Abstract
Oral carcinogenesis proceeds through a stepwise accumulation of genetic damage over time. Because the oral cavity is easy to examine and risk factors for oral cancer are known, there is great opportunity to improve patient outcomes through diagnosis and treatment of premalignant lesions before the development of invasive oral carcinoma. This review provides a summary of developments in detection and diagnosis of oral premalignant lesions and innovative approaches to management of early oral neoplasia. These technological and therapeutic advances are much needed to improve the poor outcomes associated with oral cancer due to our inability to diagnose and treat this disease at an early, curable stage.
Introduction
Oral squamous cell carcinoma (SCC) is one of the ten most common cancers worldwide. Despite therapeutic advances, survival rates for patients with oral SCC remain at approximately 50% and have not improved over several decades. Persistent failure to diagnose and treat oral cancer at an early stage is a key factor limiting advances in outcome. Improving detection, diagnosis, and treatment of precancerous changes and early asymptomatic cancers is imperative to increase survival and improve functional outcomes for persons at risk to develop oral cancer.
Oral Carcinogenesis Progression Model
Like other solid tumors, oral cancers arise from sustained, stepwise accumulations of mutations resulting in transition of normal mucosa to dysplasia to invasive carcinoma over time [1,2]. Research efforts have identified specific mutations and molecular abnormalities that occur during these transitional steps [2,3••,4]. These mutations often involve inactivation of tumor suppressor genes such as FHIT, p53, and p16 and/or activation or overexpression of regulatory molecules such as cyclin D1, epidermal growth factor receptor (EGFR), and telomerase [5,6]. This knowledge is important for developing diagnostic and therapeutic interventions to identify, prevent, or reverse these specific molecular abnormalities.
According to the theory of field cancerization, the entire lining of the upper aerodigestive tract exposed to carcinogens, such as those present in alcohol and tobacco, sustains damage and is at risk to develop squamous carcinomas [7•,8•]. The high rate (approximately 4% per year) of multiple primaries that occur in patients previously treated for oral SCC supports this theory. In these patients with chronic exposure to carcinogens, areas of clinically normal-appearing mucosa often have histologic and genetic changes [9,10]. Developing ways to identify the presence of this damage is important for early diagnosis and treatment of oral cancer.
Clinically Apparent Oral Premalignant Lesions
Genetic mutations often produce early phenotypic changes that may present as clinically apparent, recognizable lesions. An oral premalignant lesion (OPL) is an area of morphologically or genetically altered tissue that is more likely than normal tissue to develop cancer. Oral lesions that have been identified clinically as having potential for malignant conversion include leukoplakia (a predominantly white lesion), erythroplakia (a predominantly reddish lesion), lichen planus, and submucous fibrosis. Reported rates of malignant transformation of leukoplakia range from less than 1% to 18% [11,12•]. There is no accepted method to predict risk of malignant progression of an individual OPL, but various factors such as location within the oral cavity, clinical appearance (homogeneous vs heterogeneous), and presence of dysplasia have been correlated with risk of progression. The histologic finding of dysplasia is strongly associated with an increased rate of invasive cancer development [12•]. Erythroplakia, a velvety reddish mucosal lesion, is associated with a higher rate of cancer development, occurs much less frequently, and is more difficult to detect clinically than oral leukoplakia. Virtually all erythroplakic lesions contain severe dysplasia, carcinoma in situ, or early invasive carcinoma at the time of presentation [13]. Formalized classification and staging systems for OPLs have been proposed [14,15••], and their use is important to facilitate uniform reporting and data comparison.
Some molecular biomarkers with potential diagnostic relevance include DNA content and chromosome polysomy, loss of heterozygosity (LOH), nucleolar organizer regions, histo-blood group antigens, proliferation markers (such as Ki-67), increased EGFR, and decreased expression of RAR-β, p16, and p53 [16–18]. Although a reliable, validated marker panel for providing clinically useful prognostic information in OPL patients has not yet been established, the advent of high throughput genomic and proteomic analysis techniques may soon yield major advances toward a prognostically relevant molecular classification system.
Screening
Detection and diagnosis of oral neoplasia has traditionally relied heavily on the clinical experience of the examiner and his or her ability to recognize often subtle morphologic changes. However, some early malignant lesions are clinically indistinguishable from benign lesions, and some patients develop carcinomas in the absence of clinically identifiable OPLs. Furthermore, it can be difficult even for experts to determine which OPLs are at significant risk to progress to invasive carcinoma. Therefore, an accurate, objective, and noninvasive method to help identify premalignant lesions and to distinguish those at risk of malignant conversion is needed.
Diagnostic aids
Several technologies that may assist in the detection and diagnosis of oral precancer and early carcinoma are currently under investigation and are summarized in Table 1. In general, these diagnostic aids can be separated (with some overlap) into two categories: those that assist in the identification and localization of abnormal areas within the oral cavity and those that help determine the risk for malignancy in previously identified lesions. Analysis of saliva is also being evaluated to screen for the presence of genetic damage.
Table I.
