Abstract
Coupling plasminogen activators to carrier red blood cells (RBC) prolongs their life-time in the circulation and restricts extravascular side effects, thereby allowing their utility for short-term thromboprophylaxis. Unlike constitutively active plasminogen activators, single chain urokinase plasminogen activator (scuPA) is activated by plasmin proteolysis or binding to its receptor, uPAR. In this study we conjugated recombinant soluble uPAR (suPAR) to rat RBC, forming RBC/suPAR complex. RBC carrying suPAR circulated in rats similarly to naïve RBC and markedly prolonged the circulation time of suPAR. RBC/suPAR carrying ~3×104 suPAR molecules per RBC specifically bound up to 2×104 molecules of scuPA, retained ~75% of scuPA binding capacity after circulation in rats and markedly altered the functional profile of bound scuPA. RBC carrying directly conjugated scuPA adhered to endothelial cells, while showing no appreciable fibrinolytic activity. In contrast, RBC/suPAR loaded with scuPA did not exhibit increased adhesion to endothelium, while effectively dissolving fibrin clots. This molecular design, capitalizing on unique biological features of the interaction of scuPA with its receptor, provides a promising modality to deliver a pro-drug for prevention of thrombosis.
INTRODUCTION
Prevention of thrombosis remains a major medical challenge. The utility of existing agents used for thromboprophylaxis and therapeutic thrombolysis is limited by bleeding and other side effects, and their delivery requires optimization1. In particular, the need for safer and more effective short-term prevention of thrombosis is especially acute in settings where the risk of thrombosis and hemorrhage are both known to be high, e.g., in elective surgical procedures. Thromboprophylaxis is often withheld for many hours or days before and after surgery due to the risk of bleeding2. This leaves patients unprotected during the most vulnerable early post-operative period characterized by immobilization, inflammation and activation of clotting caused by operation3.
Surgical hemostasis is attained within an hour after uncomplicated interventions, while the highest risk of thrombosis persists for 24–48 hours4,5. In theory, prophylactic administration of fibrinolytics shortly after surgery could expedite thrombolysis of newly forming pathological clots, but the unfavorable pharmacokinetics of fibrinolytic plasminogen activators (circulation time <30 min) precludes their use as thromboprophylaxis. Moreover, no fibrinolytic drugs, including widely clinically used tissue-type plasminogen activator (tPA) and newly developed activators with enhanced potency and affinity for thrombi6,7, distinguish between preformed mural hemostatic clots and nascent pathological thrombi. Considerable efforts are being devoted to design of advanced systems for delivery plasminogen activators, including fibrin-targeted recombinant pro-drug versions, polymer-coupled and microbubbles-loaded tPA, streptokinase and other fibrinolytics7–10. These approaches promise improving benefit/risk ratio of fibrinolytics in emergency therapy, but less likely to provide prolongation of circulation and retention in the vascular space sufficient for prophylactic applications.
However, coupling tPA to a large biocompatible carrier, i.e., red blood cells (RBC) does fundamentally alter its pharmacokinetics and activity, thereby converting it into a safe and effective thromboprophylactic agent11. RBC-bound tPA (RBC/tPA) does not permeate or dissolve post-surgical clots, even those formed just 10 min prior to administration 11. However, RBC/tPA circulate for a prolonged time, which enables them to incorporate into, and rapidly lyse from within, intravascular clots formed hours after RBC/tPA administration, thereby preventing vascular occlusion12. Studies performed in several species have documented that RBC/tPA provides effective short-term thromboprophylaxis against pulmonary venous, peripheral arterial and cerebrovascular thrombosis without adverse effects typical of free tPA11,13.
In theory, prolonged retention of RBC-coupled plasminogen activators in the vascular lumen, through restricting side effects in tissues and enabling prophylactic thrombolysis, might also activate cognate receptors expressed on vascular and blood cells, including the urokinase plasminogen activator receptor (uPAR), low-density lipoprotein-related receptors, integrins, and variously described “tPA receptors”14,15. Engaging these cellular receptors, in particular, endothelial uPAR, may alter vasoreactivity and permeability, promote inflammation and even provoke pro-coagulant pathways as a result of intracellular signaling.
One way to avoid this complication, in theory, would be to couple a plasminogen activator to RBC via a corresponding receptor, thereby blocking interaction with its vascular counterparts. In this context, urokinase plasminogen activator (uPA) and its receptor, uPAR, represent an attractive candidate pairing, because: i) recombinant soluble uPAR (suPAR) is available; ii) previous studies revealed that binding to uPAR and suPAR converts a latent form of uPA, single chain uPA (scuPA) into a fibrinolytically active uPA; iii) activation of scuPA by suPAR is facilitated by ternary interactions of suPAR-scuPA complex with fibrin, which may enable specific activation of a pro-drug in the intended site of action; iv) the activity of scuPA-suPAR complex is down-regulated in plasma by plasminogen but is restored by fibrin; and v) the activity of the complex is relatively resistant to inhibition by its high affinity inhibitor PAI-1 that is often present at concentrations that prevent fibrinolysis at sites of vascular damage and inflammation16,17.
Therefore, in theory, coupling of scuPA to RBC/suPAR complex may provide important advantages in the context of prophylactic thrombolysis including: a) inhibition of undesirable vascular signaling; and, b) fibrin-dependent conversion of a pro-drug into an inhibitor-resistant fibrinolytic agent. In this study, we coupled suPAR to RBC, determined the biocompatibility and functionality of RBC/suPAR complex in vitro and in vivo and investigated how binding to RBC/suPAR affects scuPA’s fibrinolytic activity and interaction with endothelium.
