Abstract
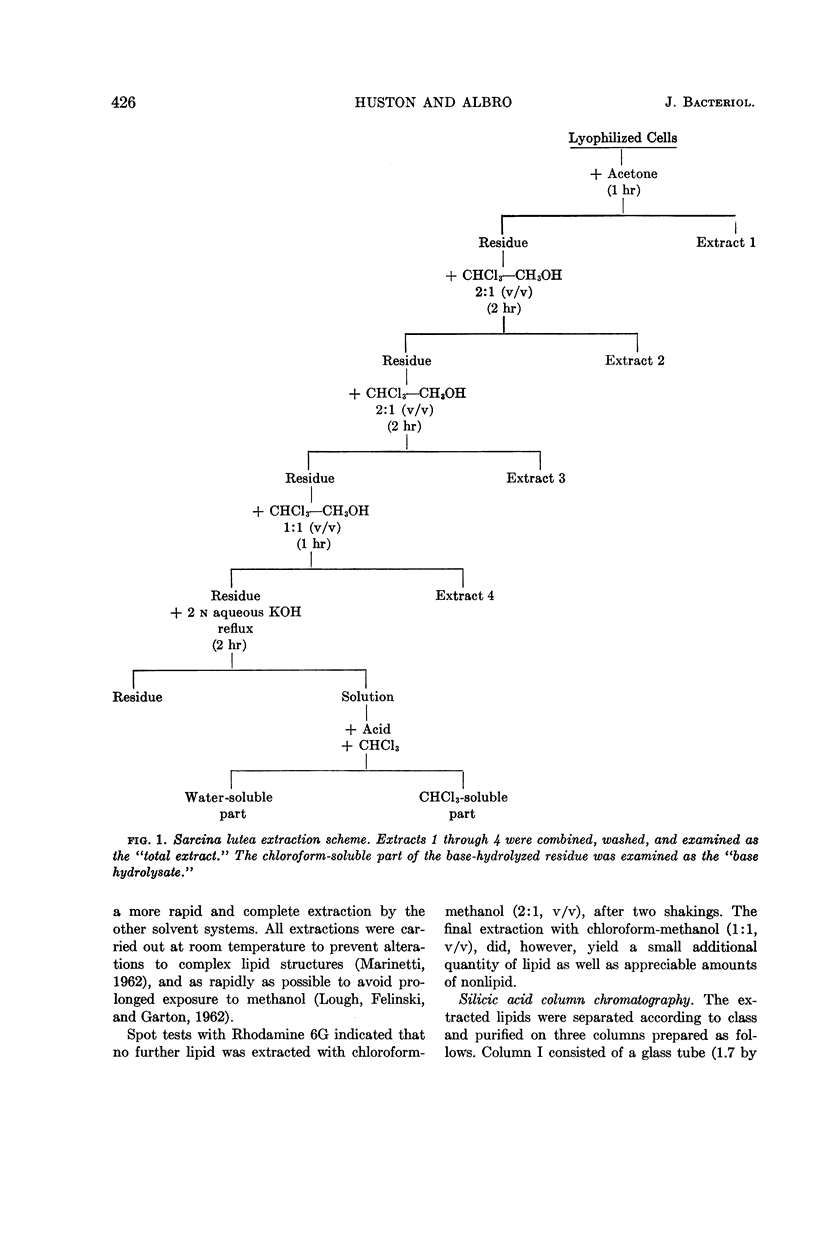
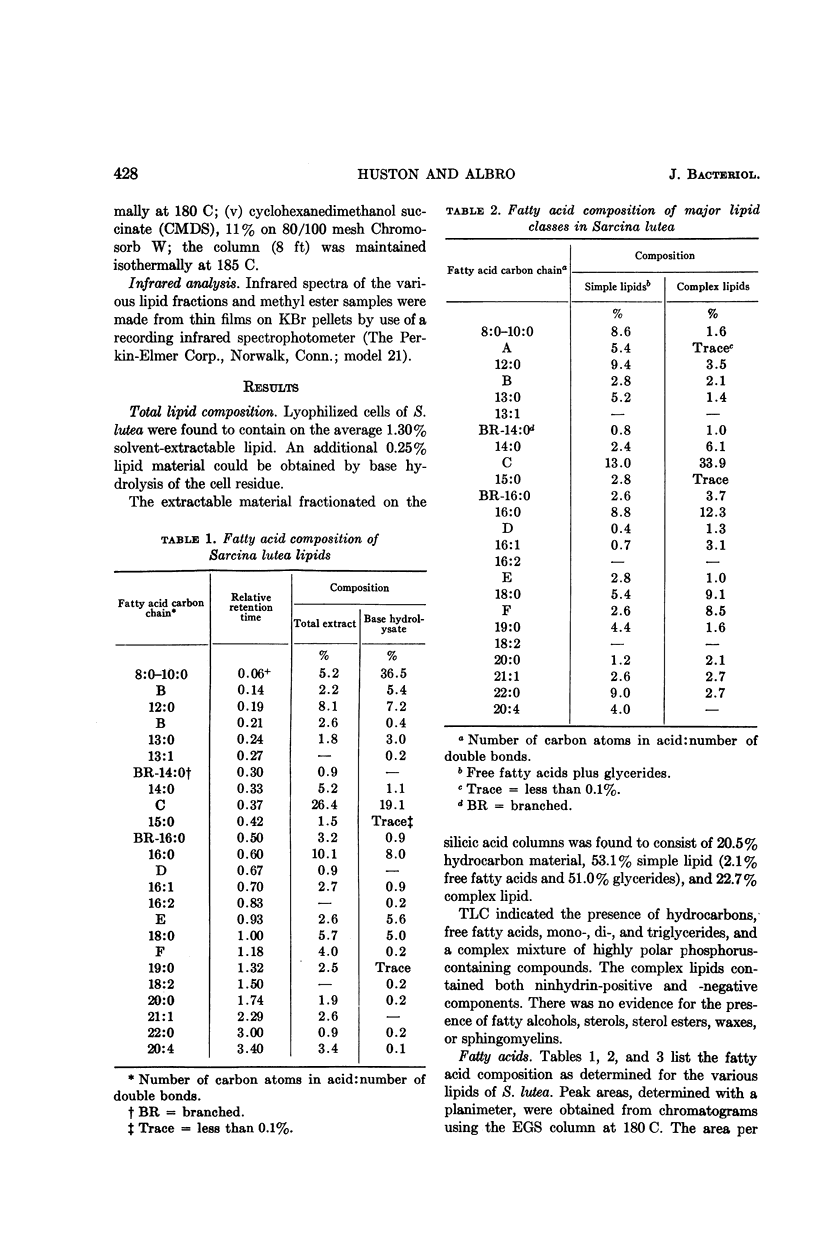
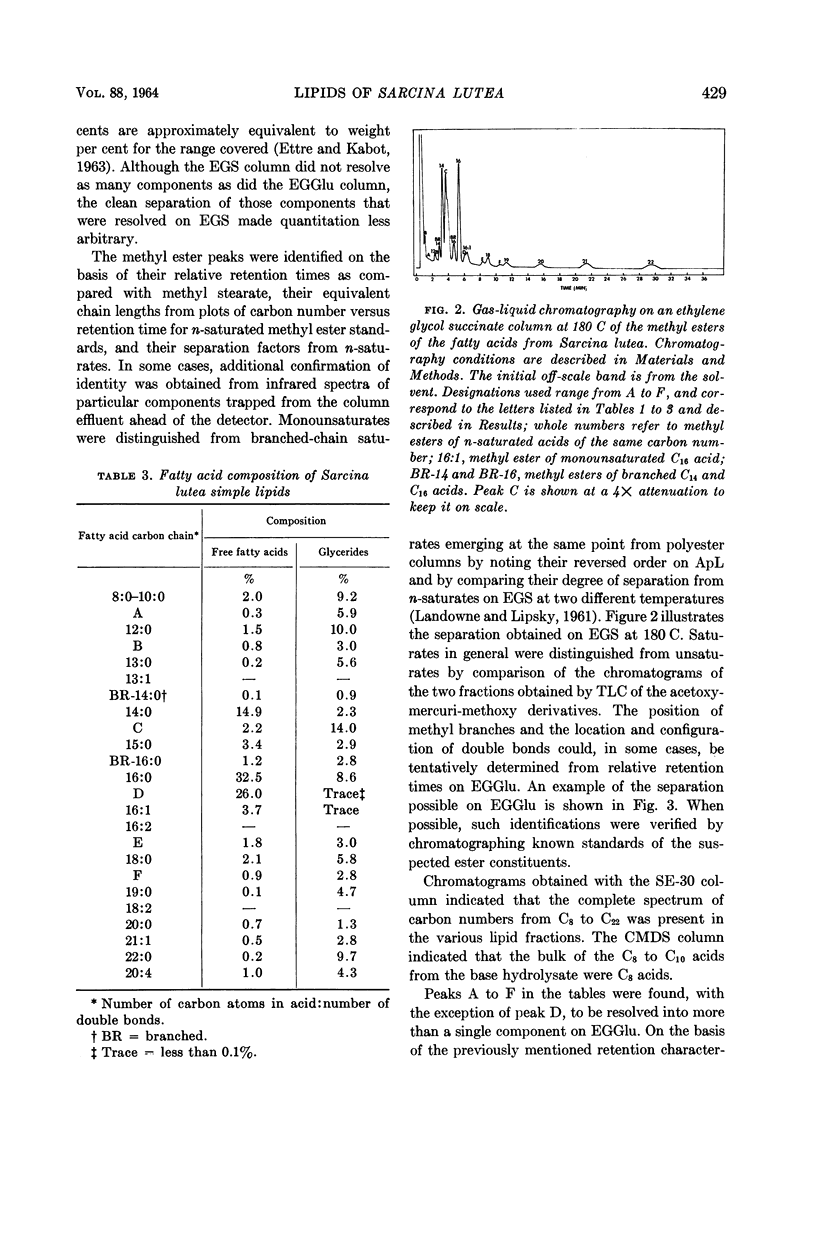
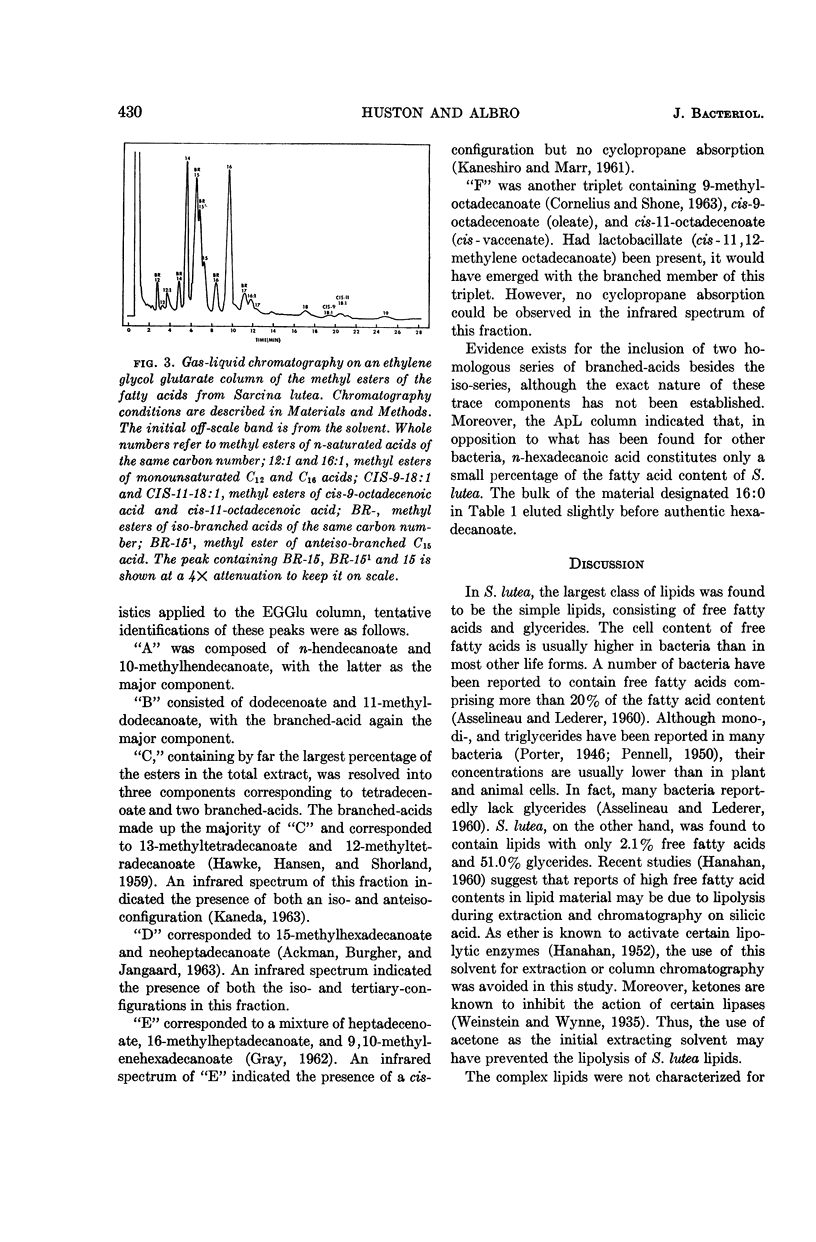
Huston, Charles K. (U.S. Army Biological Laboratories, Fort Detrick, Frederick, Md.), and Phillip W. Albro. Lipids of Sarcina lutea. I. Fatty acid composition of the extractable lipids. J. Bacteriol. 88:425–432. 1964.—The extractable lipids of Sarcina lutea were separated into several fractions by a combination of column and thin-layer chromatography. Qualitative and quantitative characterization of the fatty acid content of these lipid fractions was accomplished by means of gas-liquid chromatography and infrared analyses. Of the total extract, the lipids consisted of 2.1% free fatty acids, 51.0% glycerides, and 22.7% complex lipids; they had a fatty acid content with a complete spectrum of carbon numbers from C8 to C22. The fatty acids included a large component of branched-acids in addition to the normal straight-chain acids. The branched-acids, comprising 40% of the fatty acids analyzed, constituted a