Potential diagnostic aids
| Technology | Detection/ localization |
Diagnosis |
|---|---|---|
| Fluorescence imaging | X | — |
| Optical spectroscopy | — | X |
| Vital staining | X | X |
| Photodynamic detection | X | X |
| Chemiluminescent kit | X | — |
| Brush biopsy/cytology | — | X |
| Confocal microscopy optical coherence | — | X |
| Tomography | — | X |
One promising approach to localization of abnormal mucosa, autofluorescence imaging, uses differences in native autofluorescence properties between normal and neoplastic tissue to visually detect abnormal oral mucosal areas. Living tissues contain fluorophores such as NADH (nicotinamide adenine dinucleotide), FAD (flavin adenine dinucleotide), and collagen and elastin crosslinks that produce fluorescence after excitation with specific light wavelengths. In autofluorescence imaging, several square centimeters of tissue are illuminated using specific excitation wavelengths, and images of the emitted fluorescence are visualized and recorded, producing a spatial depiction of areas with altered fluorescence. This technique has been studied extensively for localization and detection of dysplasia and early carcinoma in the bronchial tree using the Light Induced Fluorescence Endoscopy (LIFE) System and other devices [19,20] and is being studied in other anatomic sites as well [21,22]. Kulapaditharom and Boonkitticharoen [23] compared the LIFE system to traditional white light imaging in 25 patients using the red-to-green fluorescence intensity ratio at 442 nm excitation for identification of abnormal oral areas. Abnormal oral lesions had an increased red-to-green fluorescence intensity ratio. A detection rate of 100% with a specificity of 87.5% was reported using the fluorescence imaging system, compared with a detection rate of 87.5% and specificity of 50% with standard white light. Similarly, Onizawa et al. [24] showed that fluorescence photography at 360 nm excitation, with emission above 480 nm, could be used to separate benign and malignant oral tissue with 91.1% sensitivity and 84.3% specificity. Using ex vivo surgical specimens, Svistun et al. [25] found that the best optical wavelength combinations for visually detecting the extent of oral neoplastic lesions was illumination at 400 nm with visualization at 530 nm.
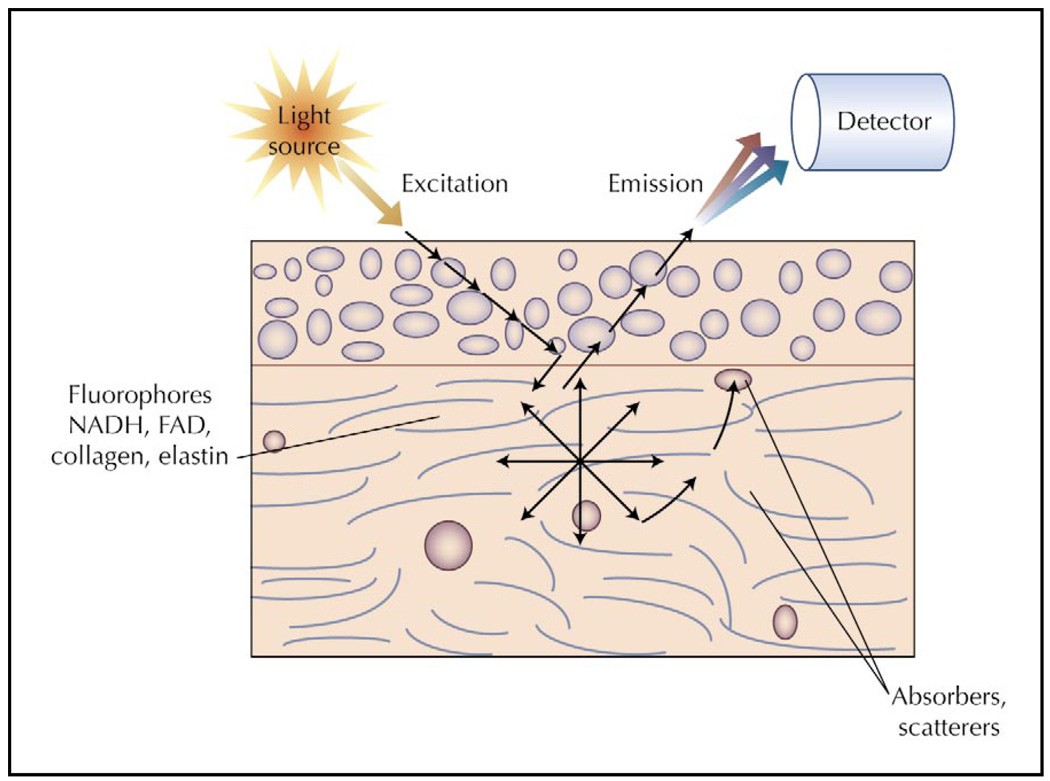
Optical spectroscopy devices using a fiberoptic probe to illuminate and collect light from a small area of tissue may provide important new diagnostic tools for noninvasive characterization of early oral lesions. Fluorescence spectroscopy can evaluate physical and biochemical properties of a specific oral site by analyzing the emitted fluorescence light (Fig. 1), providing automated, noninvasive discrimination between benign and neoplastic epithelial lesions in many anatomic sites [26,27•]. Several small clinical series [28–30] demonstrated that the fluorescence intensity from normal mucosa is generally greater than that from abnormal mucosa. Algorithms based on differences between fluorescence spectra could discriminate normal mucosa from dysplastic and carcinomatous tissue with high sensitivity and specificity [29–31]. In one study, using only a single emission wavelength of 472 nm, and 350 and 400 nm excitation, algorithm performance in the training set had 90% sensitivity and 88% specificity and in the validation set had 100% sensitivity and 98% specificity [31]. These results suggest that fluorescence spectroscopy can provide a simple, objective tool to improve in vivo identification of oral neoplasia.
Figure 1.
Diagram of the optical interactions involved in fluorescence spectroscopy. Light at a specific excitation wavelength illuminates the tissue and interacts with structures and molecules. Some light is absorbed by molecules such as hemoglobin, or scattered by nuclei or other structures, but some light reaches native fluorophores within the tissue. Fluorophores, when excited by photons in light, emit energy in the form of fluorescence, which can be detected when it reaches the tissue surface. FAD—flavin adenine dinucleotide; NADH—nicotinamide adenihsucleotide.
Diffuse reflectance spectroscopy, which measures light that is scattered within the tissue before emerging back out from the tissue surface, can provide morphologic and biochemical information about epithelial stroma. Light scattering spectroscopy is a noninvasive optical technique that can provide data on nuclear size and chromatin content in epithelial tissue [32,33]. Combinations of these techniques have shown promise for noninvasive diagnosis of dysplasia and early carcinoma in the oral cavity [34] and other anatomic sites [35,36].
