Abstract
Chronic or excessive (+)-methamphetamine (METH) use often leads to addiction and toxicity to critical organs like the brain. With medical treatment as a goal, a novel single chain variable fragment (scFv) against METH was engineered from anti-METH monoclonal antibody mAb6H4 (IgG, κ light chain, KD = 11 nM) and found to have similar ligand affinity (KD = 10 nM) and specificity as mAb6H4. The anti-METH scFv (scFv6H4) was cloned, expressed in yeast, purified and formulated as a naturally occurring mixture of monomer (~75%) and dimer (~25%). To test the in vivo efficacy of the scFv6H4, male Sprague Dawley rats (n=5) were implanted with 3-day sc osmotic pumps delivering 3.2 mg/kg/day METH. After reaching steady-state METH concentrations, an i.v. dose of scFv6H4 (36.5 mg/kg, equimolar to the METH body burden) was administered along with a [3H]-scFv6H4 tracer. Serum pharmacokinetic (PCKN) analysis of METH and [3H]-scFv6H4 showed that the scFv6H4 caused an immediate 65-fold increase in the METH concentrations and a 12-fold increase in the serum METH area under the concentration-time curve from 0–480 min after scFv6H4 administration. The scFv6H4 monomer was quickly cleared or converted to multivalent forms with an apparent t1/2λz of 5.8 min. In contrast, the larger scFv6H4 multivalent forms (dimers, trimers, etc.) showed a much longer t1/2λz (228 min), and the significantly increased METH serum molar concentrations correlated directly with scFv6H4 serum molar concentrations. Considered together these data suggested that the scFv6H4 multimers (and not the monomer) were responsible for the prolonged redistribution of METH into the serum.
Introduction
There are currently nearly 20 monoclonal antibody (mAb) medications approved by the FDA, and over 20 more in early clinical or preclinical trials (Holliger and Hudson, 2005). These medications include full length IgG mAbs; along with five mAb fragments as Fab, F(ab')2 (antigen binding fragments of IgG), or scFv (single chain variable fragment) proteins.
IgG mAbs are typically chimeric, humanized or fully human proteins and are administered to patients requiring a long-acting antagonist with minimal extravascular penetration (Bazin-Redureau et al., 1997). IgG mAb is best for this purpose since it has a terminal elimination half-life (t1/2λz) ranging from about 3–26 days. The longest t1/2λz values are usually achieved when the antibody does not bind to tissue sites and is not prematurely cleared due to antigenicity. When a short duration of action and greater extravascular penetration are needed, a significantly smaller fragment like Fab (t1/2λz ranging from 0.5–21 hrs, Trang, 1992) or scFv (t1/2λz ranging from minutes to hours, Goel et al., 2000) is used. In particular rat pharmacokinetic studies of an anti-anthrax toxin scFv report a t1/2α of ~5 min (Maynard et al., 2002). Additionally, PCKN studies of a scFv in mice report t1/2α values of 2.7 and 1 min (Pavlinkova et al., 2000; Willuda et al., 2001).
It is possible that a short-acting scFv could be used to rapidly clear the body of small molecule toxins. For example, Shelver et al. (1996) reported anti-desipramine scFv favorably altered rat serum desipramine concentrations. For these studies, a recombinant desipramine-specific scFv fragment (~27 kDa) was generated with a 15 amino acid (Gly4Ser)3 linker, using an amino terminus-VH-VL-carboxyl terminus orientation. For the preclinical test of this scFv, male rats were administered a tracer dose of [3H]-desipramine (17 nmol), followed 15 minutes later with an equimolar dose of mouse monoclonal anti-desipramine scFv. One min after scFv dosing, serum [3H]-desipramine concentrations increased 7.3-fold, while control treatment with anti-desipramine monoclonal IgG and Fab increased 13.3- and 10-fold, respectively. These antibody-induced early increases in the [3H]-desipramine serum concentrations appeared to be inversely related to the apparent volume of distribution of the three proteins (i.e., scFv > Fab > IgG). Although there were significant limitations in the study design (e.g., blood sampling was for only 15 min after antibody administration and the doses of desipramine were extremely small), these data suggest larger doses of an anti-desipramine scFv could be a helpful antidote for desipramine toxicity.
A unique property of scFv is the ability of the heavy and light chains to form non-covalent multimeric derivatives including divalent, trivalent and tetravalent proteins (Hudson and Kortt, 1999). While these monomeric and multimeric proteins are denoted by several names in the literature, for the purposes of the discussion in this manuscript we will use the terms monomeric/monovalent, and multimeric/multivalent interchangeably. These configurations are believed to result from the heavy or light chain variable regions naturally associating with the corresponding light and heavy chains (respectively) of another scFv molecule. This multimer formation in vitro is at least partially dependent on the length of linker group between heavy and light chain Fv proteins (Hudson and Kortt, 1999). Amino acid sequences linking the heavy and light chains that are >12 residues yield a predominance of monomers, while shorter linkers yield non-covalently bound multivalent scFv proteins. The in vitro ratio of monomer to multimers is often dynamic, and dependent on protein concentration and buffer conditions (Lee et al., 2002; Huang et al., 2005).
There have not been many studies elucidating the detailed pharmacokinetics of antibody fragments that target small molecules. While the effects of dimerization on in vivo tumor binding and tissue localization are reported for one scFv (Kubetzko et al., 2006), no studies have extensively examined the pharmacokinetic fate of a scFv in the presence of an antigen target that would not affect its disposition. A potential solution to this issue is to use a small molecule antigenic target like (+)-methamphetamine (METH, 151 Da), which would not be expected to affect the disposition of the much larger scFv (a ~27,000 Da protein).
In this report we describe the design, production, and functional characterization of a high affinity anti-METH scFv (designated anti-METH scFv6H4). We also report in vivo pharmacokinetic studies in rats, which show scFv6H4 quickly and dramatically increases serum concentrations of METH over an extended period of time (>5 hrs). Data from size exclusion chromatography (SEC) showed that in serum and in the presence of METH, scFv6H4 showed time-dependent in vivo conversion of the protein from monomeric to multimeric complexes without forming aggregates. We found that both monomer and multimeric complexes were functional, but the multimeric forms appeared to be primarily responsible for the prolonged redistribution of METH into the serum. These data suggest the effectiveness of scFv could be customized for specific medical indications by altering the scFv (e.g., linker length, pegylation) to maximize naturally occurring in vivo pharmacokinetics properties of the scFv.
Methods
Chemicals and Drugs
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise noted. Enzymes and E. coli strains were purchased from Invitrogen (Carlsbad, CA). [3H]-METH ((+)-2',6'-3H(n)] methamphetamine; 23.5 Ci/mmol) labeled at two metabolically stable sites on the aromatic ring structure was obtained from the National Institute on Drug Abuse (Bethesda, MD) after synthesis at the Research Triangle Institute (Research Triangle Park, NC). Haptens S-(+)-6-[4-[2-(N-methylamino)propyl]phenyl]hexanoic acid (METH (+)P6) and S-(+)-6-[3-[2-(methylamino)propyl]phenoxy]hexanoic acid (METH (+)MO6) that were used for generating and screening anti-METH scFv were synthesized by Drs. Ivy Carroll and Philip Abraham at the Research Triangle Institute. Other METH-like drugs were also obtained from the National Institute on Drug Abuse.