MATERIALS AND METHODS
The following reagents were used: recombinant human scuPA and suPAR were prepared, purified and characterized as previously described18. Fibrinogen was purchased from Enzyme Research Labs (South Bend, IN), thrombin and streptavidin (SA) from Calbiochem (San Diego, CA), Iodogen and 6-biotinylaminocaproic acid N-hydroxysuccinimide ester (long chain biotin ester BxNHS) from Pierce (Rockford, IL).
Proteins (suPAR and scuPA) were biotinylated with a 10-fold molar excess of BxNHS as previously described for other protein molecules11 and radiolabeled with Na [125I] (Perkin-Elmer, Boston, MA) using Iodogen according to the manufacturer’s recommendations. The free iodine was removed using a Bio-Spin 6 column (Bio-Rad Laboratory, Hercules CA). RBC were obtained from fresh anticoagulated rat blood and radiolabeled with [51Cr] chloride (Perkin-Elmer, Boston, MA) as described11.
Binding of biotinylated suPAR (b-suPAR) to streptavidin and scuPA
Plastic wells were coated with streptavidin (SA) by incubating 1 µg of SA in 300 µl phosphate buffer solution (PBS) for 1 hour at 20°C and then incubated with 300 µl of a 1mg/ml solution of bovine serum albumin (BSA) in PBS (BSA-PBS). 125I-b-suPAR was added to SA- or BSA-coated wells in 3% PBS-BSA, incubated for 1 h, washed 4 times and the isotope remaining bound to the wells was measured in a gamma counter. In other experiments, 125I-b-suPAR alone or mixed with 50-fold molar excess unlabeled b-suPAR was incubated for 1 h with CHO-cells stably transfected with scuPA19, washed, and binding was measured as described above. In a third set of experiments, 125I-scuPA, alone or mixed with 50-fold molar excess unlabeled scuPA, was incubated in the plastic wells coated with b-suPAR or BSA, and binding was measured as above. Fourth, to test whether b-suPAR can bind both streptavidin and scuPA simultaneously, binding of 125I-scuPA was measured to plastic wells coated with streptavidin and pre-incubated with b-suPAR.
Coupling of suPAR or scuPA to carrier RBC
Biotinylated suPAR was coupled to biotinylated carrier RBC via streptavidin following the step-wise protocol that we have previously developed for coupling other proteins to RBC without compromising RBC biocompatibility in vivo11,20. Briefly, RBC were biotinylated using 10 µM BxNHS to produce b-RBC that were coupled with streptavidin to form SA/b-RBC followed by either 125I-labeled or unlabeled b-suPAR or b-scuPA to form b-suPAR/SA/b-RBC complexes (designated hereafter as RBC/suPAR) or b-scuPA/SA/b-RBC (designated as RBC/scuPA). At each conjugation step, excess unbound reagents were removed by washing the RBC four times with a 20-fold volume of PBS-BSA. To test the capacity of RBC/suPAR to bind scuPA, 125I-scuPA was incubated with a suspension of RBC/suPAR containing ~3.7×104 b-suPAR molecules per RBC. The cells were washed four times and the RBC-bound isotope was measured.
Distribution of RBC/suPAR in vivo
125I-suPAR or 51Cr-RBC/125I-suPAR was injected in anesthetized rats via the jugular vein. At the indicated times, 200 µl aliquots of blood were taken in heparin, animals were sacrificed and radioactivity in blood and organs was determined as previously described11,20. Animal experiments have been performed accordingly to the animal protocols approved by IACUC of the University of Pennsylvania.
Ex vivo binding of scuPA to RBC/suPAR after their circulation in vivo
Anesthetized rats were injected via the jugular vein with a 0.5 ml solution of PBS containing a 10% suspension of washed 51Cr-RBC or 51Cr-RBC carrying ~3×104 suPAR per cell. One hour later, 1 ml of blood was removed in a heparinized solution and incubated with 50 nM 125I-scuPA for an hour. The cells were washed four times and the radioactivity in the RBC pellet was measured in a gamma-counter, as a measure of the binding capacity of circulating RBC/suPAR. In parallel, binding of 125I-scuPA to RBC/suPAR mixed with heparinized rat blood was measured to simulate the assay and analyze potential loss of scuPA binding by RBC/suPAR after circulating in the blood.
Fibrinolysis assay
Lysis of 125I-fibrin clots containing plasminogen was monitored by measuring the release of 125I into supernatants11. RBC/scuPA, RBC/suPAR-scuPA complexes, naïve RBC, free scuPA or PBS vehicle were added on ice to 3mg/ml solution of fibrinogen in PBS containing tracer amount of 125I-labeled fibrinogen. A mixture of CaCl2 and thrombin (final concentration 20 mM and 0.2U/ml, respectively) was added. The samples were rapidly divided into 200µl aliquots to form fibrin clots that were allowed to mature for 20 min at room temperature and subsequently overlaid with equal volume of saline. Clots were then incubated at 37°C and fibrinolysis was assessed by release of isotope-labeled fibrin degradation products in supernatant as described12.
Binding of RBC to endothelial cells
Human umbilical cord endothelial cells (HUVEC) were grown to confluence in 24-well Multiwell plates. Samples containing naïve washed RBC, RBC/scuPA, RBC/suPAR or RBC/suPAR-scuPA loaded were added to the monolayer for 1 h at 37°C and the cells were washed four times. A hypotonic lytic buffer was added for 1 h at 20°C and the concentration of hemoglobin in the lysates measured in a spectrophotometer at A412nm was used to calculate the amount of RBC bound to HUVEC compared with a calibration curve containing known amounts of RBC lysates, as described21.