Vital staining with agents such as tolonium chloride (toluidine blue) has been used to improve recognition of early neoplastic lesions for many years. Neoplastic cells preferentially take up tolonium chloride, resulting in retained blue coloring of abnormal mucosa. Clinical trials to assess whether vital staining helps to identify oral neoplasia have had varying results. A meta-analysis of clinical trials using tolonium chloride for oral neoplasia identification found sensitivities ranging from 93.5% to 97.8% and specificities ranging from 73% to 92.9% [37]. A multicenter study in 668 high-risk patients reported sensitivity for detecting carcinoma of 40% versus 96.7% for standard clinical examination, compared with tolonium chloride rinse methods and positive predictive values of 36.4% and 32.6%, respectively [38]. No data were provided regarding the sensitivity for diagnosis of oral dysplasia. Although vital staining may improve the ability of expert examiners to detect early oral carcinoma, it is not recommended for routine screening due to low specificity in unskilled hands.
Vital staining may be most useful for predicting risk of malignant progression of specific OPLs and for helping to identify the optimal site to perform an invasive biopsy in non-uniform lesions. In a study of 100 patients with OPLs followed longitudinally for about 44 months, staining of specific lesions with toluidine blue correlated with clinicopathologic and molecular risk factors and malignant progression to invasive carcinoma [39]. Staining correlated with degree of dysplasia, with only five of 19 (26%) non-dysplastic lesions compared with 16 of 17 (96%) with severe dysplasia staining blue. Significantly, only three of 64 (5%) non-staining lesions progressed to invasive SCC, compared with 12 of 36 (33%) toluidine-positive lesions. Toluidine blue staining was also associated with higher frequency of LOH. Although further investigations are needed, these findings suggest that toluidine blue staining may be useful as a prognostic marker of malignant potential of OPLs in high-risk patients.
Similar in concept to the use of toluidine chloride, hematoporphyrin derivatives are also preferentially retained in abnormal tissue and have been evaluated for localization of oral premalignant and early carcinoma lesions. Leunig et al. [40] evaluated 58 patients with suspected oral neoplasia using topical 5-aminolevulenic acid (5-ALA) and fluorescence imaging and found that the abnormal areas had increased red fluorescence. The authors reported increased sensitivity and improved ability to detect peripheral extent of dysplasia compared with standard white light examination, but specificity was only 60%. In an effort to improve specificity, Zheng et al. [41] used digital image processing to quantify the amount of fluorescence from normal and abnormal areas. Using a blue excitation light (380–450 nm), normal mucosa appeared blue, whereas lesions that retained the 5-ALA emitted red fluorescence. In a study of 49 patients, a diagnostic algorithm using red-to-blue intensity ratios yielded a sensitivity and specificity of 98% and 92% for distinguishing normal from cancerous tissue, and 92% and 96% for separating benign mucosa from dysplasia. The authors reported no photosensitivity or other complications with the procedure [41].
A kit containing a disposable chemiluminescent light and 1% acetic acid mouth rinse is also being marketed to assist in detection and diagnosis of oral neoplasia. With this technique, the patient rinses his or her mouth with the acetic acid pre-rinse, after which the chemiluminescent light is activated and the oral mucosa is examined in a dimly lit room. Under the blue light, normal tissue should appear dark, whereas abnormal areas will cause more reflection of the light and appear white. In one small pilot study the investigators reported that hyperkeratotic lesions, lesions with chronic inflammatory infiltrate, and areas with altered nuclear-cytoplasmic ratio strongly reflected the blue-white light and became clinically discernible [42]. This technique needs to be investigated further in clinical trials.
The application of molecularly targeted optical contrast agents is another interesting approach being investigated for diagnosis and characterization of OPLs [43]. Optically active contrast agents range from far-red fluorescent dyes and porphyrin derivatives to nanoparticles and quantum dots. The EGFR, which is highly overexpressed in oral neoplasia, is an ideal molecular target to image. Soukos et al. [44] conjugated indocyanin Cy5.5 fluorescent dye to a monoclonal antibody against EGFR and evaluated for fluorescence in a hamster cheek pouch model. Hsu et al. [45] used a far-red fluorescent dye, Alexa Fluor 660 (sreptavidin; Molecular Probes, Eugene, OR), coupled to an anti-EGFR monoclonal antibody and demonstrated specific EGFR labeling and the feasibility of topical application of the contrast agent using oral SCC cells and tissue constructs. Another approach is to use molecularly targeted nanoparticles to provide strong optical signals. Sokolov et al. [46] used gold nanoparticles conjugated to anti-EGFR antibodies to produce specific, highly reflectant labeling of EGFR in cervical and oral carcinoma cells [47]. Although further investigations are necessary before these optical contrast agents can be used in the clinic, the concept of molecularly targeted contrast agents has exciting possibilities for detection, localization, diagnosis, molecular characterization, and treatment monitoring of oral neoplasia [43].
Computer-assisted analysis of oral brush biopsy specimens, currently marketed under the trade name OralCDx (OraScan Laboratories, Inc., Suffern, NY), is a promising technology that may help clinicians objectively and noninvasively determine whether an identifiable oral lesion is benign or neoplastic. Exfoliated cells from a suspicious oral site obtained with a cytobrush undergo computer-assisted analysis in a central laboratory, followed by standard cytologic evaluation if any abnormalities are detected on the initial screen. Results of a multicenter trial performed by academic dental specialists demonstrated impressive sensitivity rates. However, because only clinically suspicious lesions were biopsied, the false-negative rate using this technique could not be determined [48••]. Poate et al. [49••] evaluated the OralCDx brush biopsy system in 112 subjects and found 71.4% sensitivity and 32% specificity for detecting dysplasia or carcinoma. The positive predictive value of an abnormal brush biopsy was 44.1%, and the negative predictive value was 60%. This study and a report of four cases of false-negative brush biopsy results [50] reinforce the need for further studies of this technology.