ScFv6H4 cloning
The generation, characterization and sequence determination of murine mAb6H4 (IgG1, κ light chain, KD = 11 nM) was previously reported (Byrnes-Blake et al., 2003; Peterson et al., 2007). General molecular and genetic techniques used for plasmid construction and transformation of scFv6H4 are described in (Sambrook and Russell, 2001). The coding sequences for the variable light chain (VL) residues 1–108 and variable heavy chain (VH) residues 1–112 containing the six complementarity determining regions (CDRs) and seven framework regions were amplified with specific primers designed to fuse the two domains in an amino terminus-VH-VL-carboxyl terminus orientation. A 15 amino acid (Gly4Ser)3 linker was included to join the two separate gene products, and a FLAG epitope was included at the amino terminus of the VH domain (Fig 1). This His6 affinity tag was encoded at the carboxy-terminus to aid in scFv protein purification. Briefly, the VH and VL regions of mAb6H4 were PCR amplified from the mAb6H4 cDNA (Peterson et al., 2007) heavy and light chains with the following primers synthesized by Integrated DNA Technologies (Coralville, IA) in two separate PCR reactions with Pfu turbo polymerase: primer 1 VH forward (CGGAATTCCCATGGATTACAAGGATGACGAC), primer 2 VH reverse (CAGAGCCACCTCCGCCTGAACCGCCTCCACCGGAGACGGTGACTGAGGTTC), primer 3 VL forward (GGCGGAGGTGGCTCTGGCGGTGGCGGATCCCAAATTGTTCTCACCCAGTCTCC), primer 4 VL reverse (GTC AAG CTT CCC GGG AGC CCG TTT TAT TTC C). The amplification reaction was set to 30 cycles of 94°C for 15 sec, 53°C for 30 sec, 68°C for 1 min, and a final extension at 68°C for 7 min. To produce the coding sequence of the single chain antibody, the products of the two PCR reactions were combined with primers 1 and 4 in a second reaction, which linked the two domains with the coding sequence for a (Gly4Ser)3 linker. The PCR product was gel-purified using the QIAquick gel purification kit (Qiagen, Valencia, CA). The product was then restricted with enzymes EcoR I and Hind III and ligated into matching sites of cloning vector pGEM-4Z (Promega, Madison, WI). The circularized product was then transformed into E. coli strain DH5α and the sequence was checked to assure integrity of the transformed product (University of Arkansas for Medical Sciences DNA Core Sequencing Facility).
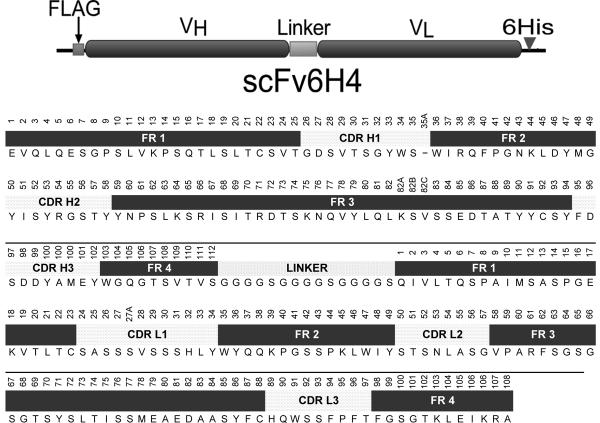
Figure 1.
Upper panel. Graphical representation of the scFv6H4 expression construct. From left to right, the amino terminus containing the FLAG epitope for protein detection, the VH and VL chains connected by a 15 amino acid (Gly4Ser)3 linker, and carboxy terminus fused to a six histidine tag for use in purification. Abbreviations are: FLAG, epitope for anti-FLAG antibody; VH, variable heavy chain region; VL, variable light chain region; and 6His, six-histidine affinity tag. Lower panel. Amino acid sequence of scFv6H4 labeled with the appropriate regions for framework and CDR residues. The sequence is in single letter amino acid notation. The Kabat numbering scheme is written above the sequence.
Subcloning and large-scale expression of anti-METH scFv6H4
For protein expression, the coding sequence of scFv6H4 was cloned into the yeast Pichia pastoris. The coding sequence was amplified by PCR with primers PP6H4.for (GCGAATTCGATTACAAGGATGACGACG) and PP6H4.rev (GCTCTAGACTAATGGTGATGGTGATGGTGGGGAGCCCGTTTT ATTTC). The PP6H4.rev primer included a coding sequence for a 6-histidine tag for use in purification after protein expression. The resulting PCR product was gel purified, restricted with EcoRI and XbaI and ligated into the plasmid pPICZα-A (Invitrogen) making the plasmid PPscFv6H4. This plasmid was transformed first into E. coli strain DH5α and then sequenced (University of Arkansas for Medical Sciences DNA Core Sequencing Facility). After this sequence confirmation, the plasmid was linearized with Sac I and used to transform P. pastoris strain GS115 (Mut+) by electroporation according to the manufacturer's instructions (Invitrogen). Potential zeocin resistant colonies were selected on YPD agar plates [1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) dextrose, and 2% agar] containing either 1 or 2 mg/ml zeocin. P. pastoris colonies that exhibited high zeocin resistance were tested for scFv expression. Briefly, colonies were picked from a freshly streaked plate and grown for 2 days in 25 ml BMGY [1% yeast extract (w/v), 2% peptone (w/v), 100 mM potassium phosphate, pH 6.0, 1.34% yeast nitrogen base (w/v), 4 × 10−5 % biotin, and 1% glycerol (v/v)] media at 28°C, the cultures were then centrifuged at 4000 × g for 15 min and resuspended in BMMY media [the same recipe as BMGY, but substitute 0.5% methanol (w/v) for glycerol] to an OD600 of 1.0. The cultures were grown at 28°C with shaking and samples were taken at 2, 24, and 72 hrs. The samples were centrifuged at 20,000 × g for 10 min and the supernatant was analyzed by SDS-PAGE and coomassie staining, or western blotting and probing with anti-His6 antibody (data not shown).
Large scale production and purification of scFv6H4
To obtain sufficient scFv6H4 for in vitro biochemical characterization and in vivo pharmacokinetic studies we expressed scFv6H4 in several large fermentation batches. Briefly described, a single P. pastoris yeast colony expressing scFv6H4 was picked from a freshly streaked YPD plate containing 100 μg/ml zeocin and used to inoculate a starter culture of 40 ml of BMGY containing 100 μg/ml zeocin. The starter culture was grown for 2 days at 30°C in a baffled flask shaken at 325 rpm. A baffled 2 L flask containing 500 ml BMGY was inoculated with 10 ml of the starter culture and incubated at 30°C for 24 hrs shaking at 325 rpm.
Fermentations were carried out in a 10 L working volume BiostatB bioreactor (Sartorius BBI, Bethlehem, PA) interfaced with Foxylogic Fermentor Control Program (v. 4.3 software, foxylogic.com). This allowed automated bioreactor control and recording of production run data. Throughout the batch and induction phases, temperature was maintained at 28°C, and a pH of 6.0 was maintained by automatic addition of a 30% solution of ammonium hydroxide. Dissolved oxygen was maintained at 30% of saturation and was controlled by a dissolved oxygen cascade of pure oxygen addition followed by an appropriate increase or decrease in the stir rate. Air supplementation was kept constant at 5 L/min. Foaming was controlled by the automatic addition of Antifoam C (Sigma, St. Louis, MO). A starting volume of 5 L of BMGY was inoculated with the 500 ml culture described above. After the initial batch phase when the original glycerol in the BMGY media was exhausted (~24 hrs), a fed batch phase was initiated to encourage cell growth. This included addition of aliquots of 50% glycerol based on dissolved oxygen levels. The fed batch phase was continued until wet cell weight reached 70 g/L; at at which point glycerol was discontinued. After all glycerol was exhausted (~6 hrs), the yeast cell culture was converted to methanol metabolism by the introduction of 50% methanol at 5 ml/hr. Methanol was then added at a rate of 10 ml/hr for the next 120 hrs. After induction, the fermentation culture pH was adjusted to 7.0 by the addition of ammonium hydroxide. The culture was centrifuged at 4000 × g and the supernatant was decanted and filtered through a 0.2 μm membrane filter. The clarified supernatant was stored at −80°C until the scFv6H4 was purified.