RESULTS
Coupling of soluble urokinase receptor (suPAR) to RBC
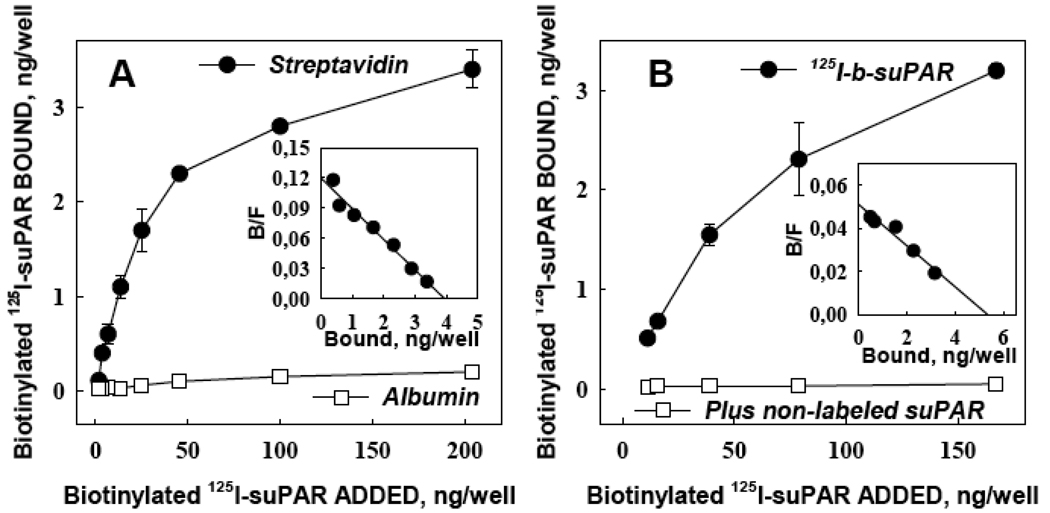
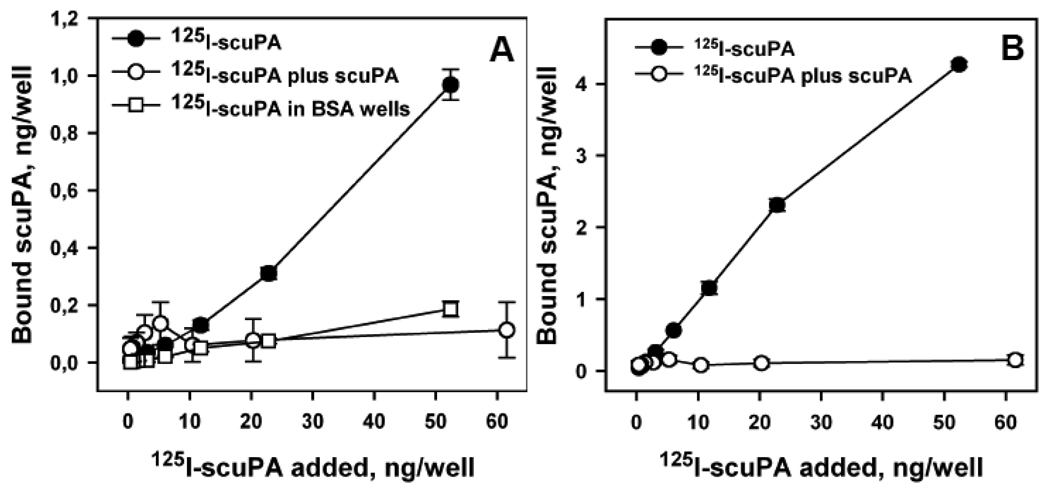
To couple suPAR to RBC, we first biotinylated and then iodinated suPAR (125I-b-suPAR) and characterized its binding properties. 125I-labeled b-suPAR bound to streptavidin with a Kd ~6.5 nM determined by Scatchard analysis (Fig.1A inset), but did not bind to albumin-coated surfaces (Fig.1A). 125I-b-suPAR also bound to scuPA-transfected CHO cells with a Kd ~4 nM (Fig.1B, upper curve). Binding was inhibited by 20-fold molar excess unlabeled suPAR, affirming the specificity of binding (Fig.1B, lower curve). Conversely, 125I-scuPA bound specifically to immobilized b-suPAR (Fig.2A) and to streptavidin-coated wells pre-incubated with b-suPAR (Fig.2B), but not to albumin coated surfaces. Therefore, b-suPAR is capable of binding simultaneously to both streptavidin and scuPA.
Figure 1. Characterization of biotinylated suPAR.
Binding of 125I-labeled b-suPAR to wells coated with streptavidin or albumin (panel A) or to scuPA-transfected CHO cells (panel B). Inhibition of binding by 20-fold excess unlabeled suPAR (lower curve) confirms specificity of suPAR binding to the cells. Insets show Scatchard analyses of the binding isotherms. All data in this and every figure are presented as M±SEM, n=3.
Figure 2. Binding of scuPA to immobilized b-suPAR.
Binding of 125I-labeled scuPA to suPAR- or albumin-coated wells (panel A) or to streptavidin-coated wells pre-incubated with b-suPAR (panel B). Specificity of binding was confirmed in both settings by inhibition with 20-fold excess unlabeled scuPA (open circles) and unspecific binding to BSA-coated wells (squares in panel A).