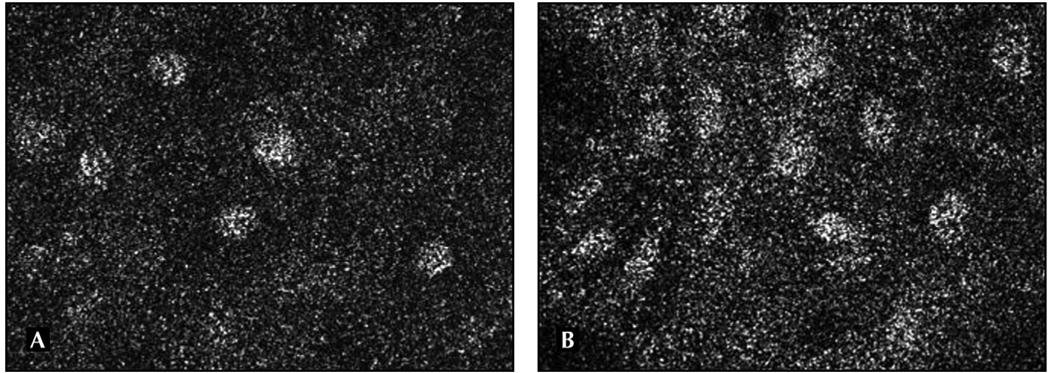
Confocal microscopy and optical coherence microscopy and tomography are techniques to directly visualize opaque tissue in vivo that may have applications in oral cancer diagnosis. For routine pathologic assessment, tissue is sliced very thinly (4 µm) and stained in order to visualize individual cells with a standard light microscope. With the confocal microscope, light reflected from a specific plane within the living tissue is focused through a pinhole and light from other planes is rejected, allowing optical sectioning of intact tissue. Contrast is produced by differences in the way specific structures, such as nuclei, reflect and scatter light. Studies in ex vivo oral tissues have demonstrated features, such as nuclear to cytoplasmic ratio, which may be useful to distinguish normal from neoplastic tissue (Fig. 2) [51]. Confocal microscopy can also be used to detect in vivo molecularly targeted optical contrast agents such as nanoparticles and quantum dots.
Figure 2.
Images of normal (A) and neoplastic (B) oral mucosa taken with a confocal microscope are shown. Nuclei are highly reflectant and appear white. Notice the irregularity of nuclear size and shape and increased nuclear-to-cytoplasmic ratio in the neoplastic mucosa (B).
Optical coherence tomography (OCT), which has become a standard diagnostic tool for retinal pathology, works similarly to ultrasound but uses infrared light waves rather than sound waves to generate high-resolution images. OCT is being evaluated for use in imaging oral tissue [52,53]. Using a hamster cheek-pouch mode of oral carcinogenesis, Wilder-Smith et al. [53] demonstrated differences between normal and dysplastic epithelium using OCT. These technologies, used alone or in combination with optical contrast agents, show great promise for diagnosing dysplasia and early oral carcinomas and are undergoing further evaluation.
Oral epithelial cells routinely shed and can be detected in saliva and oral rinses, making cytologic and molecular analysis of this fluid attractive for oral cancer screening. Several small studies have shown differences in DNA and RNA expression patterns and microsatellite motifs between groups of oral cancer patients and normal control subjects [54–56]. Identification of more sensitive and specific molecular biomarkers should greatly augment the efficacy of this approach for screening large numbers of at-risk individuals. This method, once tested and verified, would be complementary to other diagnostic technologies; saliva analysis could screen for the presence of genetically damaged oral epithelial cells, localization technologies could help to identify areas of mucosal abnormality, and more focused diagnostic aids could provide noninvasive, immediate information about the identified suspicious sites.
These new developments in diagnostic technology may someday greatly improve the ability to screen people for oral premalignancy and to detect and diagnose OPLs. However, intensive research and clinical trial investigations are needed to test the sensitivity, specificity, and negative and positive predictive ability of these technologies before they can be widely implemented.
Management of Oral Premalignant Lesions
The management of patients with OPLs can be quite challenging. Because most OPLs are asymptomatic, the primary management goal is to prevent and/or detect any cancer development early. Given that currently there is no reliable method to predict when and if an OPL will undergo malignant transformation, it is important to weigh the risks and potential morbidity carefully of any therapeutic intervention against the potential benefits. All patients with clinically apparent OPLs should have routine oral examinations and counseling to avoid known risks factors for oral cancer. Biopsy is indicated to assess for dysplasia or occult carcinoma. However, a “negative,” that is, nondysplastic pathologic result, does not exclude the presence of dysplastic changes within the oral mucosa or future malignant transformation. This may be due to several factors, including the presence of skip areas of dysplasia interspersed with histologically normal areas and lack of uniformity for diagnosing dysplasia among pathologists [15••].
Management strategies for patients with OPLs fall into three categories: close observation, surgical removal and ablation, and medical therapies [57]. The mainstay of therapy is observation using frequent clinical examinations. The frequency of examinations should be tailored to individual patient factors such as the clinical appearance and stage of lesion; presence of dysplasia; continued use of tobacco, alcohol, or Areca quid; reliability; and access to medical care.
Surgical resection is performed to remove areas at high risk to harbor foci of early carcinoma or to undergo early malignant transformation. Attempts to remove all clinically apparent areas of leukoplakia, or histologically diagnosed areas of dysplasia, are impractical in most circumstances and produce scarring and contracture that result in more morbidity than benefit to the patient. This inability to successfully eradicate all precancerous areas with surgery is due to the widespread “field effect” commonly found in the oral cavity, in which exposure to carcinogens creates premalignant changes over large areas of mucosa. Recurrence rates of 10% to 34% after surgical resection of oral leukoplakia have been reported [58,59]. Furthermore, complete excision of visibly apparent leukoplakia does not prevent development of oral carcinoma [60•,61]. In an analysis of 150 patients undergoing surgical resection of dysplastic oral lesions, the presence of dysplasia at the margins of resection did not have a significant impact on the development of cancer [60•]. Laser ablation has also been advocated for eradication of OPLs [58]. This approach offers the potential advantage of reduced scarring, but a major disadvantage is the lack of a resected specimen for histopathologic and molecular analysis.
