-miR-493-5p | 5.75 | 0.00566 |
| hsa-miR-323 | 4.01 | 0.00815 |
| hsa-miR-34a | 3.80 | 0.00018 |
| hsa-miR-196b | 2.86 | 0.00509 |
| hsa-miR-335 | 2.48 | 0.00498 |
| hsa-miR-542-5p | −7.70 | 0.00422 |
| hsa-miR-642 | −5.58 | 0.00935 |
| hsa-miR-150 | −4.41 | 0.00002 |
| hsa-miR-203 | −4.31 | 0.00061 |
| hsa-miR-139 | −4.29 | 0.00014 |
| hsa-miR-29b | −3.40 | 0.00596 |
| hsa-miR-486 | −3.31 | 0.00068 |
| hsa-miR-451 | −2.68 | 0.00456 |
| hsa-miR-10a | −2.62 | 0.00163 |
| hsa-miR-29c | −2.57 | 0.00056 |
| hsa-miR-498 | −2.38 | 0.01260 |
Leiomyoma versus myometrium
MicroRNA Expression Validation
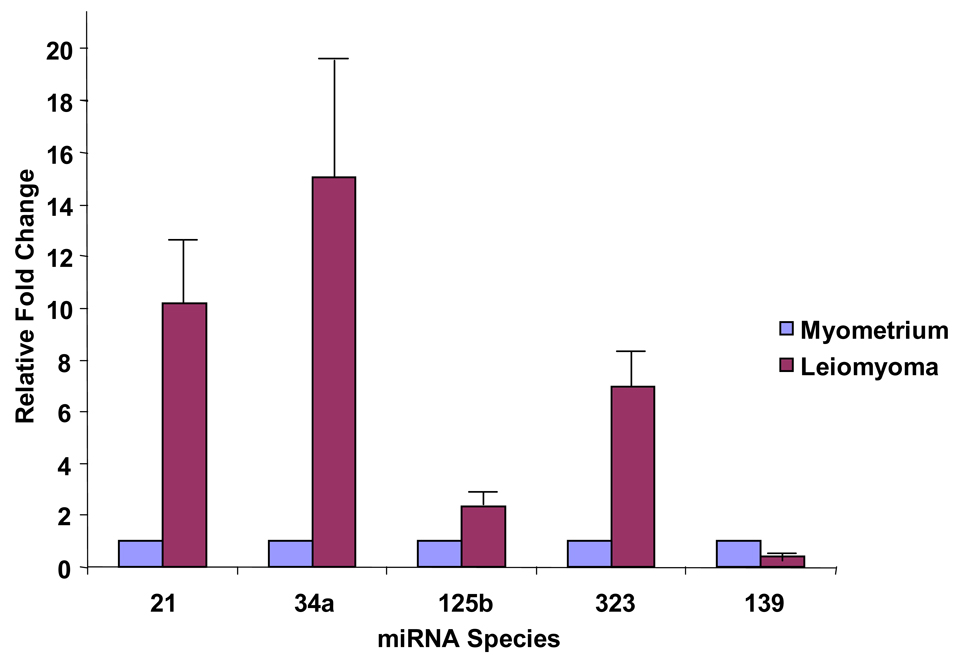
Using real-time RT-PCR employing commercially available primers (Applied Biosystems, Foster City, California), we validated the differential expression of miR-21 (10.2 ±3.3 fold increase), miR 125b (2.39±0.51 fold increase) miR-34a, (15.07±5.56 fold increase) miR-323 (6.95±1.62 fold increase), and miR-139 (2.44±0.06 fold decrease) in a set of experiments in ten matched leiomyoma and myometrium pairs (five pairs that were used in the microarray analysis and five pairs, on which the microarray had not been performed). These data are represented in Figure 2 and validate the microarray results shown in Table 2. The possible roles of some of these miRNAs are discussed below.
Figure 2.
Real time RT PCR Validation of Microarray. Real time semiquantitative RT PCR of 5 different miRNA species (miR-21, miR-34a, miR-125b, miR-139 and miR-323) across ten subjects. miR-21, miR-34a, miR-125b and miR-323 were over-expressed in leiomyoma in the ten pairs tested and miR-139 was under-expressed, consistent with the microarray results.
Discussion
In this study, we have used miRNA microarray technology to demonstrate that miRNAs are differentially expressed in leiomyoma versus adjacent myometrium. Real time RT-PCR analysis verified the results of the microarray and showed that the microarray actually underestimated the true fold change.
