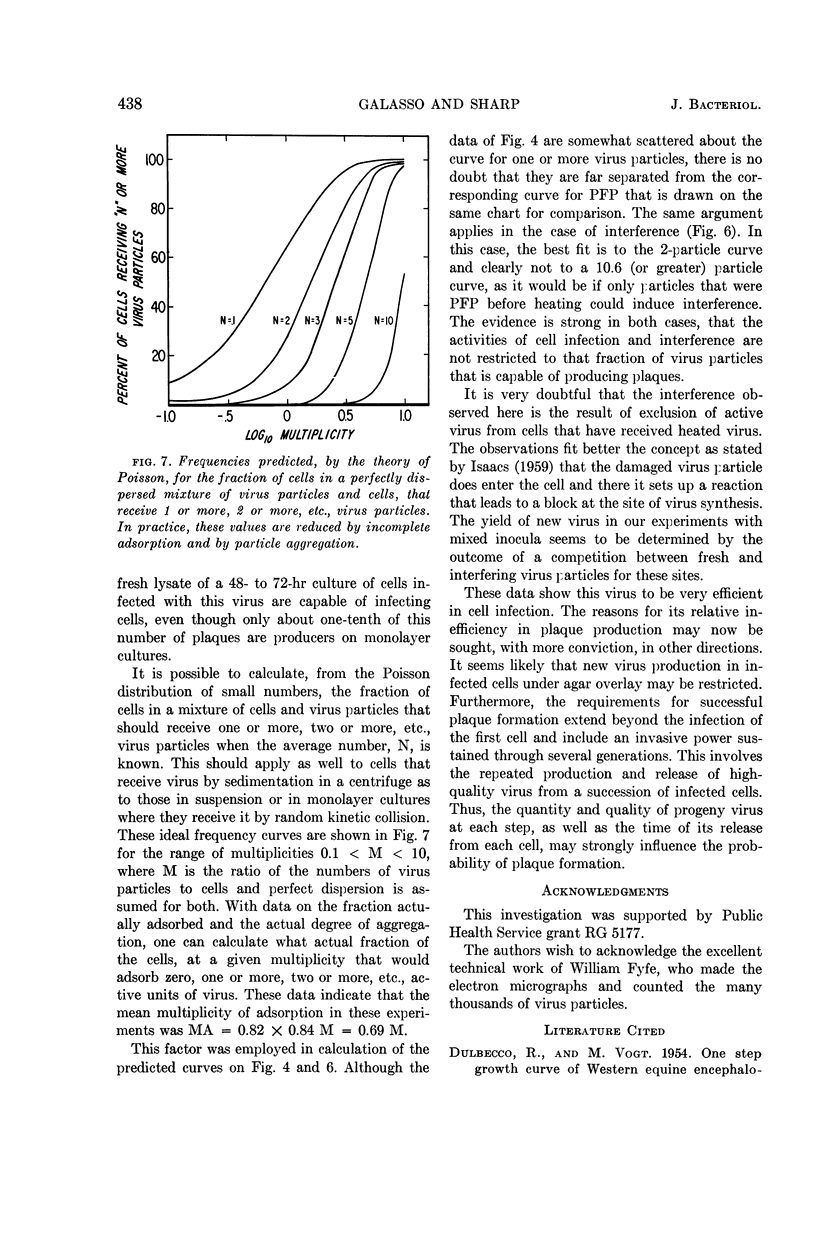
Abstract
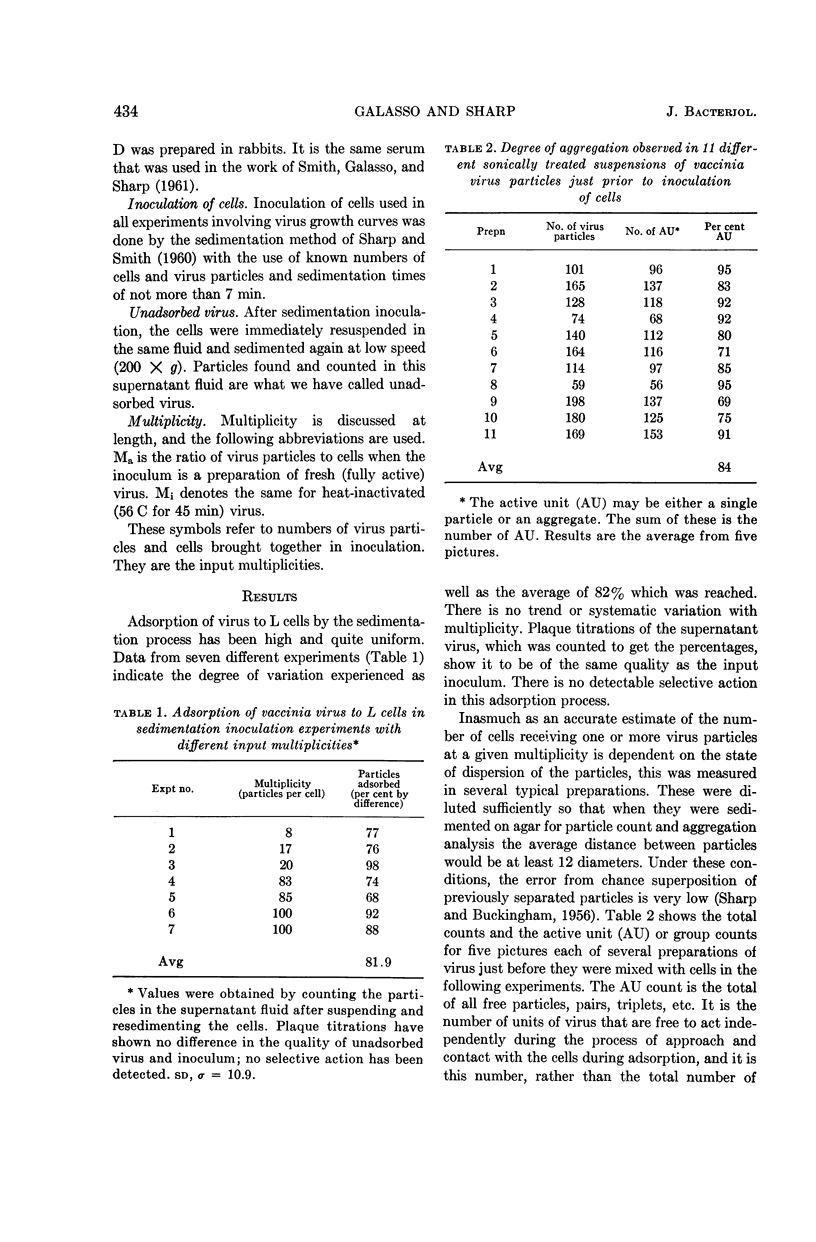
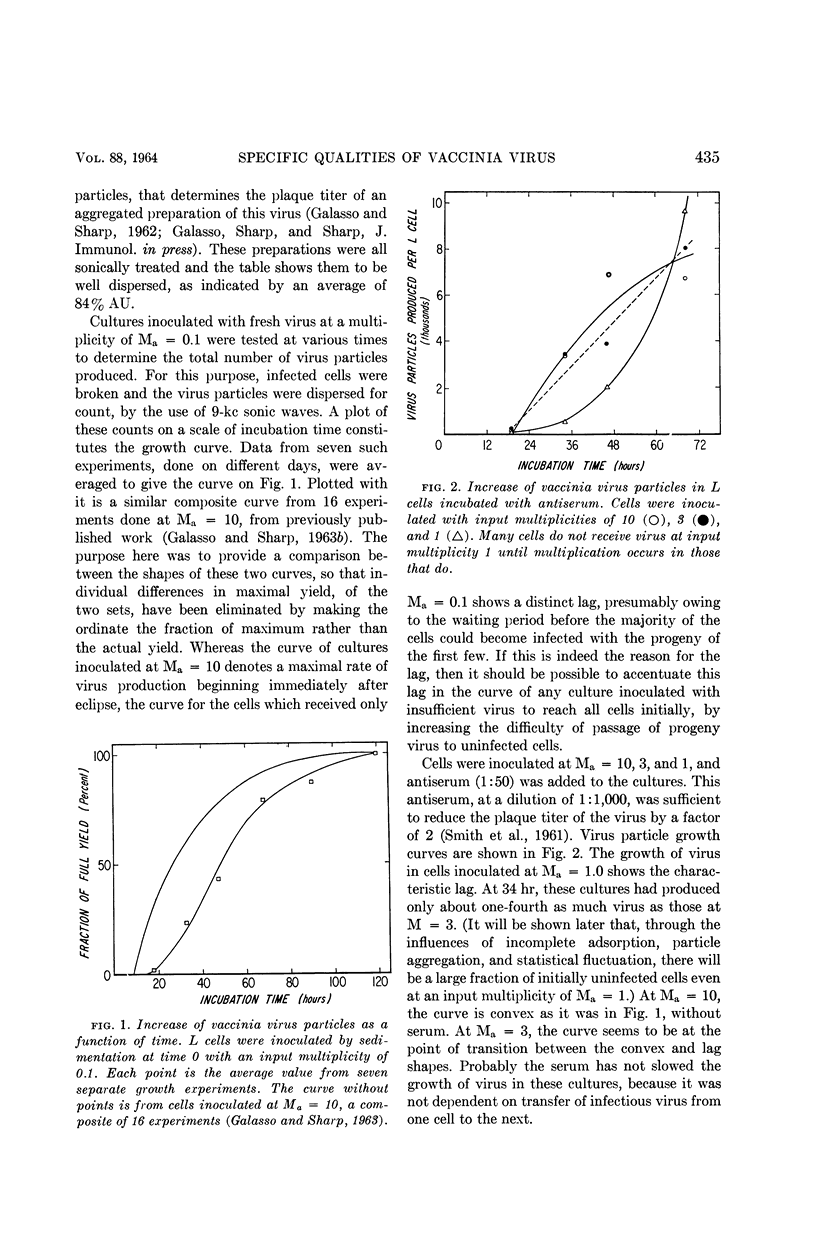
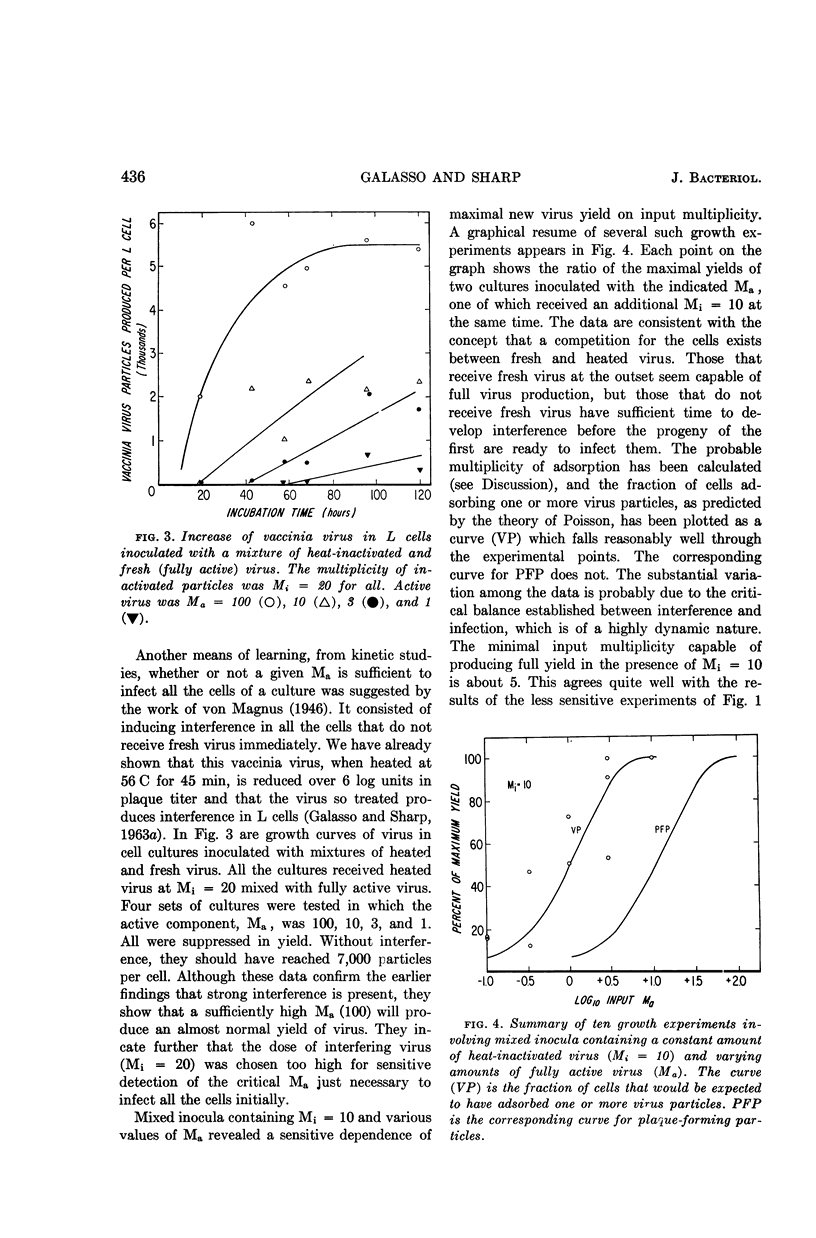
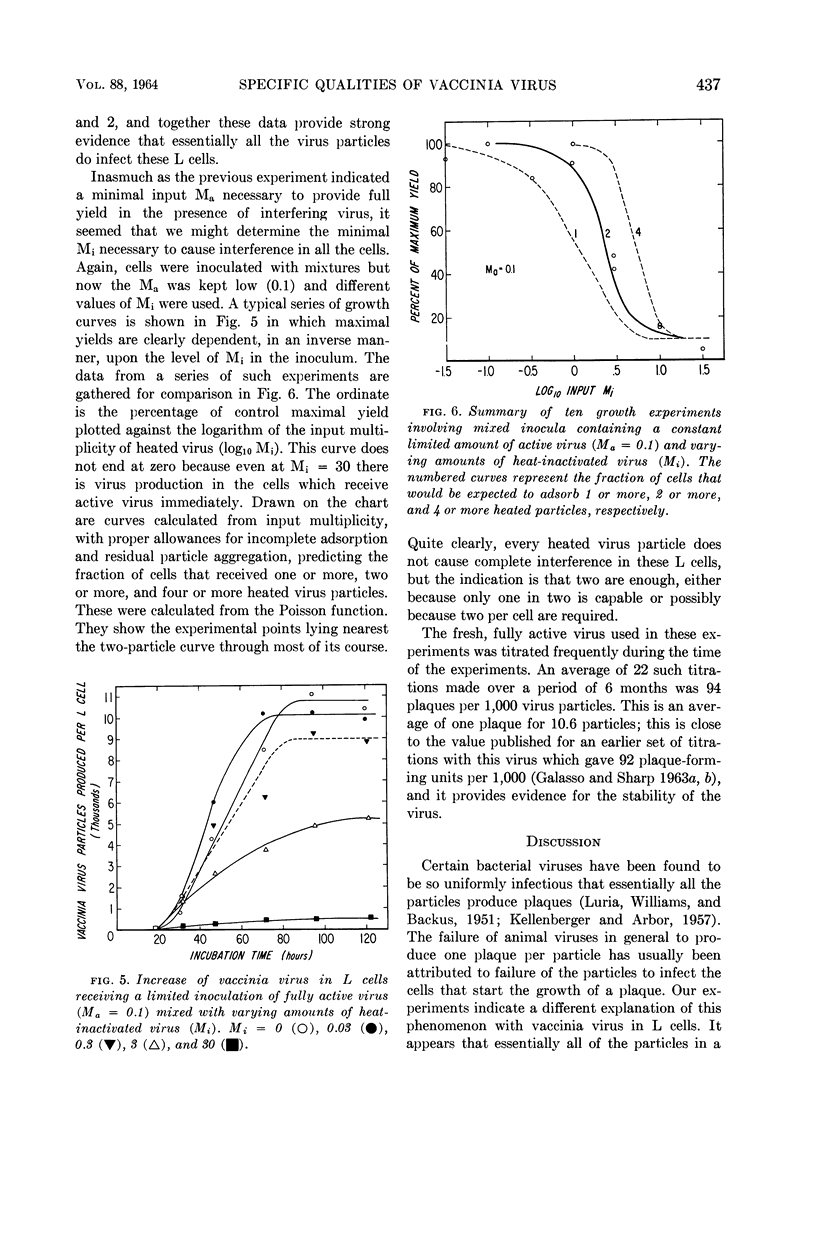
Galasso, G. J. (University of North Carolina School of Medicine, Chapel Hill), and D. G. Sharp. Relative plaque-forming, cell-infecting, and interfering qualities of vaccinia virus. J. Bacteriol. 88:433–439. 1964.—The growth of vaccinia virus in slant cultures of L cells inoculated with different multiplicities