Abstract
Fatty acid amide hydrolase (FAAH) activity is known to mediate the tone of endogenous fatty acid amides including the endocannabinoid anandamide (AEA). FAAH is a potential therapeutic target becuase genetic or pharmacological ablation of FAAH promotes analgesia and anxiolytic effects without disrupting motor coordination. Little is known about the endogenous temporal fluctuations of brain FAAH activity. This is the first comprehensive study examining temporal fluctuations in mouse brain FAAH activity. Regional mouse brain homogenates were generated at the midpoint of the light (“noon”) and dark (“midnight”) cycles. While immunoblots revealed no significant change (P>0.05) in regional activity between these two time points, in vitro activity assays detected a subtle 10% reduction (P<0.05) in cerebellar FAAH activity at midnight. A novel ex vivo autoradiography technique permitted the study of eleven different brain regions, many of which cannot be studied using traditional in vitro methods. The cerebellum and the PAG both exhibited significant (P<0.05) reductions in regional FAAH activity in ‘midnight’ brains. These data confirm the need to account for temporal changes in FAAH activity when therapeutically targeting FAAH.
Keywords: anandamide, endocannabinoid, FAAH, circadian
Introduction
The endocannabinoid system mediates numerous physiological processes, including reproduction 12, metabolism 3, immunity 36, and digestion 1. Through the activation of cannabinoid receptor 1 (CB1R), endocannabinoids play a central role in CNS-mediated functions including nociception 17, motor coordination 11, memory 11, and vision 42. Signaling of the endocannabinoid arachidonoyl ethanolamide (AEA) is terminated by its hydrolysis by fatty acid amide hydrolase (FAAH). Because FAAH knockout mice posses elevated endogenous fatty acid amide levels and are unable to efficiently metabolize exogenously administered AEA 4, FAAH is the principal enzyme mediating the in vivo metabolism of AEA other fatty acid amides (for review, see 25). FAAH also hydrolyzes the endocannabinoid 2-arachidonoylglycerol (2-AG) in vitro 15, although its contribution toward 2-AG inactivation in vivo is currently not known.
As expected, CB1R and FAAH are distributed throughout the mouse brain, with robust immunoreactivity in the hippocampus and dorsal tenia tecta. Additional FAAH immunoreactivity occurs in the olfactory bulb, cerebral cortex, thalamus, and cerebellum, predominantly in dendrites and somata post-synaptic to CB1R immunoreactive axons 9. This expansive immunoreactivity pattern suggests that FAAH plays a critical role in mediating AEA signaling in the brain.
Selective FAAH inhibitors produce a subset of the behavioral effects observed with CB1R agonists. For example, i.p. administration of URB597 increased endogenous AEA levels and produced analgesia and anxiolytic effects, without promoting catalepsy, hypothermia, or hypophagia in rats 19. Similarly, administration of the selective FAAH inhibitor OL-135 produced analgesic effects in mice as determined by hotplate and tail immersion tests 22. Therefore, FAAH activity mediates endocannabinoid tone in brain regions responsible for pain and anxiety. Cannabinoid-mediated effects such as catalepsy, hypothermia, and hypophagia are likely regulated by enzymes other than FAAH, as these behaviors were unaffected by inhibitor administration.
While FAAH is principally responsible for AEA inactivation, several other enzymes are also capable of metabolizing AEA. Cyclooxygenase-2 metabolizes AEA in in vitro assays 10, 43 and its activity limits endocannabinoid signaling in the hippocampus 21. FAAH-2 39 and N-acylethanolamine-hydrolyzing acid amidase 35 hydrolyze AEA, although they are more effective in metabolizing oleamide and palmitoylethanolamide, respectively. The in vivo contributions of FAAH-2 and NAE-hydrolyzing acid amidase remain unknown, and their CNS contributions are likely minimal. NAE-hydrolyzing acid amidase is expressed primarily in the periphery (lungs, spleen, small intestine, thymus and immune cells) 35, and may mediate fatty acid amide inactivation in these tissues. In primates and several other species, FAAH-2 is widely expressed in peripheral tissues including kidney, liver, lung, prostate, heart, and ovary. It is important to note that FAAH-2 has not been found in the brain of any species examined, nor is it expressed in mice or rats 39.