It is not surprising that miRNAs are differentially expressed in leiomyomas versus myometrium. Several reviews have described differential expression of miRNAs in neoplastic versus normal tissue (9, 15). Furthermore, there is a growing body of literature that is elucidating the function of these cancer associated- miRNAs. For example, miRNAs miR125b and miR-34, which were increased in leiomyoma, have also been shown to be increased in thyroid papillary cancer versus normal thyroid tissue (16). MicroRNA-21, which was found to be increased 1.97 fold in leiomyoma (P<0.0004), has been shown to be differentially expressed in glioblastoma cells and act as an antiapoptotic factor (17, 18). This oncogenic role of miR-21 has been demonstrated to at least partially be mediated through regulation of bcl-2 (19). Bcl-2 was previously shown to be a progesterone-regulated gene that plays a key role in controlling apoptosis in leiomyoma cells (20). MicroRNA-139, which has been shown to be decreased in pancreatic adenocarcinoma, is also decreased in leiomyoma 4.29-fold (P<0.0001) (Table 2) (21). In addition, certain miRNAs show increased expression with estrogen and progesterone receptor positivity in breast and prostate cancers (22). Leiomyomas are known to grow in response to estrogen and progesterone. We demonstrated that many of these sex-steroid receptor associated miRNA were increased in leiomyoma, including miRNA-21, miR-34a, miR-125b, miR-150 (22).
Uterine leiomyomata represent a significant public health problem and are the leading cause of hysterectomy in the United States. They represent a common, chronic and steroid (estrogen/progesterone) and growth factor (TGFβ)-dependent disease with a complex multifactorial etiology that involves abnormal expression of hundreds to thousands of genes (11–13). Since miRNA –mediated changes in transcript levels arising from some 25,000 genes in the human are predicted to account for almost 33% of all human gene regulation it is highly likely that miRNAs play an important role in the etiology of leiomyomata. The clinical implication of this lies in the use of miRNA not only as biomarkers for various neoplasms but also as therapeutic in vivo targets. In fact, targeting of specific miRNAs has been shown to yield lasting physiological changes as has been shown in rodent studies (23).
To this end, we have studied the expression of this novel level of gene regulation. Specifically, using microarray technology, we have determined that specific miRNA species are differentially expressed in leiomyomata versus myometrium. Some of these miRNAs have been shown to be dysregulated in other neoplastic tissues as discussed above. We have also identified miRNA species, the biological roles of which have not been previously implicated in disease states (see Table 2). We are conducting follow up studies that will elucidate the function of the differentially expressed miRNA in leiomyoma gene dysregulation. We anticipate that by developing a better understanding of the molecular underpinnings of leiomyoma pathogenesis through identification of differentially expressed miRNAs and defining their functions, therapeutic targets for fibroids may be identified that will result in noninvasive less morbid treatment alternatives for women who suffer from this complex and prevalent condition.
Acknowledgments
This work was supported by NIH Grant HD46260 and the Friends of Prentice of Northwestern Memorial Hospital; E.E.M received support from Eleanor Wood-Prince Grant of the Women’s Board of Northwestern Memorial Hospital and the ACOG/Ortho Women's Health Fellowship in Academic Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capsule:
Microarray and PCR studies of matched human leiomyoma and myometrium reveal that microRNAs are differentially expressed between the two tissues and may thus play a role in leiomyoma development.
References
- 1.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson GF, Shaber RE, Armstrong MA, Hung YY. Hysterectomy rates for benign indications. Obstet Gynecol. 2006;107:1278–1283. doi: 10.1097/01.AOG.0000210640.86628.ff. [DOI] [PubMed] [Google Scholar]
- 3.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195:955–964. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development (Cambridge, England) 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 5.Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I, et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004;14:2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. Embo J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 11.Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14–18. doi: 10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 12.Stewart EA, Morton CC. The genetics of uterine leiomyomata: what clinicians need to know. Obstet Gynecol. 2006;107:917–921. doi: 10.1097/01.AOG.0000206161.84965.0b. [DOI] [PubMed] [Google Scholar]
- 13.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes, chromosomes & cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 16.Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri MT, Troncone G, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer. 2006;13:497–508. doi: 10.1677/erc.1.01209. [DOI] [PubMed] [Google Scholar]
- 17.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 18.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 19.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2006 doi: 10.1038/sj.onc.1210083. EPub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82:293–299. doi: 10.1210/jcem.82.1.3650. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, DM, Hughes JL, Lee JH, Kavanagh JJ. Metastasis to sigmoid colon mucosa and submucosa from serous borderline ovarian tumor: response to hormone therapy. Int J Gynecol Cancer. 2006;16:295–299. doi: 10.1111/j.1525-1438.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 22.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]