of counted particles suggests a higher incidence of cell infection than can be accounted for by the number of plaque-forming units. From cultures containing antiserum or heated virus to limit the passage of progeny to uninfected cells, the data clearly indicate the ability of all the particles to infect cells even though the plaque titer is only one-tenth of this number. Analogous experiments show that an average of two heat-inactivated (56 C, 45 min) particles induce interference in L cells. There is nothing yet to show whether the few plaque-forming particles are different from the majority or whether they are just statistically fortunate in the complex process of plaque formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Homologous inhibition with heated and ultraviolet-treated vaccinia virus in cultures of L cells. Virology. 1963 May;20:1–13. doi: 10.1016/0042-6822(63)90135-1. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Quality and yield of vaccinia virus from L cell cultures. Proc Soc Exp Biol Med. 1963 May;113:43–47. doi: 10.3181/00379727-113-28271. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Virus particle aggregation and the plaque-forming unit. J Immunol. 1962 Mar;88:339–347. [PubMed] [Google Scholar]
- ISAACS A. Particle counts and infectivity titrations for animal viruses. Adv Virus Res. 1957;4:111–158. doi: 10.1016/s0065-3527(08)60597-7. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., WILLIAMS R. C., BACKUS R. C. Electron micrographic counts of bacteriophage particles. J Bacteriol. 1951 Feb;61(2):179–188. doi: 10.1128/jb.61.2.179-188.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP D. G., BUCKINGHAM M. J. Electron microscopic measure of virus particle dispersion in suspension. Biochim Biophys Acta. 1956 Jan;19(1):13–21. doi: 10.1016/0006-3002(56)90381-x. [DOI] [PubMed] [Google Scholar]
- SHARP D. G., SMITH K. O. Rapid adsorption of vaccinia virus on tissue culture cells by centrifugal force. Proc Soc Exp Biol Med. 1960 May;104:167–169. doi: 10.3181/00379727-104-25767. [DOI] [PubMed] [Google Scholar]