Photodynamic therapy is another ablative approach being investigated for management of OPLs. A photosensitizing agent, such as hematoporphyrin derivatives or 5-ALA, that preferentially targets neoplastic cells is administered either intravenously or topically. The tissue to be targeted is then exposed to a specific wavelength of light, which activates the photosensitizer, causing it to transfer energy to molecular oxygen, generating reactive oxygen species locally and subsequent tissue damage [62]. Several small clinical studies have shown promising results for treating epithelial premalignant lesions and superficial carcinomas with this technique [63–65]. The major limiting side effect has been cutaneous photosensitivity, but newer photosensitizing agents such as 5-ALA and topical administration have markedly reduced the incidence and severity of this complication. Whether photodynamic therapy will prove more successful than standard surgical and laser approaches will need to be determined in future clinical trials.
Chemoprevention is the use of natural or synthetic substances to halt, delay, or reverse malignant progression in tissues at risk to develop invasive cancer. Retinoids are the most extensively studied agents for chemoprevention of oral cancer. Hong et al. [66••] reported the first randomized clinical trial using high-dose 13-cis-retinoic acid (13cRA) in 44 assessable patients with OPL. 13cRA given for only 3 months produced a clinical response rate of 67% versus 10% for placebo. However, toxicities were considerable, and a very high rate of relapse within 3 months of stopping treatment was reported. Subsequent studies with retinoids in patients with OPLs have confirmed clinical and pathologic response rates, though toxicities remain a concern [67–69]. However, translational studies showed that molecular abnormalities persisted in some patients with complete clinical and pathologic response to retinoid therapy [70], suggesting that cancer development may be delayed rather than prevented by these agents.
Other agents that have been assessed in clinical trials for chemoprevention activity in oral leukoplakia patients include vitamin E [71], Bowman-Birk inhibitor concentrate (BBIC) derived from soybeans [72], curcumin [73], and green tea polyphenol epigallocatechin-3-gallate (EGCG). Small clinical trials using oral BBIC revealed no significant toxicity and a 32% response rate [72]. In addition, a trial of an attenuated adenovirus (ONYX-015), delivered topically as a mouthwash, was performed in 22 patients with OPLs. ONYX-015 is thought to be selectively toxic to cells harboring defects in the p53 tumor suppressor gene. Of the 19 evaluable patients, seven (37%) achieved histologic resolution of dysplasia, although most responses were temporary. Only limited toxicity was noted [74].
Attention is focused now on development of agents targeted to specific steps in the molecular progression from normal to oral premalignancy to invasive carcinoma. Examples of molecularly targeted agents that have shown promise in vitro, in animal models, or in early clinical trials include cyclooxygenase (COX)-2 inhibitors and EGFR inhibitors [75•,76•,77,78].
Data from several sources suggest that the cyclooxygenase pathway is a good target for oral cancer prevention. COX-2 is overexpressed in head and neck squamous carcinoma (HNSCC) [79], and COX-2 inhibitors prevented oral cancer development in animal models [80]. A randomized placebo-controlled trial of the COX-2 inhibitor ketorolac administered as an oral rinse in oral leukoplakia patients revealed that the treatment was well tolerated but did not result in greater clinical response than placebo [81]. However, analysis of the results of this trial are confounded somewhat by the high response rate (32%) in the placebo arm and difficulty in determining whether topical delivery of the agent allowed penetration to the damaged cells. The future of COX-2 inhibitors as chemoprevention agents will also depend on determination of the extent of risk for cardiac toxicities associated with this class of agents.
The epidermal growth factor receptor is also a promising molecular target for intervention in oral malignant progression [78]. EGFR is a receptor tyrosine kinase that is overexpressed in oral dysplasia and invasive cancer and associated with worse prognosis in patients with HNSCC [82,83]. EGFR inhibitors, alone or in combination with chemotherapy and radiotherapy, have shown activity against HNSCC in clinical trials, and toxicities were generally well tolerated [84]. Evidence has suggested that combination therapy targeting COX-2 and EGFR may be efficacious [78,85]. Although chemoprevention appears to be a promising approach to managing oral premalignancy, prospective clinical trials using specific agents, and strong corollary translational and laboratory investigations, are needed to evaluate clinical, histologic, and molecular efficacy. In the future it may be possible and necessary to individualize medical therapy to specific genetic abnormalities detected within the oral mucosa.
Conclusions
The detection, diagnosis, and management of oral premalignant lesions is complex. Promising technologies are being rapidly developed to assist in localization of abnormal oral mucosa, in noninvasive and objective diagnosis and characterization of identified mucosal lesions, and in therapy of patients with OPLS. Intensive evaluation of these technologies in prospective clinical trials is needed to find the best ways to incorporate them into clinical practice to improve outcome and survival of persons at risk for oral cancer.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Vogelstein B, Kinzler KW. The multistep nature of cancer trends. Genet (England) 1993;9:38–41. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 2.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 3. Braakhuis BJM, Leemans CR, Brakenhoff RH. A genetic progression model of oral cancer: current evidence and clinical implications. J Oral Pathol Med. 2004;33:317–322. doi: 10.1111/j.1600-0714.2004.00225.x. An excellent, thoughtful review of current knowledge on the molecular progression associated with oral carcinogenesis and the potential role of a genetically altered stem cell population.
- 4.Lippman SM, Hong WK. Molecular markers of the risk of oral cancer. N Engl J Med. 2001;344:1323–1326. doi: 10.1056/NEJM200104263441710. [DOI] [PubMed] [Google Scholar]
- 5.Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol. 2005;23:346–356. doi: 10.1200/JCO.2005.09.128. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee AG, Bhattacharyya I, Vishwanatha JK. Identification of genes and molecular pathways involved in the progression of premalignant oral epithelia. Mol Cancer Ther. 2005;4:865–875. doi: 10.1158/1535-7163.MCT-05-0033. [DOI] [PubMed] [Google Scholar]
- 7. Slaughter OP. Multicentric origin of intraoral carcinoma. Surgery. 1946;20:133–146. This report, along with that of Strong et al. [8•], are the classic descriptions leading to the acceptance of the concept of “field cancerization” and “field effect” in oral and head and neck carcinogenesis.