homologous series of iso-acids from C12 to C19. Two 18-carbon unsaturates were found cis-9-octadecenoate and cis-11-octadecenoate. A relatively high percentage (20.5%) of the extractable material from S. lutea was found to be hydrocarbon. This material was not further characterized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKMAN R. G., BURGHER R. D., JANGAARD P. M. Systematic identification of fatty acids in the gas-liquid chromatography of fatty acid methyl esters: a preliminary survey of seal oil. Can J Biochem Physiol. 1963 Jul;41:1627–1641. [PubMed] [Google Scholar]
- AGRE C. L., CASON J. Complexity of the mixture of fatty acids from tubercle bacillus. Acids with less than twenty carbon atoms. J Biol Chem. 1959 Oct;234:2555–2559. [PubMed] [Google Scholar]
- ASSELINEAU J. [On various applications of gas phase chromatography to the study of bacterial fatty acids]. Ann Inst Pasteur (Paris) 1961 Jan;100:109–119. [PubMed] [Google Scholar]
- CASON J., TAVS P. Separation of fatty acids from tubercle bacillus by gas chromatography: identification of oleic acid. J Biol Chem. 1959 Jun;234(6):1401–1405. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GRAY G. M. The cyclopropane-ring fatty acids of Salmonella typhimurium. Biochim Biophys Acta. 1962 Nov 19;65:135–141. doi: 10.1016/0006-3002(62)90157-9. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J. The enzymatic degradation of phosphatidyl choline in diethyl ether. J Biol Chem. 1952 Mar;195(1):199–206. [PubMed] [Google Scholar]
- HOFMANN K., LIU T. Y. Lactobacillic acid biosynthesis. Biochim Biophys Acta. 1960 Jan 15;37:364–365. doi: 10.1016/0006-3002(60)90252-3. [DOI] [PubMed] [Google Scholar]
- HOFMANN K., SAX S. M. The chemical nature of the fatty acids of Lactobacillus casei. J Biol Chem. 1953 Nov;205(1):55–63. [PubMed] [Google Scholar]
- HOFMANN K., TAUSIG F. On the identity of phytomonic and lactobacillic acids; a reinvestigation of the fatty acid spectrum of Agrobacterium (Phytomonas) tumefaciens. J Biol Chem. 1955 Mar;213(1):425–432. [PubMed] [Google Scholar]
- HOFMANN K., TAUSIG F. The chemical nature of the fatty acids of a group C Streptococcus species. J Biol Chem. 1955 Mar;213(1):415–423. [PubMed] [Google Scholar]