Very few studies have examined temporal changes in endocannabinoid levels and their metabolism. With respect to FAAH, Valenti et al. determined regional endogenous AEA levels increased, and rat brain FAAH activity decreased, at midnight relative to midday brain samples 37. These data are complemented by a second study indicating oleamide, palmitoylethanolamide, and AEA levels are highest in rat cerebrospinal fluid during the “lights-off” period 27. Epithelial cells of the rat choroid plexus, a region known to produce cerebrospinal fluid, are FAAH-IR 8, suggesting FAAH activity in these cells may also vary temporally. Mounting evidence indicates that AEA modulates sleep (for review, see 26), so temporal changes in regional FAAH activity may also affect sleep patterns.
In this manuscript, we utilized a novel ex vivo autoradiography technique to examine temporal changes in regional FAAH activity (See 14 for a detailed characterization of this assay). Unlike in vitro homogenate assays and RT-PCR, this novel autoradiography technique takes into account both FAAH activity levels and the capacity of AEA trafficking mechanisms such as FABPs18, as FAAH activity is measured following substrate delivery in intact tissue. In addition, brain regions impossible to accurately dissect, and regions too small for homogenate generation, can easily be studied. Temporal changes in FAAH activity were examined throughout mouse brain. Mice were intravenously administered [3H]AEA at the midpoint of the light (“noon”) or dark (“midnight”) cycle. Differences in regional tritium distribution patterns, representing changes in regional [3H]AEA hydrolysis capacity between these two time points, were determined. Data from these autoradiography studies were compared to traditional in vitro homogenate assay and immunoblot data to confirm the results and techniques of this study.
Experimental Procedures
Animals
C57BL/6 mice (Taconic Farms, Germantown, NY) weighing approximately 25g were kept in LD 12:12. noon mice were studied at the midpoint of the light cycle, while midnight mice were studied at the midpoint of the dark cycle. All mice were kept in the same room and were maintained with free access to food and water according to the federal guidelines for the care and use of animals. These studies were approved by the Institutional Review Committee.
Regional FAAH western blots
Six hours after the onset of the light and dark period, regional brain dissections were performed on a chilled surface. Following tissue homogenization on ice in Tris + 1mM ethylenediaminetetraacetic acid (EDTA) + complete Mini EDTA acid -free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), protein concentrations were determined by BCA protein assay (Pierce Chemical, Rockford, IL). 40 μg of protein/lane were run on a 10% SDS-PAGE gel. Following transfer to a nitrocellulose membrane at 100V for 25 min, blots were blocked for one hour in 5% non-fat dry milk in PBS Tween (PBST). The blots were probed for one hour while shaking with polyclonal rabbit FAAH antibody directed against aa 561–579 of rat FAAH (Alexis Biochemicals, San Diego, CA) at a final concentration of 1:250 or monoclonal mouse anti-β actin antibody directed against aa 1–14 of Xenopus laevis actin (AbCam, Inc. Cambridge, MA) at a final concentration of 1:10,000. The blots were rinsed three times with PBST followed by incubation with goat anti-mouse or goat anti-rabbit IgG HRP-conjugated antibodies (Molecular Probes, Eugene, OR) for 1 hour. The blots were rinsed three times with PBST, developed using the Immun-star HRP substrate (Bio-Rad, Hercules, CA) and exposed to film. To determine changes in FAAH protein levels between noon and midnight samples, FAAH levels were normalized to actin levels within the same lane, and analyzed by densitometry using Image J software. Significance was determined by paired 2-Tailed T-test using Excel (Microsoft).