- 8. Strong MS, Incze J, Vaughan CN. Field cancerization in the aerodigestive tract - its etiology, manifestation, and significance. J Otolaryngol. 1984;13:1–6. This report, along with that of Slaughter et al. [7•], are the classic descriptions leading to the acceptance of the concept of “field cancerization” and “field effect” in oral and head and neck carcinogenesis.
- 9.Incze J, Vaughan CW, Jr, Lui P, et al. Premalignant changes in normal appearing epithelium in patients with squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1982;144:401–405. doi: 10.1016/0002-9610(82)90411-1. [DOI] [PubMed] [Google Scholar]
- 10.Westra WH, Sidransky D. Phenotypic and genotypic disparity in premalignant lesions: of calm water and crocodiles. J Natl Cancer Inst. 1998;90:1500–1501. doi: 10.1093/jnci/90.20.1500. [DOI] [PubMed] [Google Scholar]
- 11.Reibel J. Prognosis of oral pre-malignant lesions: significance for clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14(1):47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 12. Silverman S, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: a follow-up study of 257 patients. Cancer. 1984;53:563–568. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. This is a classic description of patients with oral leukoplakia documenting the clinical follow-up over time and the development of oral carcinoma.
- 13.Reichart PA, Philipsen HP. Oral erythroplakia: a review. Oral Oncol. 2005;41:551–561. doi: 10.1016/j.oraloncology.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Axell T, Pindborg JJ, Smith CJ, van der Waal I. Oral white lesions with special reference to precancerous and tobacco-related lesions: conclusions of an international symposium held in Uppsala, Sweden, May 18–21 1994. International Collaborative Group on Oral White Lesions. J Oral Pathol Med. 1996;25:49–54. doi: 10.1111/j.1600-0714.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 15. Van der Waal I, Schepman KP, van der Meij EH. A modified classification and staging system for oral leukoplakia. Oral Oncol. 2000;36:264–266. doi: 10.1016/s1368-8375(99)00092-5. This report describes the staging system frequently cited for use in oral leukoplakia patients.
- 16.Scully C, Sudbo J, Speight PM. Progress in determining the malignant potential of oral lesions. J Oral Pathol Med. 2003;32:251–256. doi: 10.1034/j.1600-0714.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 17.Sudbo J, Kildal W, Risberg B, et al. DNA content as a prognostic marker in patients with oral leukoplakia. N Engl J Med. 2001;344:1270–1278. doi: 10.1056/NEJM200104263441702. [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–1710. [PubMed] [Google Scholar]
- 19.Lam S, MacAulay C, leRiche JC, Palcic B. Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer. 2000;89(11 Suppl):2468–2473. doi: 10.1002/1097-0142(20001201)89:11+<2468::aid-cncr25>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.Stanzel F. Fluorescent bronchoscopy: contribution for lung cancer screening? Lung Cancer. 2004;45 Suppl 2:S29–S37. doi: 10.1016/j.lungcan.2004.07.995. [DOI] [PubMed] [Google Scholar]
- 21.Bogaards A, Varma A, Zhang K, et al. Fluorescence image-guided brain tumor resection with adjuvant metronomic photodynamic therapy: pre-clinical model and technology development. Photochem Photobiol Sci. 2005;4:438–442. doi: 10.1039/b414829k. [DOI] [PubMed] [Google Scholar]
- 22.Kara MA, Smits ME, Rosmolen WD, et al. A randomized crossover study comparing light-induced fluorescence endoscopy with standard videoendoscopy for the detection of early neoplasia in Barrett’s esophagus. Gastrointest Endosc. 2005;61:671–678. doi: 10.1016/s0016-5107(04)02777-4. [DOI] [PubMed] [Google Scholar]
- 23.Kulapaditharom B, Boonkitticharoen V. Laser-induced fluorescence imaging in localization of head and neck cancers. Ann Otol Rhinol Laryngol. 1998;107:241–246. doi: 10.1177/000348949810700310. [DOI] [PubMed] [Google Scholar]
- 24.Onizawa K, Saginoya H, Furuya Y, et al. Usefulness of fluorescence photography for diagnosis of oral cancer. Int J Oral Maxillofac Surg. 1999;28:206–210. [PubMed] [Google Scholar]
- 25.Svistun E, Alizadeh-Naderi R, El-Naggar A, et al. Vision enhancement system for detection or oral cavity neoplasia based on autofluorescence. Head Neck. 2004;26:205–215. doi: 10.1002/hed.10381. [DOI] [PubMed] [Google Scholar]
- 26.Wagnieres GA, Star WM, Wilson BC. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem Photobiol. 1998;68:603–632. [PubMed] [Google Scholar]
- 27. Ramanujam N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia. 2000;2:89–117. doi: 10.1038/sj.neo.7900077. An excellent overview of principles behind fluorescence spectroscopy and some potential clinical applications of this technology.