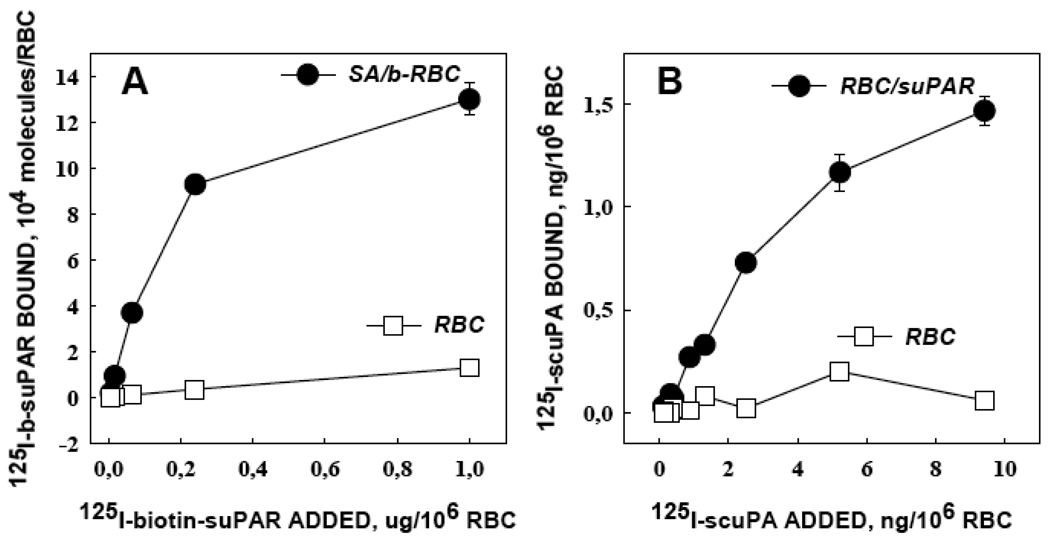
We have previously shown that RBC incubated with 10 µM biotin succinimide ester generates biocompatible b-RBC and allows streptavidin-mediated attachment of up to 105 molecules b-tPA per cell11. Similarly, streptavidin coupled 125I-b-suPAR bound specifically to b-RBC, but not to naive RBC, with a loading capacity approaching ~105 b-suPAR molecules per cell (SA/b-RBC; Fig.3A). The resultant b-suPAR/SA/b-RBC complex (indicated hereafter as RBC/suPAR) bound 125I-scuPA specifically, whereas binding to naive RBC was not observed (Fig.3B).
Figure 3. Coupling of b-suPAR to b-RBC and subsequent loading of scuPA on RBC/suPAR complex.
Panel A: binding of 125I-labeled b-suPAR to biotinylated RBC coated with streptavidin (circles, upper curve) or naïve RBC (squares, lower curve). Panel B: binding of 125I-labeled scuPA to RBC/suPAR or naïve RBC (same symbols).
Circulation of suPAR/RBC in rats
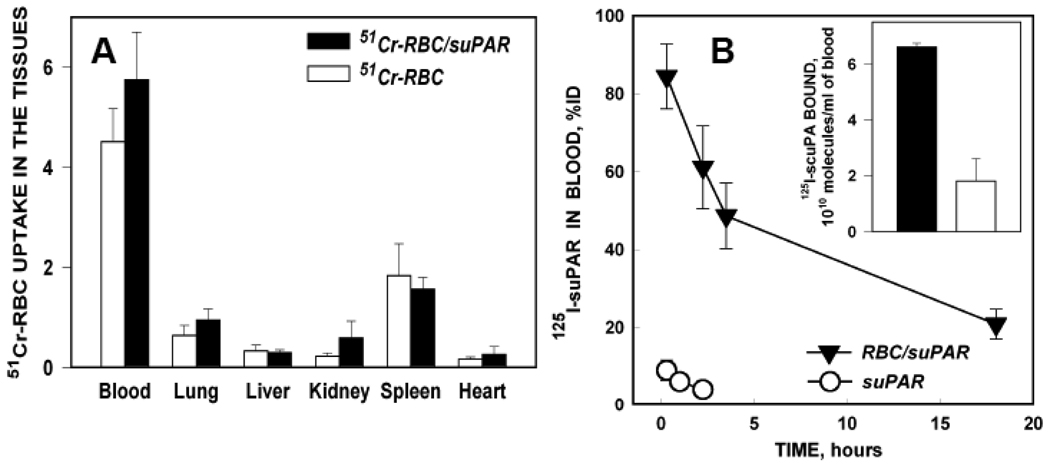
RBC induced to carry up to 5×104 molecules of tPA or IgG per cell using this approach circulate in animals with a life-span comparable to naïve RBC11,12,20. In agreement with these previous observations utilizing prototype cargoes, the distribution of 51Cr-RBC/suPAR among the major organs after iv injection in rats was also similar to naïve 51Cr-labeled RBC (Fig. 4A). Of note, the proportion of labeled RBC in the blood approached 80% of the injected dose, and uptake in the liver, spleen and kidneys was not enhanced, indicating that coupling of suPAR to the carrier RBC did not accelerate their elimination (Fig. 4A). Nor was 51Cr-RBC/suPAR retained preferentially in the lungs, indicating an ability to traverse the extensive capillary network found in that organ.
Figure 4. Biodistribution, circulation and functional activity of RBC/suPAR in vivo.
Biodistribution of 51Cr-labeled naïve washed rat RBC (open bars) or RBC/suPAR (closed bars) one hour after iv injection in rats (A). Kinetics of free (open circles) or RBC conjugated 125I-labeled b-suPAR (closed triangles) in blood after iv injection in anesthetized rats. Inset shows binding of 125I-scuPA to RBC obtained from rats injected with RBC/suPAR (closed bars) vs RBC (open bars).
On the other hand, coupling suPAR to RBC markedly prolonged its circulation. Within 15 min after iv injection, <10% of injected dose of 125I-suPAR remained in blood, whereas ~50% of injected RBC/125I-suPAR was detected 3 hours after iv injection (Fig. 4B). Even 18 hours after injection, approximately 90% of 125Iodine recovered in blood samples from rats injected with RBC/125I-suPAR was associated with the RBC pellet (not shown).