ScFv6H4 was purified by metal affinity chromatography using an AKTA Explorer 100 FPLC system and a 20 ml HisPrep 16/10 metal affinity column (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) in five 1 L batches. Briefly, the column was equilibrated with five column volumes of binding/wash buffer (20 mM NaPO4, pH 7.4, 500 mM NaCl, 20 mM imidazole) at 7 ml/min. The supernatant from the fermentation was loaded onto the column and the unbound sample was washed out with three column volumes of binding/wash buffer. The scFv6H4 was eluted from the column with elution buffer (binding/wash buffer plus 500 mM imidazole) and collected in 12 ml fractions. Fractions were analyzed for scFv6H4 content by SDS-PAGE. Fractions from all purification runs containing scFv6H4 were pooled, exchanged into scFv administration buffer (15 mM NaPO4, 150 mM NaCl, pH 7.4) and concentrated to 1.77 mg/ml.
[3H]-scFv6H4 was synthesized similarly to a previously reported method for radiolabeling anti-PCP Fab (McClurkan et al., 1993) using 4.4 mg of scFv6H4 and labeling with [3H]-NSP (250 μCi) (GE Healthcare, Piscataway, NJ). After labeling, [3H]-scFv6H4 was separated from unincorporated [3H]-NSP by SEC and dialysis into scFv administration buffer (see later section).
The in vitro stability of scFv6H4 was analyzed at concentrations ranging from 0.25–4.6 mg/ml of unlabeled scFv6H4. The concentration of 4.6 mg/ml was near the limit of solubility of the scFv. As for the in vivo studies, the [3H]-scFv6H4 was included as an experimental tracer. In all cases the SEC profile remained relatively constant and similar to the results shown in Fig. 2.
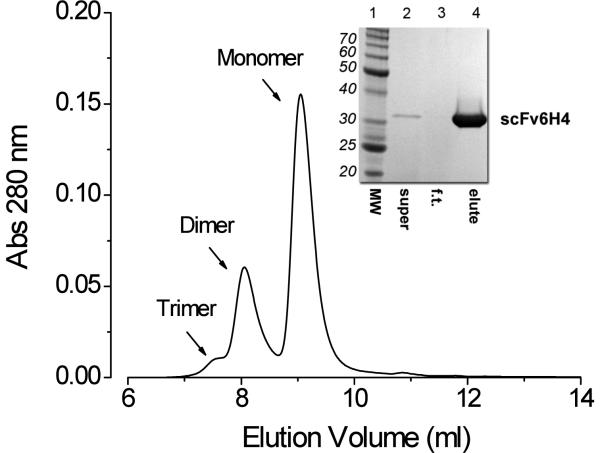
Figure 2.
Inset. Coomassie stained SDS-PAGE reducing gel of purified scFv6H4, indicating a monovalent scFv6H4 of approximately 98% purity. Graph. SEC analysis of the same purified and formulated scFv6H4. These data suggest the scFv existed in vitro as dimeric and monomeric species shown by the two prominent peaks eluted at 73 kDa and 34 kDa. There was also trace quantity of a possible trimeric species eluting at about 7.5 ml.
The monomer to multimer ratios of scFv6H4 were analyzed by SEC under three different conditions. First, the scFv6H4 was analyzed in situ, in Pichia expression media. Secondly, the purified and concentrated form was analyzed after in vitro incubation at 4°C, 25°C, and 37°C for 1 hr. Finally, 5 μl of [3H]-scFv6H4 was incubated in 200 μl of administration buffer or rat serum at 37°C for 1 hr. After the incubation periods, 20 μl of each of the samples was analyzed by SEC to determine if there were changes in monomer to dimer ratio.
Bead-based Radioimmunoassays (RIA) for determination of KD values for METH and METH-like drugs
RIA were performed similarly to the method of Owens et al. (1988) with the following changes: a 20 μl aliquot containing 100 ng of purified scFv6H4 was incubated with 50,000 DPM of [3H]-METH in the presence of varying concentrations of unlabelled METH. A 20 μl aliquot of a 50% slurry of TALON beads (Clontech, Mountain View, CA) in RIA buffer (0.05 M Tris pH 7.6, 150 mM NaCl, 2% BSA, 0.2% NaN3, 0.05% Tween-20) was added, and the final volume was adjusted to 220 μl with RIA buffer. The solutions were incubated overnight at 4°C with end-over-end rotation. The next day, the tubes were centrifuged at 1,000 × g, the beads were washed twice with 1 ml of ice-cold RIA buffer and resuspended in 2 ml scintillation fluid for quantification by liquid scintillation spectrophotometry.
Pharmacokinetic studies of METH and scFv6H4 in rats
For METH and scFv6H4 PCKN studies, adult male Sprague Dawley rats (about 250 g) were purchased from Charles River Laboratories (Raleigh, NC) with surgically implanted dual jugular vein catheters (Silastic medical-grade tubing, 0.020 in inner diameter and 0.037 in outer diameter; Dow Corning, Midland, MI) that were used for drug administration and blood sampling, respectively. Catheters were placed in the subcutaneous tissue for transport from the vendor and were kept there until the day before the first experimental procedure, when catheters were exposed under halothane anesthesia. Catheter patency was maintained by daily injection of saline containing 25 U of heparin.
Animals were housed individually in a light-controlled environment (12 hr light/dark cycle). They received water ad libitum and were fed approximately 20 g of food pellets daily, which maintained their body weights between 250–280 g. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health, and were performed with the prior approval of the Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
On day -2 of the study, pre-METH blood samples were taken and the rats were implanted with Alzet 3 day micro-osmotic pumps (Durect Corp, Cupertino, CA) delivering 3.2 mg/kg/day METH. On day -1, blood samples (200 μl) were taken at 10 am, 1 pm and 5 pm to allow determination of METH control steady-state concentrations. On day 0, the rats were given either 36.5 mg/kg anti-METH scFv6H4 at 1.77 mg/ml containing a tracer dose of 1 × 106 dpm of [3H]-scFv6H4 in antibody administration buffer, or an equal volume of buffer without scFv6H4 (controls). Immediately following administration of the scFv6H4 or buffer, blood samples (200 μl) were taken at: 1, 5, 10, 30, 60, 90, 120, 240, 480, and 1440 min. Following each blood collection, the cannula was filled with saline or heparinized saline (if the time between blood collections was greater than 1 hr). The saline was removed prior to collection of the next sample to prevent volume dilution of the blood sample.
ScFv6H4 blood to plasma ratios were determined using blood samples collected during the PCKN studies. The blood samples were collected at time points from 1–120 min from the rats. A small aliquot of whole blood was immediately used to determine the hematocrit in heparinized hematocrit tubes by standard procedures. Duplicate whole blood samples (10 μl) were then added to scintillation fluid and the [3H]-scFv6H4 concentration was determined by liquid scintillation spectrophotometry. After centrifugation of the remaining blood, duplicate 10 μl aliquots of the plasma were used to determine the [3H]-scFv6H4 concentration in plasma, as just described for the whole blood. The ratio of the concentration in the whole blood to the concentration in the plasma (the so-called blood/plasma ratio) was used to represent the distribution of scFv6H4 between red blood cells and plasma.
The concentration of [3H]-scFv6H4 in serum and urine samples was determined by SEC similar to a previous method developed in our laboratory (Proksch et al., 1998). For the analysis of [3H]-scFv6H4 protein concentrations, a TSK-GEL G2000SWxl 30 cm size exclusion column (Toso Haas, Montgomeryville, PA) was connected to a Waters HPLC system (Waters, Corp., Milford, MA) consisting of an autoinjector in series with a multi-solvent delivery system, a UV absorbance detector and a fraction collector. The Millennium software package (Waters Corp.) was used to control the HPLC system and to collect all HPLC chromatography data. The column was equilibrated with buffer (50 mM NaPO4, 100 mM NaSO4, pH 6.7) at a flow rate of 1 ml/min. The column elution profile was determined using size exclusion standards (Sigma) consisting of blue dextran (2000 kDa, to determine void volume), β-amylase (200 kDa), bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), cytochrome C (12.4 kDa), and phenol red (to determine the column inclusion volume). Samples were centrifuged at 20,000 × g for 10 min prior to chromatography to remove any precipitated material or debris. The UV Abs280 was used to detect the real-time elution of serum proteins in each sample. Careful monitoring of the profile allowed detection of any potential elution shift either related to column degradation or instrument malfunction. Additionally, reproducibility was monitored using blank serum and urine samples spiked with a known amount of [3H]-scFv6H4. These quality control scFv samples were analyzed with each batch of urine and serum samples. Liquid scintillation spectrophotometry was used to quantitate the radioactivity in each 0.25 ml HPLC fraction.