- 28.Schantz SP, Kolli V, Savage HE, et al. In vivo native cellular fluorescence and histological characteristics of head and neck cancer. Clin Cancer Res. 1998;4:1177–1182. [PubMed] [Google Scholar]
- 29.Dhingra JK, Perrault DF, Jr, McMillan K, et al. Early diagnosis of upper aerodigestive tract cancer by autofluorescence. Arch Otolaryngol Head Neck Surg. 1996;122:1181–1186. doi: 10.1001/archotol.1996.01890230029007. [DOI] [PubMed] [Google Scholar]
- 30.Gillenwater AM, Jacob R, Ganeshappa R, et al. Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Arch Otolaryngol Head Neck Surg. 1998;124:1251–1258. doi: 10.1001/archotol.124.11.1251. [DOI] [PubMed] [Google Scholar]
- 31.Heintzelman D, Utzinger U, Fuchs H, et al. Optimal excitation wavelengths for in vivo detection of oral neoplasia using fluorescence spectroscopy. Photochem Photobiol. 2000;72:103–113. doi: 10.1562/0031-8655(2000)072<0103:OEWFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Sokolov K, Drezek R, Gossage K, Richards-Kortum R. Reflectance spectroscopy with polarized light: is it sensitive to cellular and nuclear morphology? Opt Expr. 1999;5:302–317. doi: 10.1364/oe.5.000302. [DOI] [PubMed] [Google Scholar]
- 33.Gurjar RS, Backman V, Perelman LT, et al. Imaging human epithelial properties with polarized light-scattering spectroscopy. Nat Med. 2001;7:1245–1248. doi: 10.1038/nm1101-1245. [DOI] [PubMed] [Google Scholar]
- 34.Muller MG, Valdez TA, Georgakoudi I, et al. Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer. 2003;97:1681–1692. doi: 10.1002/cncr.11255. [DOI] [PubMed] [Google Scholar]
- 35.Georgakoudi I, Jacobson BC, Van Dan J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2001;120:1620–1629. doi: 10.1053/gast.2001.24842. [DOI] [PubMed] [Google Scholar]
- 36.Chang SK, Mirabal YN, Atkinson EN, et al. Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt. 2005;10:024–031. doi: 10.1117/1.1899686. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg Oral Med Oral Pathol. 1987;67:621–627. doi: 10.1016/0030-4220(89)90286-7. [DOI] [PubMed] [Google Scholar]
- 38.Epstein JB, Scully C, Spinelli JJ. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160–163. doi: 10.1111/j.1600-0714.1992.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Williams M, Poh CF, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65:8017–8021. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]
- 40.Leunig A, Betz CS, Mehlmann M, et al. Detection of squamous cell carcinoma of the oral cavity by imaging 5-aminolevulinic acid-induced protoporphyrin IX fluorescence. Laryngoscope. 2000;1100:78–83. doi: 10.1097/00005537-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Zheng W, Olivo M, Soo KC. The use of digitized endoscopic imaging of 5-ALA-induced PPIX fluorescence to detect and diagnose oral premalignant and malignant lesions in vivo. Int J Cancer. 2004;110:295–300. doi: 10.1002/ijc.20080. [DOI] [PubMed] [Google Scholar]
- 42.Huber MA, Bsoul SA, Terezhalmy GT. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: pilot study. Quintessence Int. 2004;35:378–384. [PubMed] [Google Scholar]
- 43.Sokolov K, Aaron J, Hsu B, et al. Optical systems for in vivo molecular imaging of cancer. Technol Cancer Res Treat. 2003;2:491–504. doi: 10.1177/153303460300200602. [DOI] [PubMed] [Google Scholar]
- 44.Soukos NS, Hamblin MR, Keel S, et al. Epidermal growth factor receptor-targeted immunophotodiagnosis and photoimmunotherapy of oral precancer in vivo. Cancer Res. 2001;61:4490–4496. [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu ER, Anslyn EV, Dharmawardhane S, et al. A far-red fluorescent contrast agent to image epidermal growth factor receptor expression. Photochem Photobiol. 2004;79:272–279. doi: 10.1562/fr-03-15.1. [DOI] [PubMed] [Google Scholar]
- 46.Sokolov K, Follen M, Aaron J, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- 47.El-Sayed IH, Huang X, El-Sayed AM. Surface plasmon resonance scattering and absorption of anti-EGF antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 48. Sciubba JJ. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. U.S. Collaborative OralCDx Study Group. J Am Dent Assoc. 1999;130:1445–2457. doi: 10.14219/jada.archive.1999.0055. This paper reports the findings of a large multicenter trial conducted by academic dental specialists evaluating the efficacy of the OralCDx brush biopsy for noninvasive diagnosis of oral lesions. Surgical biopsies were only performed when the brush biopsy was atypical or positive; therefore, there is no way to document whether there were any false-negative results.
- 49. Poate TWJ, Buchanan JAG, Hodgson TA, et al. An audit of the efficacy of the oral brush biopsy technique in a specialist Oral Medicine unit. Oral Oncol. 2004;40:829–834. doi: 10.1016/j.oraloncology.2004.02.005. An important study documenting the sensitivity, specificity, and positive and negative predictive ability of the OralCDx brush biopsy.
- 50.Potter TJ, Summerlin D-J, Campbell JH. Oral malignancies associated with negative transepithelial brush biopsy. J Oral Maxillofac Surg. 2003;61:674–677. doi: 10.1053/joms.2003.50136. [DOI] [PubMed] [Google Scholar]
- 51.Clark AL, Gillenwater AM, Collier TC, et al. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin Cancer Res. 2003;9:4714–4721. [PubMed] [Google Scholar]
- 52.Colston BW, Jr, Everett MJ, Sathyam US, et al. Imaging of the oral cavity using optical coherence tomography. Mogr Oral Sci. 2000;17:32–55. doi: 10.1159/000061643. [DOI] [PubMed] [Google Scholar]
- 53.Wilder-Smith, Jung WG, Brenner M, et al. In vivo optical coherence tomography for the diagnosis of oral malignancy. Laser Surg Med. 2004;35:269–275. doi: 10.1002/lsm.20098. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, St. John MAR, Zhou X, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 55.El-Naggar AK, Mao L, Staerkel G, et al. Genetic heterogeneity in saliva from patients with oral squamous carcinomas: implications in molecular diagnosis and screening. J Mol Diagn. 2001;3:164–170. doi: 10.1016/S1525-1578(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spafford MF, Koch WM, Reed AL, et al. Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysis. Clin Cancer Res. 2001;7:607–612. [PubMed] [Google Scholar]
- 57.Tradati N, Grigolat R, Calabrese L, et al. Oral leukoplakias: to treat or not? Oral Oncol. 1997;33:317–321. doi: 10.1016/s1368-8375(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 58.Van der Hem PS, Nauta JM, van der Wal JE, Roodenburg JLN. The results of CO2 laser surgery in patients with oral leukoplakia: a 25 year follow up. Oral Oncol. 2005;41:31–37. doi: 10.1016/j.oraloncology.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Vedtofte P, Holmstrup P, Hjorting-Hansen E, Pindborg JJ. Surgical treatment of premalignant lesions of the oral mucosa. Int J Oral Maxillofac Surg. 1987;16:656–664. doi: 10.1016/s0901-5027(87)80049-8. [DOI] [PubMed] [Google Scholar]
- 60. Sudbo J, Lippman SM, Lee JJ, et al. The influence of resection and aneuploidy on mortality in oral leukoplakia. N Engl J Med. 2004;350:1405–1413. doi: 10.1056/NEJMoa033374. An important paper documenting the impact of aneuploidy in oral leukoplakic lesions on patient survival rates after subsequent oral cancer development in a high-risk population in Norway.