Circulating RBC/suPAR retained its ability to bind scuPA. RBC obtained from rats injected with RBC/suPAR bound three times more scuPA than RBC obtained from controls (Fig.4B, inset). Using a comparative analysis of binding radiolabeled scuPA ex vivo vs in vitro, as described in the Methods, we found that fresh RBC/suPAR mixed in blood bound approximately 2×104 scuPA molecules per RBC, whereas circulating RBC/suPAR bound approximately 1.5×104 molecules of scuPA per RBC. Thus, circulating RBC/suPAR retained approximately 75% of its original scuPA-binding capacity.
RBC/suPAR carrying scuPA do not adhere to endothelial cells
Interaction of RBC/suPAR carrying scuPA with endothelial cells expressing uPAR might promote untoward RBC adhesion leading to vascular occlusion or activate potentially injurious procoagulant or inflammatory processes. To address this issue, we measured endothelial adhesion of RBC carrying directly conjugated scuPA vs scuPA anchored to RBC/suPAR.
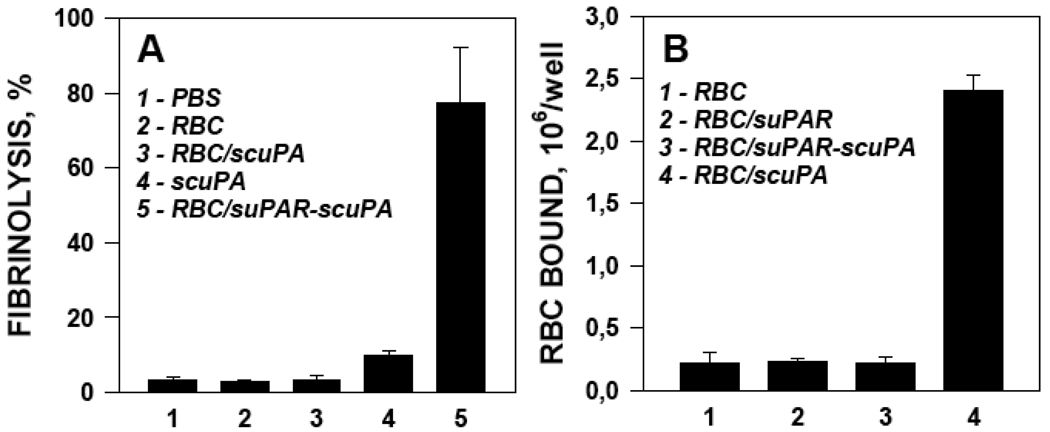
We found that binding of RBC/suPAR to human endothelial cells was comparable to naïve RBC (Fig.5A). This result agrees with the outcome of the prior in vivo studies that revealed no excess retention of RBC/suPAR in the lungs, i.e., the organ with the most extensive vasculature (Fig. 4A). RBC/scuPA bound to endothelial cells, presumably via interaction with endothelial uPAR (Fig. 5A), whereas adhesion of RBC/suPAR carrying scuPA was comparable to naïve RBC. Therefore, coupling scuPA to RBC via conjugated suPAR abolished its affinity for endothelial cells, likely by occupying the uPAR binding site.
Figure 5. Binding of scuPA to RBC/suPAR alters its functional profile.
Binding of RBC carrying indicated proteins to HUVEC (A). Lysis of fibrin clots by RBC carrying indicated proteins or by free scuPA (B).
Binding of latent scuPA to RBC/suPAR generates an active fibrinolytic complex, RBC/suPAR-scuPA
We then measured the fibrinolytic activity of scuPA coupled to RBC directly or to RBC/suPAR by detection of dissolution of fibrin clots, as described in Methods. Addition of naïve RBC to fibrin clots did not enhance the rate of spontaneous lysis (3–5% fibrinolysis at 1 hour) (Fig.5B). Of note, scuPA coupled directly to RBC also failed to augment fibrinolysis, whereas soluble scuPA caused a modest, approximately 10% increase in fibrinolysis (p<0.05 vs PBS, naïve RBS and RBC/scuPA). Most likely, scuPA diffuses more freely through the fibrin meshwork than RBC/scuPA and is more susceptible to activation by trace amounts of plasmin formed on the clot surface. In contrast, RBC/suPAR-scuPA caused approximately 80% fibrinolysis, although its diffusion in fibrin meshwork is likely impeded to the same extent as RBC/scuPA.
DISCUSSION
RBC represent potential biocompatible carriers for intravascular drug delivery22. Coupling therapeutics to RBC can dramatically enhance their bioavailability in the blood by prolonging their circulation and reducing the propensity to cause side effects in tissues23–26. RBC are far larger and show greater intravascular retention, i.e. longer lifespan in the circulation, compared with liposomes27, albumin, lipoproteins28, polymers and other carriers that might be useful for therapeutic drug delivery29. Based on its capacity to restrict extravasation into tissue, drugs that act within the blood are good candidates for vascular delivery using RBC.
Recent studies in various animal models establish that carriage on RBC provides the potential to convert plasminogen activators from problematic therapeutics into safe and effective agents for thromboprophylaxis. RBC carriage prevents the diffusion of PAs into mural hemostatic clots and tissues, yet their long time in the circulation permits inclusion of the enzymes into nascent clots at the incipient stages of formation. This strategy helps to ameliorate the daunting problem of poor diffusion of fibrinolytics into cross-linked occlusive thrombi, which restricts their therapeutic effect and demands for high and potentially toxic plasma levels to achieve fibrinolysis30. Thus, RBC carriage of fibrinolytics provides a paradigm-shifting approach for prophylactic thrombolysis.