To validate our analytical method, the serum protein SEC elution profile was monitored by Abs280 for each sample. The serum profile of the rats exhibited at least four characteristic UV280 peaks, the most prominent eluting at ~8.07 min. Based on our standard curve generated with molecular weight standards, this peak was calculated to be ~67 kDa, corresponding in abundance and size to rat serum albumin. Since this protein was in the molecular weight range of scFv monomers and multimers (25 kDa to 150 kDa), this peak was used as an internal reference for elution times and, based on this, the analytical retention times were quite reliable (elution time CV,% = 0.3 for all samples n = 49). The relative concentrations of scFv6H4 monomer and dimer were calculated and plotted against the time of collection (Figs. 5 and 6). [3H]-scFv6H4 concentrations in the SEC fractions eluting after the monomer peak (at ~9 min) were considered to be small molecular weight degradation products that did not contribute to METH binding. Thus, they were not considered further.
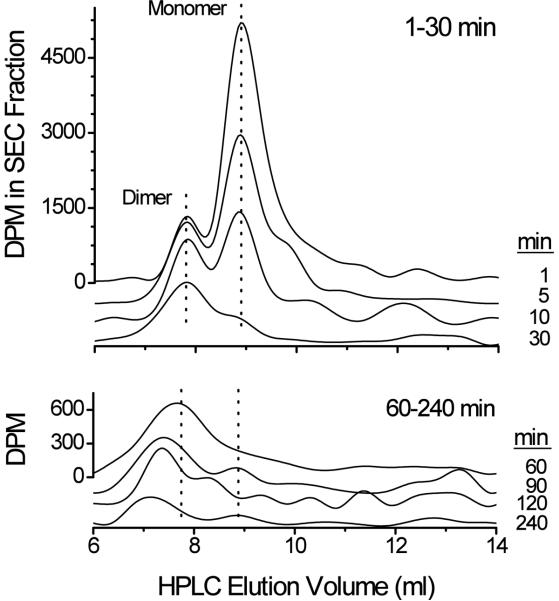
Figure 5.
A representative plot of SEC time-lapsed analysis of monomeric and multimeric forms of scFv6H4. Serum samples separated by SEC show the proportion of monomeric and multimeric [3H]-scFv6H4 at the indicated time points. Dotted lines indicate the approximate retention time for the monomeric and dimeric scFv6H4 species. Early and later serum samples are represented with different ordinate scales for clarity.
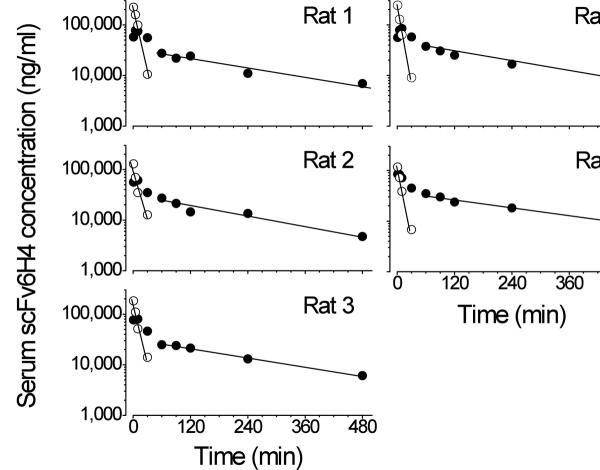
Figure 6.
Individual plots of scFv6H4 concentrations in rat serum vs. time after an iv bolus dose of anti-METH scFv6H4 (37 mg/kg) along with a tracer dose of anti-METH [3H]-scFv6H4 (1 × 106 dpm). After analysis of scFv6H4 by SEC, dpm peak heights of monomeric (open circles) and multimeric (closed circles) forms were plotted.
To determine the molar concentration of unlabeled scFv6H4 in the serum after SEC fractionation, the known amount of [3H]-scFv6H4 radioactivity in the serum or urine was converted to the relative molar concentration of scFv6H4. The calculations were based on a 1 × 106 DPM [3H]-scFv6H4 radiolabeled dose and a 36.5 mg/kg dose of unlabeled scFv6H4 per rat. For the calculation, a molecular weight of 27,000 grams/mol for scFv6H4 was assumed.
METH and AMP concentrations in serum and urine were determined by liquid chromatography-tandem mass spectrometry as previously described (Hendrickson et al., 2004).
Data Analysis
An IC50 value for METH binding to scFv6H4 was determined from the RIA [3H]-METH percent bound vs. METH concentration curve (Akera and Cheng, 1977) using the computer software program Origin (Origin Lab, Northampton, MA). Pharmacokinetic analysis was performed with the software package WinNonlin (Pharsight Corp., Mountain View, CA). PCKN parameters for scFv6H4 were derived from model-independent analysis of the concentration vs. time curves of scFv6H4 monomer and dimer. The PCKN calculations included terminal elimination rate constant (λz), terminal elimination half-life (t1/2λz), systemic clearance (CLs), volume of distribution at steady-state (VSS, calculated as a product of the mean residence time and CLs), and area under the concentration-time curve (AUC).
To assess the effect of the presence or absence of scFv6H4 on METH serum concentrations at each time point, a repeated measures two-way ANOVA statistical analysis was performed. If significant differences were found, a Tukey's post hoc test was performed. Statistical differences were set at the P < 0.05 level. All tests were performed using SigmaStat computer software (Systat Software, Jose, CA).
Results
Cloning and expression of anti-METH scFv6H4
The scFv6H4 was initially expressed in E. coli, but during purification it was found to form >90% insoluble inclusion bodies. Attempts at refolding the insoluble protein only modestly improved the yield of functional scFv6H4, thus an alternative yeast Pichia pastoris expression system was utilized.
The scFv6H4 coding sequence was ligated into a Pichia expression vector so that the coding sequence was in-frame behind the cleavable alpha-mating factor. This allowed protein secretion into the media during methanol-induced protein expression, thereby decreasing the potential for formation of insoluble protein inclusion bodies. The scFv6H4 yield after a 98 hr yeast fermentation production run and downstream purification was approximately 12.4 mg/L.
The combination of a single gene-derived protein product, a media-secreted protein and an affinity tag on the protein allowed us to purify the scFv6H4 to over 98% homogeneity in one purification step (Fig. 2, SDS-PAGE inset). Although the SDS-PAGE analysis showed a single protein band of <30 kDa, SEC analysis indicated that the purified and buffer-exchanged scFv6H4 product migrated as three peaks (Figure 2, chromatogram). When plotted against molecular weight protein standards, the apparent molecular weights for the monomer and dimer were 34 and 73 kDa. These estimated values were in agreement with actual molecular size of these two species, calculated from the protein sequence analysis of the monomeric (27.4 kDa) and dimer (54.8 kDa) of scFv6H4 (Figure 1).