- KANESHIRO T., MARR A. G. cis-9,10-Methylene hexadecanoic acid from the phospholipids of Escherichia coli. J Biol Chem. 1961 Oct;236:2615–2619. [PubMed] [Google Scholar]
- KATES M., KUSHNER D. J., JAMES A. T. The lipid composition of Bacillus cereus as influenced by the presence of alcohols in the culture medium. Can J Biochem Physiol. 1962 Jan;40:83–94. [PubMed] [Google Scholar]
- LANDOWNE R. A., LIPSKY S. R. A simple method for distinguishing between unsaturated and branched fatty acid isomers by gas chromatography. Biochim Biophys Acta. 1961 Mar 4;47:589–592. doi: 10.1016/0006-3002(61)90555-8. [DOI] [PubMed] [Google Scholar]
- MACLEOD P., BROWN J. P. FATTY ACID COMPOSITION OF LIPIDS FROM STREPTOCOCCUS CREMORIS AND STREPTOCOCCUS LACTIS VAR. MALTIGENES. J Bacteriol. 1963 May;85:1056–1060. doi: 10.1128/jb.85.5.1056-1060.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'leary W. M. THE FATTY ACIDS OF BACTERIA. Bacteriol Rev. 1962 Dec;26(4):421–447. doi: 10.1128/br.26.4.421-447.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