Present study extends this paradigm by demonstrating that allowing scuPA to bind onto RBC carrying its receptor, suPAR, may offer important additional advantages. Data shown in this paper indicate in vivo RBC/suPAR complexes show biodistribution and blood level similar to those of control unmodified RBC (Fig.4), bind latent scuPA (Fig.3& 4), and thereby alter its functional profile. In particular, scuPA bound to RBC/suPAR does not bind to endothelium, but exerts plasminogen activator activity and causes fibrinolysis (Fig.5).
Urokinase is synthesized by many cell types in the body, including endothelial cells, as a single chain 54 kD protein (scuPA) composed of several domains including the amino-terminal growth factor domain that binds to the urokinase receptor (uPAR), the regulatory kringle domain and the C-terminal serine protease domain 31,32. Importantly, scuPA exerts minimal plasminogen activator activity 33, until cleaved by plasmin at Leu158-Ile159 to yield a fully enzymatically active, disulfide-linked two-chain molecule (tcuPA) that, in turn, cleaves plasminogen at Arg561-Val562 to form plasmin 34,35. Plasminogen activator inhibitor PAI-1 rapidly binds to tcuPA with high affinity, thereby “irreversibly” inactivating tcuPA, whereas PAI-1 binding to scuPA is of lower affinity and reversible 36.
Several groups, including our own, have reported that binding of scuPA to its cellular receptor (uPAR) or to recombinant soluble uPAR (suPAR) generates catalytic activity 37–39 . Of note, scuPA/suPAR is relatively resistant to PAI-1 in contrast to tcuPA or tcuPA/suPAR 16,17 and thereby exerts greater fibrinolytic activity in vitro and in vivo at sites where PAI-1 levels are increased by cell damage and inflammation, among other processes 40. Further, the kringle domains of plasminogen interact with the scuPA/suPAR complex, thereby restraining its activity in plasma under physiological conditions 41. Fibrin not only reverses plasminogen-mediated regulation of scuPA/suPAR 41 but augments enzymatic activity of pro-urokinase 42, helping to explain why scuPA/suPAR is enzymatically active against nascent fibrin clots and offers a mechanism for site-selective activation of long-circulating scuPA-suPAR formulations, such as RBC-carried complex.
These features of scuPA/suPAR complex are attractive from the standpoint of enhancing its therapeutic potency by RBC carriage. In theory, an interaction between RBC-bound scuPA and uPAR expressed on vascular cells could lead to such side effects as abnormal vasoreactivity, permeability and inflammation 14,18,43,44. Thus, scuPA directly bound to RBC increases adhesion endothelium without expressing appreciable fibrinolytic activity (Fig. 5). In contrast, scuPA bound to RBC/suPAR is fibrinolytically active and does not show untoward adhesion to endothelium (Fig. 5). The most likely explanation for this outcome is that anchoring to RBC/suPAR blocks scuPA’s ability to bind with high affinity to its endothelial uPAR counterpart.
In analysis of the physiological and potential therapeutic relevance of this study, it should be noted that the hemodynamic factors play a very important role in the mechanisms of thrombosis45,46, and, presumably, in delivery and effects of RBC-coupled PAs in vivo and in thromboses. Analysis of the literature suggests that hemodynamic factors, propelling RBC towards the blood mainstream47–49, are favorable in terms of safety, as this distribution in the flow restricts drug contact with vascular walls and mural hemostatic clots, thereby minimizing adverse effects of drug interaction with endothelial receptors and hemorrhages. We have studied effect of flow on dissolution of clots by RBC/PA in vitro and in vivo, and demonstrated that RBC/PA possess an affinity to fibrin and effectively dissolve nascent fibrin clots in both model systems21. However, in the envisioned applications, the concern whether affinity of adhesion of RBC/PA complex to fibrin clots is sufficient to produce fibrinolysis seems less relevant, because RBC/PA will be used in prophylactic regimen, in which drug delivery is driven not by fibrin affinity, but rather passive inclusion of drug-loaded RBC into nascent pathological clots forming within the vascular lumen, providing their expedited dissolution from within. Animal experiments in mice employing different types of intravascular clots formed in the pulmonary microvasculature11, large arteries (carotid)11,50 and in the cerebral vasculature13 documented that RBC/PA provides a very effective and safe prophylactic fibrinolysis in all these settings. Therefore, several studies unequivocally verified intended fibrinolytic effect of RBC/PA under physiological flow conditions in vitro and in vivo.
Theoretically, this new paradigm of drug delivery may be used to provide prophylactic thrombolysis by injecting either fully preloaded RBC/suPAR-scuPA complex, or RBC/suPAR and scuPA separately. Ex vivo conjugation to RBC, such as described in this paper has the potential to be useful in clinical settings where transfusion is common and acceptable. However, we realize practical limitations associated with this approach for our strategy, such as danger of blood-transmitted infections and limited shelf-life of the drug, typical of all hemotransfusion approaches. To solve this concern, RBC can also be invested with uPA binding capacity by injecting suPAR conjugated with antigen-binding fragments of antibodies that recognize circulating RBC50 as a more universal and practical alternative for clinical administration. We designed, produced and currently test in animals a series of recombinant proteins combining mutant forms of plasminogen activators including uPA fused with antigen-binding fragments of antibodies permitting safe loading of these drugs on circulating RBC. Our pilot data demonstrate that loading of these murine fusion proteins onto circulating RBC affords a very effective and safe prophylactic thrombolysis in mice. We believe that this approach, utilizing a recombinant biotherapeutics that can be stored in a lyophilized form for a prolonged time and does not require ex vivo loading onto RBC, will greatly enhance practical utility of RBC-PA strategy. Thus, molecular design of RBC-based thromboprophylaxis systems holds the potential to help optimize treatment of patients in clinical settings associated with a high propensity for intravascular thrombosis.