Bead-based RIA for determination of KD values for METH and METH-like drugs
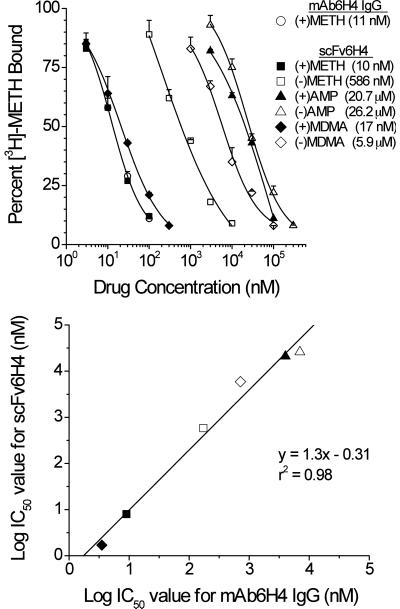
To determine the IC50 values and specificity of the purified scFv6H4 for METH-like analogs, we performed a bead-based RIA. Initially we used an ELISA to determine IC50 values of scFv6H4. However we found these assays to be less accurate and less reproducible when compared to the results from a double antibody-based RIA used to characterize mAbs in our laboratory. Therefore, we developed a new RIA procedure that utilized the His6 affinity tag at the carboxy-terminus of the VL region of scFv6H4 (Fig. 1). Binding an affinity bead to this region oriented the scFv6H4 METH binding site distal to the bead, allowing sterically unhindered access to METH and possibly helping to stabilize the protein. This bead-based assay also allowed rapid separation of bound and free [3H]-METH by centrifugation and excellent signal-to-noise ratios for the measurement of [3H]-METH binding. The results of the ligand binding characterization showed the apparent KD for METH (10 nM) was nearly identical to that previously determined for the parent mAb6H4 (11 nM; Byrnes-Blake et al., 2003) (Fig. 3, upper panel). The scFv6H4 also retained the same stereo-specificity for (+)-isomers of METH-like compounds as found with the parent IgG mAb6H4. For instance, there was a 70-fold greater affinity for (+)METH than for (−)METH. Importantly, scFv6H4 had almost equal affinity for (+)MDMA (17 nM) and (+)METH (10 nM). We also attempted to determine the IC50 values of structurally related compounds (i.e., pseudoephedrine, norepinephrine, dopamine, and serotonin) and found that scFv6H4, like the parent antibody, had no significant cross reactivity with these compounds even at 100 μM concentrations. IC50 values from the scFv6H4 RIA (Fig 3, upper panel) were compared with IC50 values of mAb6H4 IgG (Byrnes-Blake et al., 2003) using linear regression analysis (Fig. 3, lower panel). These data indicated a strong linear relationship for the binding specificities of the two antibodies.
Figure 3.
scFv6H4 binds to METH and (+/−) isomers of METH-like compounds with same affinity and specificity as parent IgG. Upper panel. [3H]-METH inhibition curves using the parent IgG monoclonal antibody mAb6H4 (open circle, designated “mAb6H4 IgG”) or scFv6H4. For these studies, METH and five other METH-like drugs were used as inhibitors of [3H]-METH binding. Abbreviations are (+)METH, (+)-methamphetamine; (−)METH, (−)-methamphetamine; (+)AMP, (+)-amphetamine; (−)AMP, (−)-amphetamine; (+)MDMA, (+)-3,4-methylenedioxymethamphetamine; (−)MDMA, (−)-3,4-methylenedioxymethamphetamine. Lower panel. Relationship between anti-METH scFv6H4 binding specificity versus mAb6H4 binding specificity. These data are derived from the IC50 values for [3H]-METH binding to scFv6H4 (see upper panel) and IC50 values for [3H]-METH binding to mAb6H4 IgG (data from Byrnes-Blake et al., 2003). Symbols for the test drugs correspond to those in the upper panel. Values for the linear regression best-fit line and r2 value are shown on the graph.
Single chain antibodies have been reported to lose activity when incubated in serum or buffer at the physiologically relevant temperature of 37°C (Benhar and Pastan, 1995; Helfrich et al., 1998), thus we were concerned with potential instabilities of the scFv6H4 during rat in vivo studies. To determine the in vitro functional stability of the scFv6H4 in buffer and serum, a bead-based RIA was performed with METH at 4°C, 22°C and 37°C after 24 hr incubation. The binding curves and IC50 values appeared unaffected by these temperatures (data not shown), indicating that scFv6H4 was stable in vitro in serum and buffer over a wide range of temperatures, including physiological temperatures.
Serum pharmacokinetics of METH with and without scFv6H4
We utilized [3H]-scFv6H4 as a tracer for determining scFv6H4 PCKN. Prior to adding the [3H]-scFv6H4 to the unlabeled scFv6H4, the stability of the radiolabeled protein was characterized in vitro. SEC indicated that the [3H]-scFv6H4 elution profile was unchanged after incubation in buffer or rat serum for 24 hrs at 37°C, however, after a similar incubation in rat urine, the [3H]-scFv6H4 was largely degraded (data not shown).
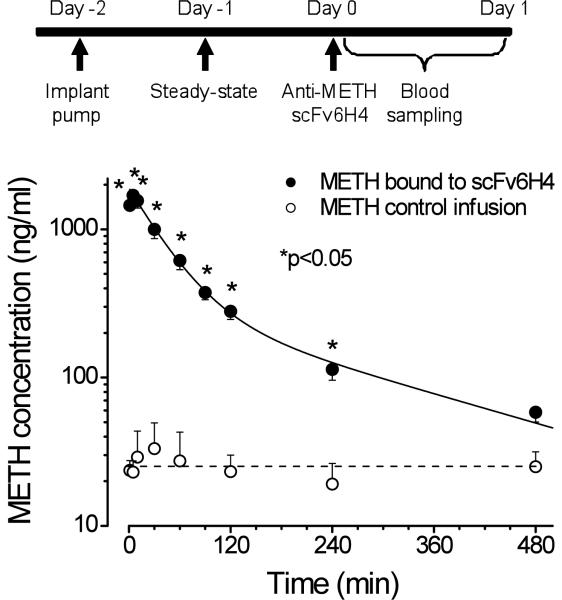
To determine the ability of anti-METH scFv6H4 to alter serum concentrations of METH, sc osmotic mini pumps were used to deliver METH at 3.2 mg/kg/day (Fig. 4, upper panel), which resulted in an average steady-state serum METH concentration of 25 ng/ml after 24 hrs (Fig. 4, lower panel). Administration of anti-METH scFv6H4 led to dramatic changes in serum METH concentrations. The serum METH concentration after scFv6H4 administration was significantly greater than vehicle treated controls from 1–240 min (P<0.05). The area under the METH serum concentration vs. time curve (AUC) from 1–480 min increased from 11,120 ng*min*ml in vehicle treated controls (without scFv6H4) to 133,144 ng*min*ml for the scFv6H4 treated animals.
Figure 4.
scFv6H4 significantly alters serum METH concentrations in vivo. Upper panel. Schematic representation of the dosing regimen used for the in vivo PCKN studies. Lower panel. Average concentration versus time profiles for METH in serum with scFv6H4 antibody administered at t = 0 min (closed circles) or with a control injection of buffer (open circles). The solid-line (closed circles) represents a non-linear regression best-fit line to the METH log concentrations (ng/ml) in the presence of scFv6H4 vs. time curve. The dashed line (open circles) is the average steady state concentration of METH (without scFv6H4). Both groups of rats (n=5 per group) received a sc METH infusion of 3.2 mg/kg/day. All values are represented as the mean ± S.D. per time point; n=5. Asterisks indicate significant difference from control (P<0.05).
Pharmacokinetics of monovalent and multivalent forms of scFv6H4
When we initially analyzed the raw serum counts of the [3H]-scFv tracer, the concentration-time profile appeared to follow a traditional biphasic distribution and elimination process. However, after SEC separation of the scFv6H4 in serum samples (Fig. 5), we realized neither a two compartment model nor a model-dependent analysis would be an acceptable interpretation. We reasoned a model-dependent pharmacokinetic analysis would only be appropriate if we had found a single, unaltered monomeric scFv6H4 species. As our data showed there is a diversity of monomer and multimers that appear progressively over time (Figures 5 and 6). Therefore we conducted model-independent analysis of these data sets, which does not make compartmental assumptions about the data that may not be correct. By analogy, a model-dependent analysis would not be appropriate to follow total parent drug and active metabolite concentrations as if they were one species. We also did not assume that the various phases of the scFv6H4 disposition were simply distribution followed by elimination. This is because our data showed some of the apparent elimination or clearance of monomer and dimer was likely a reformation into larger multimers, which were also able to redistribute METH into the serum through high affinity binding.