- 61.Zhang L, Poh CF, Lam WL, et al. Impact of localized treatment in reducing risk of progression of low-grade oral dysplasia: Molecular evidence of incomplete resection. Oral Oncol. 2001;37:505–512. doi: 10.1016/s1368-8375(00)00140-8. [DOI] [PubMed] [Google Scholar]
- 62.Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–386. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 63.Kubler A, Haase T, Rheinwald M, et al. Treatment of oral leukoplakia by topical application of 5-aminolevulinic acid. Int J Oral Maxillofac Surg. 1998;27:466–469. doi: 10.1016/s0901-5027(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 64.Fan KF, Hopper C, Speight PM, et al. Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer. 1996;78:1374–1383. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1374::AID-CNCR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 65.Sieron A, Adamek M, Kawczyk-Krupka A, et al. Photodynamic therapy (PDT) using topically applied g-aminolevulinic acid (ALA) for the treatment of oral leukoplakia. J Oral Pathol Med. 2003;32:330–336. doi: 10.1034/j.1600-0714.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 66. Hong WK, Endicott J, Itri LM, et al. 13-cis retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. This landmark paper documented the first prospective, randomized, placebo-controlled trial showing efficacy of chemoprevention to reverse premalignant changes in oral mucosa.
- 67.Lippman SM, Bastsakis JG, Toth BB, et al. Comparison of low-dose isoretinoin therapy with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 68.Papadimitrikopoulou VA, Hong WK, Lee JS, et al. Low dose isoretinoin versus beta carotene to prevent oral carcinogenesis: long term follow up. J Natl Cancer Inst. 1997;89:257–258. doi: 10.1093/jnci/89.3.257. [DOI] [PubMed] [Google Scholar]
- 69.Chiesa F, Tradati N, Marazza M, et al. Fenretinide (4-HPR) in chemoprevention of oral leukoplakia. J Cell Biochem Suppl. 1993;17F:255–261. doi: 10.1002/jcb.240531038. [DOI] [PubMed] [Google Scholar]
- 70.Mao L, El-Naggar AK, Papadmitrakopoulou V, et al. Phenotype and genotype of advanced premalignant head and neck lesions after chemopreventive therapy. J Natl Cancer Inst. 1998;90:1545–1551. doi: 10.1093/jnci/90.20.1545. [DOI] [PubMed] [Google Scholar]
- 71.Benner SE, Winn RJ, Lippman SM, et al. Regression of oral leukoplakia with alpha-tocopherol: a community clinical oncology program chemoprevention study. J Natl Cancer Inst. 1993;85:44–47. doi: 10.1093/jnci/85.1.44. [DOI] [PubMed] [Google Scholar]
- 72.Armstrong WB, Kennedy AR, Wan XS, et al. Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial. Clin Cancer Res. 2000;6:4684–4691. [PubMed] [Google Scholar]
- 73.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 74.Rudin CM, Cohen EEX, Papdimitrakopoulou VA, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol. 2003;21:4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Papadimitrakopoulou VA. Chemoprevention of head and neck cancer: an update. Curr Opin Oncol. 2002;14:318–322. doi: 10.1097/00001622-200205000-00011. A good review of recent chemoprevention trials.
- 76. Rhee JC, Khuri FR, Shin DM. Advances in chemoprevention of head and neck cancer. Oncologist. 2004;9:302–311. doi: 10.1634/theoncologist.9-3-302. A good review of recent chemoprevention trials.
- 77.Wang Z. The role of COX-2 in oral cancer development, and chemoprevention/treatment of oral cancer by selective COX-2 inhibitors. Curr Pharm Des. 2005;11:1771–1777. doi: 10.2174/1381612053764887. [DOI] [PubMed] [Google Scholar]
- 78.Lippman SM, Gibson N, Subbaramaiah K, Dannenberg AJ. Combined targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways. Clin Cancer Res. 2005;11:6097–6099. doi: 10.1158/1078-0432.CCR-05-1217. [DOI] [PubMed] [Google Scholar]
- 79.Chan G, Boyle JO, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 80.Nishimura N, Manno Y, Takaoka K, et al. Increased expression of cyclooxygenase (COX)-2 in DMBA-induced hamster cheek pouch carcinogenesis and chemopreventive effect of a selective COX-2 inhibitor celecoxib. J Oral Pathol Med. 2004;33:614–621. doi: 10.1111/j.1600-0714.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 81.Mulshine JL, Atkinson JC, Greer RO, et al. Randomized, double-blind, placebo-controlled phase IIb trial of the cyclooxygenase inhibitor ketorolac as an oral rinse in oropharyngeal leukoplakia. Clin Cancer Res. 2004;10:1565–1573. doi: 10.1158/1078-0432.ccr-1020-3. [DOI] [PubMed] [Google Scholar]
- 82.Shin DM, Ro JY, Hong WK, Hittleman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54:3153–3159. [PubMed] [Google Scholar]
- 83.Dassonville O, Formento JL, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol. 1993;11:1873–1878. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 84.Pomerantz RG, Grandis JR. The epidermal growth factor receptor signaling network in head and neck carcinogenesis and implications for targeted therapy. Semin Oncol. 2004;31:734–743. doi: 10.1053/j.seminoncol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 85.Choe MS, Zhang X, Shin HJ, et al. Interaction between epidermal growth factor receptor- and cyclooxygenase 2-mediated pathways and its implications for the chemoprevention of head and neck cancer. Mol Cancer Ther. 2005;4:1448–1455. doi: 10.1158/1535-7163.MCT-04-0251. [DOI] [PubMed] [Google Scholar]