Acknowledgements
Authors thank Dr. Elena Atochina (University of Pennsylvania) for help in animal experiments. This study was supported by HL076406, NS053410, HD57355, HL081864, PENN Institute for Translational Medicine and Therapeutics (DC), HL66442, HL090697 and PENN Research Foundation Award (VM) and grant from Fondo de Investigaciones Sanitarias PI081795 (JC-M). CNIC is supported by the Spanish Ministry of Health and Consumer Affairs and the Pro-CNIC Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Motlekar NA, Youan BB. The quest for non-invasive delivery of bioactive macromolecules: a focus on heparins. J Control Release. 2006;113:91–101. doi: 10.1016/j.jconrel.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin ME, Kloecker GH, Laber DA. Argatroban for anticoagulation during cardiac surgery. Eur J Haematol. 2007;78:161–166. doi: 10.1111/j.1600-0609.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 3.Spyropoulos AC. Bridging of oral anticoagulation therapy for invasive procedures. Curr Hematol Rep. 2005;4:405–413. [PubMed] [Google Scholar]
- 4.Selim M. Perioperative stroke. N Engl J Med. 2007;356:706–713. doi: 10.1056/NEJMra062668. [DOI] [PubMed] [Google Scholar]
- 5.Hertfelder HJ, Bos M, Weber D, Winkler K, Hanfland P, Preusse CJ. Perioperative monitoring of primary and secondary hemostasis in coronary artery bypass grafting. Semin Thromb Hemost. 2005;31:426–440. doi: 10.1055/s-2005-916678. [DOI] [PubMed] [Google Scholar]
- 6.Zhang RL, Zhang L, Jiang Q, Zhang ZG, Goussev A, Chopp M. Postischemic intracarotid treatment with TNK-tPA reduces infarct volume and improves neurological deficits in embolic stroke in the unanesthetized rat. Brain Res. 2000;878(1–2):64–71. doi: 10.1016/s0006-8993(00)02693-7. [DOI] [PubMed] [Google Scholar]
- 7.Reed GL, Houng AK, Liu L, et al. A catalytic switch and the conversion of streptokinase to a fibrin-targeted plasminogen activator. Proc Natl Acad Sci U S A. 1999;96:8879–8883. doi: 10.1073/pnas.96.16.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang VC, Naik SS, Song H, Dombkowski AA, Crippen G, Liang JF. Construction and characterization of a t-PA mutant for use in ATTEMPTS: a drug delivery system for achieving targeted thrombolysis. J Control Release. 2005;110:164–176. doi: 10.1016/j.jconrel.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Cheng MB, Wang JC, Li YH, et al. Characterization of water-in-oil microemulsion for oral delivery of earthworm fibrinolytic enzyme. J Control Release. 2008;129:41–48. doi: 10.1016/j.jconrel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J Control Release. 2006;114:89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21:891–896. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 12.Ganguly K, Krasik T, Medinilla S, et al. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther. 2005;312:1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 13.Danielyan K, Ganguly K, Ding BS, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118:1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nassar T, Haj-Yehia A, Akkawi S, et al. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem. 2002;277:40499–40504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- 15.Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AA. LRP and alphavbeta3 mediate tPA activation of smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1351–1359. doi: 10.1152/ajpheart.01042.2005. [DOI] [PubMed] [Google Scholar]
- 16.Higazi AA, Mazar A, Wang J, et al. Single-chain urokinase-type plasminogen activator bound to its receptor is relatively resistant to plasminogen activator inhibitor type 1. Blood. 1996;87:3545–3549. [PubMed] [Google Scholar]
- 17.Bdeir K, Kuo A, Sachais BS, et al. The kringle stabilizes urokinase binding to the urokinase receptor. Blood. 2003;102:3600–3608. doi: 10.1182/blood-2003-03-0949. [DOI] [PubMed] [Google Scholar]
- 18.Haj-Yehia A, Nassar T, Sachais BS, et al. Urokinase-derived peptides regulate vascular smooth muscle contraction in vitro and in vivo. Faseb J. 2000;14:1411–1422. doi: 10.1096/fj.14.10.1411. [DOI] [PubMed] [Google Scholar]
- 19.al-Roof Higazi A, Aceto JF, Kniss D, et al. Unesterified long chain fatty acids inhibit the binding of single chain urokinase to the urokinase receptor. Biochemistry. 1996;35:6884–6890. doi: 10.1021/bi9514774. [DOI] [PubMed] [Google Scholar]
- 20.Muzykantov VR, Murciano JC, Taylor RP, Atochina EN, Herraez A. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal Biochem. 1996;241:109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]
- 21.Ganguly K, Goel MS, Krasik T, et al. Fibrin affinity of erythrocyte-coupled tissue-type plasminogen activators endures hemodynamic forces and enhances fibrinolysis in vivo. J Pharmacol Exp Ther. 2006;316:1130–1136. doi: 10.1124/jpet.105.093450. [DOI] [PubMed] [Google Scholar]
- 22.Magnani M, Rossi L, Fraternale A, et al. Erythrocyte-mediated delivery of drugs, peptides and modified oligonucleotides. Gene Ther. 2002;9:749–751. doi: 10.1038/sj.gt.3301758. [DOI] [PubMed] [Google Scholar]
- 23.Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100:111–119. doi: 10.1016/j.jconrel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118:145–160. doi: 10.1016/j.jconrel.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Millan CG, Marinero ML, Castaneda AZ, Lanao JM. Drug, enzyme and peptide delivery using erythrocytes as carriers. J Control Release. 2004;95:27–49. doi: 10.1016/j.jconrel.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Chambers E, Mitragotri S. Long circulating nanoparticles via adhesion on red blood cells: mechanism and extended circulation. Exp Biol Med (Maywood) 2007;232:958–966. [PubMed] [Google Scholar]