For the monomer form of the scFv6H4, the concentration in the first four time points could be determined, because after 30 min the concentration of the [3H]-scFv6H4 was too low to accurately measure. These data indicated the monomer had a t1/2λz of 5.8 ± 0.8 min (see Table 1 for complete PCKN parameters). This monomer t1/2λz is in agreement with rat PCKN of an anti-anthrax toxin scFv t1/2α phase of ~5 min ((Maynard et al., 2002). Additionally, PCKN studies in mice report even faster t1/2α values of 2.7 (Pavlinkova et al., 2000) and 1 min ((Pavlinkova et al., 2000; Willuda et al., 2001). The multimeric forms of scFv6H4 exhibited a much longer serum kinetic profile. Interestingly, in four of the five rats, the concentrations of the divalent form did not rapidly decrease in the first 10 min (Fig. 6). After 30 min, the multivalent scFv6H4 concentrations appeared to more rapidly decrease and exhibited an apparent biphasic curve. This biphasic shape was likely due to changes in scFv6H4 distribution and elimination, as well as formation of new scFv6H4 multimers.
Table 1.
Average PCKN values from rats (n=5) for monovalent and multivalent forms of scFv6H4 determined in the presence of steady-state METH infusions. These PCKN values for scFv6H4 in serum were determined based on the serum concentrations of [3H]-scFv6H4 determined by SEC and liquid scintillation spectrophotometry quantitation of the [3H]-scFv6H4. See Fig. 7 for the scFv6H4 serum concentration-time data. All data are shown as mean ± S.D.
| Pharmacokinetic parameters | ||||
|---|---|---|---|---|
| ScFv6H4 form | λz (min−1) | t1/2λz (min) | Vss (ml/kg) | CLs (ml/min/kg) |
| Monovalent | 0.07 | 5.8 | 780 | 9.4 |
| S.D. | 0.02 | 0.8 | 234 | 2.8 |
| CV, % | 22.9 | 13.7 | 30 | 29.6 |
| Multivalent | 0.0031 | 228 | 346 | 1.1 |
| S.D. | 0.0004 | 38 | 33 | 0.2 |
| CV, % | 14.1 | 16.8 | 9.6 | 21.0 |
Since the native scFv6H4 was administered as a mixture of monomer and dimer (see Fig. 2), we assessed the PCKN profile of the scFv6H4 monomeric (alone) and other multivalent forms (as a composite). Fractionation of the serum samples from each time point showed that the monomer was almost completely eliminated from the serum in <30 min, but the divalent and other apparent multivalent larger proteins persisted for >240 min (Fig. 5 and 6). Due to the complexity of these multivalent changes, we calculated the individual PCKN values for the monomer, and calculated the PCKN values of the multimers (i.e., dimer and trimer) together. Based on the analysis of the terminal elimination data in Fig. 6, the overall t1/2λz of multivalent scFv6H4 forms was 228 ± 38 min.
Studies of in vitro scFv6H4 stability at different protein concentrations, incubation times, and temperatures (see Methods for specific details) indicated that there was excellent stability and a constant ratio of monomer to dimer, similar to the profile shown in Figure 2. Studies of the in vivo blood to plasma concentration ratios of scFv6H4 indicated an average ratio of 0.52 ± 0.04 and a hematocrit of 0.42 ± 0.01. Since the average packed red blood cell volume (i.e., hematocrit) for each ml of blood was 0.42 ml, this would have resulted in an average plasma volume of 0.58 ml (1 minus the red blood cell volume). This estimated average value of 0.58 ml for the plasma volume was in very close agreement with the scFv6H4 blood to plasma ratio of 0.52 ml, therefore these data suggested the scFv6H4 was confined to the plasma compartment, with little or no protein distributed into the red blood cells.
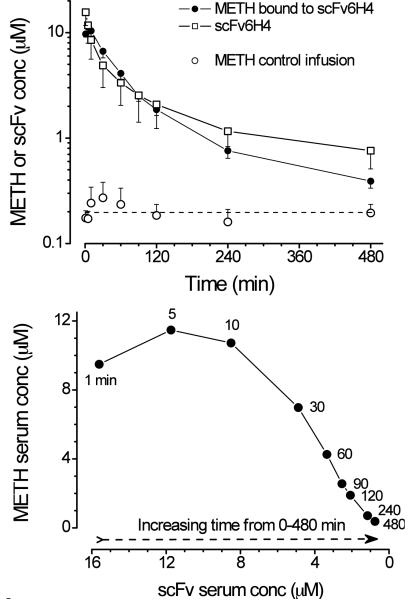
When METH concentration in the presence of scFv6H4 (Fig. 7, upper panel solid circles) and total scFv6H4 protein concentrations (Fig. 7, upper panel open squares) were plotted as their respective molar concentrations, the relationship was nearly one-to-one. When the concentrations of METH and scFv6H4 were plotted on opposite axes, the resulting curve is sigmoidal (Fig. 7, lower panel) with maximum METH concentrations occurring from 1–10 min after scFv6H4 dosing.
Figure 7.
Upper panel: METH concentrations in the presence of scFv6H4 (closed circles), scFv6H4 protein concentrations (monomer and other multivalent forms), and METH steady-state concentration without scFv. All concentration values are shown as μM concentrations versus time. METH concentrations in the presence (solid circles), or absence (open circles) of scFv6H4 were replotted from the data in Fig. 4 (lower panel). Lower panel: Relationship between METH concentration (μM, in the presence of scFv6H4) versus scFv6H4 concentration (μM). In this graph, the samples were collected from 0–480 min after scFv dosing. The time of sample collection (in min) is noted by each data point.
Although the serum PCKN data suggested profound redistribution of METH in the presence of scFv6H4, we found no intact scFv6H4 in the urine and the METH renal clearance was not affected by scFv6H4. This finding was not unexpected, since our preliminary in vitro studies showed scFv6H4 was not stable when incubated in urine for 24 hrs at 37°C. However, we plan to address METH and scFv6H4 renal clearance in METH overdose studies using intravenous bolus doses of METH followed by scFv6H4 treatment. We have performed similar studies to determine anti-PCP Fab elimination (Proksch et al., 1998), and this experimental design will be more appropriate for studying METH elimination since continuous replacement of METH during the infusions limited our ability to reliably and accurately monitor METH renal clearance during discrete time intervals.
Discussion
The genetic re-engineering of mAb6H4 IgG into scFv6H4 changed the protein from a ~150 kDa protein with two anti-METH binding sites to a ~27.4 kDa protein with one METH binding site. It also converted the original IgG from a two gene product (from heavy and light chain coding sequences) into a single gene product, without affecting its affinity or specificity for METH and other ligands (Fig. 3).
We constructed the scFv6H4 by joining the light and heavy chain variable domains of the parent mAb6H4 with a 15 amino acid linker (Fig. 1). Previous studies suggest that linkers shorter than 12 amino acids can result in multimeric complexes (diabody, triabody, etc.) due to “domain swapping,” and that transition between monovalent and divalent scFv can be somewhat controlled by linker length (Atwell et al., 1999; Volkel et al., 2001). Considering these findings, it was our intention to design a predominately monomeric scFv. However, we found the purified and formulated scFv6H4 existed in situ as a mixture of monomer (~75%) and dimer (~25%), with traces of a possible trimer (Fig. 2).
Like the parent mAb6H4, the scFv6H4 had nearly the same affinity for (+)MDMA and (+)METH with little cross-reactivity against (−) isomers (17 nM vs. 10 nM, Fig 3). (+/−) MDMA, or ecstasy, is abused as a racemic mixture with the (+) form acting predominately on the dopaminergic system and the (−) form acting predominately on the serotonergic system (Cho and Segal, 1994). Some studies suggest (+)MDMA is the more potent of the stereoisomers in vivo (Nichols and Oberlender, 1989; Nichols and Oberlender, 1990). Thus, the scFv6H4 we developed has potential clinical utility for treatment of medical problems caused by two abused drugs (METH and MDMA).
To test the ability of scFv6H4 to alter METH disposition in vivo, we first chronically infused METH (Fig. 4, upper panel). The 3.2 mg/kg/day dose allowed us to have mild METH pharmacological effects, and easily measurable METH PCKN changes in the presence or absence of scFv6H4. We did not measure behavioral changes because it would have interfered with the PCKN experiments, however we did note modest increases in locomotor activity in the METH-treated rats.
We calculated that a dosing regimen of 3.2 mg/kg/day of METH equates to a METH body burden of 0.2 mg/kg at steady-state. On day 2, after steady-state METH concentration was achieved, we administered a single bolus dose of scFv6H4 (36.5 mg/kg) that was equimolar in binding sites to the steady-state METH body burden. By comparison, this dose was one third of the dose of IgG (102 mg/kg) that would be needed to achieve the same number of METH antibody binding sites. Thus, use of scFv6H4 allowed a significantly lower protein load for the equivalent number of antibody binding sites. It is important to note that a single dose of scFv6H4 was administered, but the METH infusion continued to replace the drug at the rate of 50% of the body burden per hour based on a 1 hr METH t1/2λz (Riviere et al., 2000).
Since METH was infused to steady-state before scFv6H4 was administered, METH was already equilibrated in tissues (Riviere et al., 2000). Nevertheless, the scFv6H4 quickly bound METH in serum and caused a statistically significant redistribution of the drug for at least 4 hrs (Fig. 4, lower panel). Indeed, (+)METH serum concentrations increased 65-fold within 1 min, and the METH increased 13-fold, compared to the control group. The nearly 1:1 ratio of scFv6H4 and METH concentration in the presence of scFv6H4 over time (Fig 7, upper panel) suggested that the scFv6H4 was responsible for the dramatic increases in METH concentrations during the 8 hr study.
When scFv6H4 in the serum samples was separated by SEC, it was apparent that monomer and other multivalent scFv6H4 forms continuously changed over time (Fig 5). Since both the monovalent (27.4 kDa) and divalent (54.8 kDa) scFv6H4 are below or near the molecular weight cut-off point for glomerular filtration,(~50 kDa; Arend and Silverblatt, 1975), we expected a fairly rapid clearance of both proteins. Unexpectedly, there was an apparent time-dependent clearance of monovalent scFv6H4, and a progressive formation and clearance of other multivalent forms of scFv6H4 (Fig. 5). Early time changes in the monovalent form of scFv6H4 suggested the t1/2λz for the monomer was only 5.8 min (see Fig. 5 for time–lapsed comparisons and Fig. 7 and Table 1 for PCKN analysis). These time-dependent changes in scFv6H4 suggested the clearance of monomeric scFv6H4 resulted from a combination of loss and conversion to dimers and other multivalent forms of scFv6H4.
This hypothesis is supported by the fact that the concentration of the dimer from 1–30 min changed much slower than the monomer (Fig. 5) and that the multivalent scFv6H4 concentration-time profile (without the monomer, solid circles Fig. 6) showed peak concentration at about 5–10 min. We think that during the first 10 min following scFv6H4 delivery, the monomer was undergoing simultaneous elimination, distribution and conversion to dimer and other multivalent forms. At the same time, the dimer was undergoing elimination and formation of larger multivalent isoforms. After 10 min, the monomer concentrations were below accurate detection levels (Fig. 5 and Fig. 6 upper panel) and the remaining multivalent pool of scFv6H4 then followed an apparent biphasic PCKN profile. Since preliminary studies in rat serum (pH 7.35 and 37°C) showed no change in the ratio of monomer to dimer during a 24 hr incubation, the in vivo conversion of scFv6H4 to larger multivalent forms was apparently due to in vivo mechanisms. We also hypothesize that the presence of METH helped to stabilize scFv6H4 and promote continued binding.
While these suggested changes are primarily based on the time-dependent scFv6H4 concentrations, they are also supported by the time-dependent changes in METH concentrations (Fig. 4, lower panel; Fig. 7, upper and lower panels) in the presence of scFv6H4. When we plotted the micromolar concentrations of METH (in the presence of scFv6H4, determined by LC/MS/MS) vs. the micromolar concentrations of scFv (determined by SEC), there was a complex sigmoidal relationship (Fig. 7, lower panel). From 1–10 min, the serum METH concentrations (solid circles) were very high and did not substantially change. This was also the time period in which monomeric and dimeric scFv6H4 concentrations were highest (Figs. 5 and 6). From about 30–120 min the relationship between the METH and scFv6H4 micromolar concentration was fairly linear (Fig. 7, lower panel). From 240–480 min, as the METH and scFv6H4 concentrations significantly decreased the termination of the sigmoidal curve was found.
Dimer formation is common in scFv proteins, and relative amounts of dimer and monomer can vary depending on linker length, pH, ionic strength, and presence or absence of antigen (Arndt et al., 1998). Based on structural evidence there are two major conformations that scFv dimers can adopt. The first conformation is the result of domain interactions between the VL of one scFv to the VH of another (Hudson and Souriau, 2003). After the protein is translated and folded this conformation requires the “opening” of the scFv molecule around the linker and binding to another unhinged scFv, requiring a transition state that is unable to bind to antigen. The other conformation that scFv dimers can adopt is a “back-to-back” conformation. Since the constant regions of the intact IgG that are normally adjacent to the variable region are absent in scFv, these regions are free to form other protein-protein interactions. Since transition from monomer to dimer does not require intra-VH and VL domains to dissociate, these dimers can theoretically associate and dissociate without perturbing the antigen binding site. Residues involved in back-to-back dimer formation of an anti-tumor scFv, MFE-23,have been compared to several other dimerizing scFv (Lee et al., 2002). When we compared the salient dimer-forming sequence features of the MFE-23 and other back-to-back dimers to scFv6H4, we found scFv6H4 possesses the same linker sequence and framework residues that favor back-to-back dimer formation, specifically Pro-L40 and Gly-L41 in the VL domain, and Pro-H41 and Glu/Gly-H42 in the VH domain (Fig. 1). In light of these structural features, the rapid transition from monomer to dimer and other multivalent scFv forms in serum (Fig. 5), along with no apparent loss of binding to METH (Fig. 7), we hypothesized that scFv6H4 forms a back-to-back dimer configuration.
In conclusion, these studies show the design, expression, purification, and preclinical characterization of a high affinity therapeutic scFv6H4 against METH. The scFv was stable for an extended period of time in vivo and was able to significantly redistribute METH into serum for at least 4 hrs. PCKN data suggested that the multivalent forms of scFv6H4 were primarily responsible for the longer term METH binding in serum, even though the scFv6H4 dose was primarily composed of monomer (75%). More studies are needed to confirm our hypotheses and we have begun crystal structure studies and behavioral studies that will hopefully add to our understanding of the pharmacological consequence of multivalent forms of scFv6H4. However, we think anti-METH scFv6H4 is a promising step toward developing a clinical therapy for treatment of METH overdose.
Acknowledgements
We thank Jon Hubbard, Rachel Tawney, Sally Huey, Sherri Wood, and Melinda Gunnell for valuable technical assistance.