- 27.Gupta AS, Huang G, Lestini BJ, Sagnella S, Kottke-Marchant K, Marchant RE. RGD-modified liposomes targeted to activated platelets as a potential vascular drug delivery system. Thromb Haemost. 2005;93:106–114. doi: 10.1160/TH04-06-0340. [DOI] [PubMed] [Google Scholar]
- 28.Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Control Release. 2007;124:163–171. doi: 10.1016/j.jconrel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Elbayoumi TA, Torchilin VP. Liposomes for targeted delivery of antithrombotic drugs. Expert Opin Drug Deliv. 2008;5:1185–1198. doi: 10.1517/17425240802497457. [DOI] [PubMed] [Google Scholar]
- 30.Sakharov DV, Rijken DC. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92:1883–1890. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- 31.Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. Faseb J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 32.Rabbani SA, Mazar AP, Bernier SM, et al. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J Biol Chem. 1992;267:14151–14156. [PubMed] [Google Scholar]
- 33.Husain SS. Single-chain urokinase-type plasminogen activator does not possess measurable intrinsic amidolytic or plasminogen activator activities. Biochemistry. 1991;30:5797–5805. doi: 10.1021/bi00237a024. [DOI] [PubMed] [Google Scholar]
- 34.Urano T, de Serrano VS, Gaffney PJ, Castellino FJ. The activation of human [Glu1]plasminogen by human single-chain urokinase. Arch Biochem Biophys. 1988;264:222–230. doi: 10.1016/0003-9861(88)90588-7. [DOI] [PubMed] [Google Scholar]
- 35.Pannell R, Gurewich V. Activation of plasminogen by single-chain urokinase or by two-chain urokinase--a demonstration that single-chain urokinase has a low catalytic activity (pro-urokinase) Blood. 1987;69:22–26. [PubMed] [Google Scholar]
- 36.Zhang L, Strickland DK, Cines DB, Higazi AA. Regulation of single chain urokinase binding, internalization, and degradation by a plasminogen activator inhibitor 1-derived peptide. J Biol Chem. 1997;272:27053–27057. doi: 10.1074/jbc.272.43.27053. [DOI] [PubMed] [Google Scholar]
- 37.Higazi A, Cohen RL, Henkin J, Kniss D, Schwartz BS, Cines DB. Enhancement of the enzymatic activity of single-chain urokinase plasminogen activator by soluble urokinase receptor. J Biol Chem. 1995;270:17375–17380. doi: 10.1074/jbc.270.29.17375. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Mazar A, Quan N, Schneider A, Henkin J. Plasminogen activation by pro-urokinase in complex with its receptor--dependence on a tripeptide (Spectrozyme plasmin) Eur J Biochem. 1997;247:256–261. doi: 10.1111/j.1432-1033.1997.00256.x. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz BS, Espana F. Two distinct urokinase-serpin interactions regulate the initiation of cell surface-associated plasminogen activation. J Biol Chem. 1999;274:15278–15283. doi: 10.1074/jbc.274.21.15278. [DOI] [PubMed] [Google Scholar]
- 40.Higazi AA, Bdeir K, Hiss E, et al. Lysis of plasma clots by urokinase-soluble urokinase receptor complexes. Blood. 1998;92:2075–2083. [PubMed] [Google Scholar]
- 41.Higazi AA, Ajawi F, Akkawi S, Hess E, Kuo A, Cines DB. Regulation of the single-chain urokinase-urokinase receptor complex activity by plasminogen and fibrin: novel mechanism of fibrin specificity. Blood. 2005;105:1021–1028. doi: 10.1182/blood-2004-03-0995. [DOI] [PubMed] [Google Scholar]
- 42.Liu JN, Gurewich V. Fragment E-2 from fibrin substantially enhances pro-urokinase-induced Glu-plasminogen activation. A kinetic study using the plasmin-resistant mutant pro-urokinase Ala-158-rpro-UK. Biochemistry. 1992;31:6311–6317. doi: 10.1021/bi00142a021. [DOI] [PubMed] [Google Scholar]
- 43.Gyetko MR, Chen GH, McDonald RA, et al. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans. A murine transgenic model. J Clin Invest. 1996;97:1818–1826. doi: 10.1172/JCI118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen TL, Yong K, Pedersen JO, Hansen NE, Dano K, Plesner T. Impaired migration in vitro of neutrophils from patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1996;95:45–51. [PubMed] [Google Scholar]
- 45.Sagnella S, Kwok J, Marchant RE, Kottke-Marchant K. Shear-induced platelet activation and adhesion on human pulmonary artery endothelial cells seeded onto hydrophilic polymers. J Biomed Mater Res. 2001;57:419–431. doi: 10.1002/1097-4636(20011205)57:3<419::aid-jbm1185>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 46.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 47.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann Biomed Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 48.Graham DA, Huang TC, Keyt BA, Alevriadou BR. Real-time measurement of lysis of mural platelet deposits by fibrinolytic agents under arterial flow. Ann Biomed Eng. 1998;26:712–724. doi: 10.1114/1.46. [DOI] [PubMed] [Google Scholar]
- 49.Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev. 1998;33:141–164. doi: 10.1016/s0169-409x(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 50.Zaitsev S, Danielyan K, Murciano JC, et al. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108:1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]