This research was supported by National Institute on Drug Abuse grants DA11560, DA14361, F32 DA018039, and from the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Abbreviations
- AMP
(+)-amphetamine
- (−)AMP
(−)-amphetamine
- CLs
systemic clearance
- CDR
complementarity determining region
- AUC
area under the concentration-time curve
- DPM
disintegrations per minute
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin gamma
- mAb
monoclonal antibody
- MDMA
(+)-3,4-methylenedioxymethamphetamine
- (−)MDMA
(−)-3,4-methylenedioxymethamphetamine
- METH
(+)-methamphetamine
- (−)METH
(−)-methamphetamine
- PCP
phencyclidine
- PCR
polymerase chain reaction
- RIA
radioimmunoassay
- scFv
single chain variable fragment
- SEC
size exclusion chromatography
- t1/2 λz
terminal elimination half-life
- VH
variable heavy region
- VL
variable light region
- VSS
volume of distribution at steady-state
References
- Akera T, Cheng VK. A simple method for the determination of affinity and binding site concentration in receptor binding studies. Biochim Biophys Acta. 1977;470:412–423. doi: 10.1016/0005-2736(77)90132-8. [DOI] [PubMed] [Google Scholar]
- Arend WP, Silverblatt FJ. Serum disappearance and catabolism of homologous immunoglobulin fragments in rats. Clin Exp Immunol. 1975;22:502–513. [PMC free article] [PubMed] [Google Scholar]
- Arndt KM, Muller KM, Pluckthun A. Factors influencing the dimer to monomer transition of an antibody single-chain Fv fragment. Biochemistry. 1998;37:12918–12926. doi: 10.1021/bi9810407. [DOI] [PubMed] [Google Scholar]
- Atwell JL, Breheney KA, Lawrence LJ, McCoy AJ, Kortt AA, Hudson PJ. scFv multimers of the anti-neuraminidase antibody NC10: length of the linker between VH and VL domains dictates precisely the transition between diabodies and triabodies. Protein Eng. 1999;12:597–604. doi: 10.1093/protein/12.7.597. [DOI] [PubMed] [Google Scholar]
- Bazin-Redureau MI, Renard CB, Scherrmann JM. Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab')2 and Fab after intravenous administration in the rat. J Pharm Pharmacol. 1997;49:277–281. doi: 10.1111/j.2042-7158.1997.tb06795.x. [DOI] [PubMed] [Google Scholar]
- Benhar I, Pastan I. Identification of residues that stabilize the single-chain Fv of monoclonal antibodies B3. J of Biol Chem. 1995;270:23373–23380. doi: 10.1074/jbc.270.40.23373. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol. 2003;461:119–128. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- Cho AK, Segal DS. Amphetamine and Its Analogs: Psychopharmacology, Toxicology, and Abuse. Academic Press; San Diego: 1994. [Google Scholar]
- Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, Batra SK. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: improved biodistribution and potential for therapeutic application. Cancer Res. 2000;60:6964–6971. [PubMed] [Google Scholar]
- Helfrich W, Kroesen BJ, Roovers RC, Westers L, Molema G, Hoogenboom HR, de Leij L. Construction and characterization of a bispecific diabody for retargeting T cells to human carcinomas. Int J Cancer. 1998;76:232–239. doi: 10.1002/(sici)1097-0215(19980413)76:2<232::aid-ijc11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hendrickson HP, Milesi-Halle A, Laurenzana EM, Owens SM. Development of a liquid chromatography-tandem mass spectrometric method for the determination of methamphetamine and amphetamine using small volumes of rat serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:81–87. doi: 10.1016/j.jchromb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Huang BC, Foote LJ, Lankford TK, Davern SM, McKeown CK, Kennel SJ. A diabody that dissociates to monomer forms at low concentration: effects on binding activity and tumor targeting. Biochem Biophys Res Commun. 2005;327:999–1005. doi: 10.1016/j.bbrc.2004.12.114. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999;231:177–189. doi: 10.1016/s0022-1759(99)00157-x. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Souriau C. Engineered antibodies. Nat Med. 2003;9:129–134. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
- Kubetzko S, Balic E, Waibel R, Zangemeister-Wittke U, Pluckthun A. PEGylation and multimerization of the anti-p185HER-2 single chain Fv fragment 4D5: effects on tumor targeting. J Biol Chem. 2006;281:35186–35201. doi: 10.1074/jbc.M604127200. [DOI] [PubMed] [Google Scholar]
- Lee YC, Boehm MK, Chester KA, Begent RH, Perkins SJ. Reversible dimer formation and stability of the anti-tumour single-chain Fv antibody MFE-23 by neutron scattering, analytical ultracentrifugation, and NMR and FT-IR spectroscopy. J Mol Biol. 2002;320:107–127. doi: 10.1016/S0022-2836(02)00403-5. [DOI] [PubMed] [Google Scholar]
- Maynard JA, Maassen CB, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat Biotechnol. 2002;20:597–601. doi: 10.1038/nbt0602-597. [DOI] [PubMed] [Google Scholar]
- McClurkan MB, Valentine JL, Arnold L, Owens SM. Disposition of a monoclonal anti-phencyclidine Fab fragment of immunoglobulin G in rats. J Pharmacol Exp Ther. 1993;266:1439–1445. [PubMed] [Google Scholar]
- Nichols DE, Oberlender R. Structure-activity relationships of MDMA-like substances. NIDA Res Monogr. 1989;94:1–29. [PubMed] [Google Scholar]
- Nichols DE, Oberlender R. Structure-activity relationships of MDMA and related compounds: a new class of psychoactive drugs? Ann N Y Acad Sci. 1990;600:613–623. doi: 10.1007/978-1-4613-1485-1_7. discussion 623-615. [DOI] [PubMed] [Google Scholar]
- Owens SM, Zorbas M, Lattin DL, Gunnell M, Polk M. Antibodies against arylcyclohexylamines and their similarities in binding specificity with the phencyclidine receptor. J Pharmacol Exp Ther. 1988;246:472–478. [PubMed] [Google Scholar]
- Pavlinkova G, Colcher D, Booth BJ, Goel A, Batra SK. Pharmacokinetics and biodistribution of a light-chain-shuffled CC49 single-chain Fv antibody construct. Cancer Immunol Immunother. 2000;49:267–275. doi: 10.1007/s002620000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, Liu H, Owens SM. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J Pharmacol and Exp Ther. 2007;322:30–39. doi: 10.1124/jpet.106.117150. [DOI] [PubMed] [Google Scholar]
- Proksch JW, Gentry WB, Owens SM. Pharmacokinetic mechanisms for obtaining high renal coelimination of phencyclidine and a monoclonal antiphencyclidine antigen-binding fragment of immunoglobulin G in the rat. J Pharmacol Exp Ther. 1998;287:616–624. [PubMed] [Google Scholar]
- Riviere GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2001. [Google Scholar]
- Shelver WL, Keyler DE, Lin G, Murtaugh MP, Flickinger MC, Ross CA, Pentel PR. Effects of recombinant drug-specific single chain antibody Fv fragment on [3H]-desipramine distribution in rats. Biochem Pharmacol. 1996;51:531–537. doi: 10.1016/0006-2952(95)02233-3. [DOI] [PubMed] [Google Scholar]
- Trang J. Pharmacokinetics and Metabolism of Therapeutic and Diagnostic Antibodies. In: Ferraiolo B, Mohler M, Gloff C, editors. Protein Pharmacokinetics and Metabolism. Plenum Press; New York: 1992. pp. 223–270. [Google Scholar]
- Volkel T, Korn T, Bach M, Muller R, Kontermann RE. Optimized linker sequences for the expression of monomeric and dimeric bispecific single-chain diabodies. Protein Eng. 2001;14:815–823. doi: 10.1093/protein/14.10.815. [DOI] [PubMed] [Google Scholar]
- Willuda J, Kubetzko S, Waibel R, Schubiger PA, Zangemeister-Wittke U, Pluckthun A. Tumor targeting of mono-, di-, and tetravalent anti-p185(HER-2) miniantibodies multimerized by self-associating peptides. J Biol Chem. 2001;276:14385–14392. doi: 10.1074/jbc.M011669200. [DOI] [PubMed] [Google Scholar]