In vitro FAAH enzyme activity assays
Six hours after the onset of the light and dark period, regional brain dissections were performed. Following tissue homogenization in Tris + 1mM EDTA on ice, protein concentrations were determined by BCA protein assay (Pierce Chemical, Rockford, IL). FAAH activity assays were performed according to published methods6. Briefly, 300 μg of regional homogenate, 500μg fatty acid-free bovine serum albumin, 100 μM AEA (Cayman Chemical, Ann Arbor, MI) plus 0.22 μCi arachidonoyl ethanolamide [ethanolamine-1-3H] (a gift from the National Institute on Drug Abuse) were incubated in Tris + 1mM EDTA pH 7.6 at 37°C in triplicate for fifteen minutes while shaking. The reactions were terminated by the addition of 2 volumes of 1:1 chloroform/methanol. Samples were centrifuged, and the aqueous phase measured by liquid scintillation counting. The rates of AEA hydrolysis/mg protein/hr were compared between noon and midnight regional homogenates. Significance was determined by paired 2-Tailed T-test using Excel (Microsoft). Data were graphed using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA).
Ex vivo autoradiography of brain FAAH activity
Radiolabeled AEA [arachidonoyl 5,6,8,9,11,12,14,15-3 H] was a generous gift from the NIDA Drug Inventory Supply Program. AEA (Cayman Chemical) and [3H]AEA were dissolved in a 1:1:18 mixture of ethanol: emulphor: saline. Mice were i.v administered 1 mg/kg AEA + 2 mCi/kg [3H]AEA. Fifteen minutes following injection, mice were sacrificed and their brains promptly transferred to a beaker containing iced saline, in order to minimize further diffusion and protein-mediated processes. Half of the cerebellum and a blood sample were homogenized, extracted in 1:1 chloroform:methanol, and analyzed by scintillation counting and thin layer chromatography (solvent system was 6:3:1 ethylacetate:hexane: acetic acid6), in order to quantify total tritium accumulation, and [3H]AEA breakdown in these tissues. Chilled brains were transferred into iced 2% formaldehyde + 2% glutaraldehyde in PB for one hour. The brains were then washed three times with iced PB, and cryoprotected at 4°C in 30% sucrose in PB. Serial 15μm cryosections were generated, and images acquired for ten hours using the BetaImager (Biospace, Paris, France).
Autoradiography analysis
Serial sections were analyzed, and regions of interested identified using a commercially available mouse brain atlas26 as a reference. Surface radioactivity (cpm/mm2) in regions of interest was quantified using Beta Vision image analysis software (Biospace Mesures, France). Data were entered into Excel (Microsoft Corp., Seattle, WA), and average regional tritium accumulation/mm2, representing AEA and its metabolites, was determined. Six mice were used for each condition. Regional surface activities of each mouse were normalized by dividing the activity of each region over that of the pontine nuclei (the region of lowest tritium accumulation) of the same mouse. Normalization enabled regional comparison among animals with slightly different levels of brain tritium uptake. Standard error was calculated using Excel. Data were graphed, and one-way analysis of variance with Dunnett’s post test was performed to determine significance between noon and midnight samples using GraphPad Prism 4 (Graph Pad Software Inc., San Diego, CA).
Results
Immunoblot analysis
To examine regional changes in FAAH protein levels, regional brain dissections were performed at the midpoint of the light (“noon”) and dark (“midnight”) cycles, and regional homogenates generated. First, immunoblots were run (Fig. 1) and densitometry analyses of FAAH protein levels were performed following normalization to β-actin (Fig. 2). Immunoblots detected no significant (P>0.05) changes in normalized FAAH protein levels in the cortex, hippocampus, cerebellum, striatum, or thalamus.
Fig. 1. Regional FAAH western blots.
Immunoblots for FAAH and actin were run against homogenates generated at the midpoint of the light (“noon”) and dark (“midnight”) cycles.
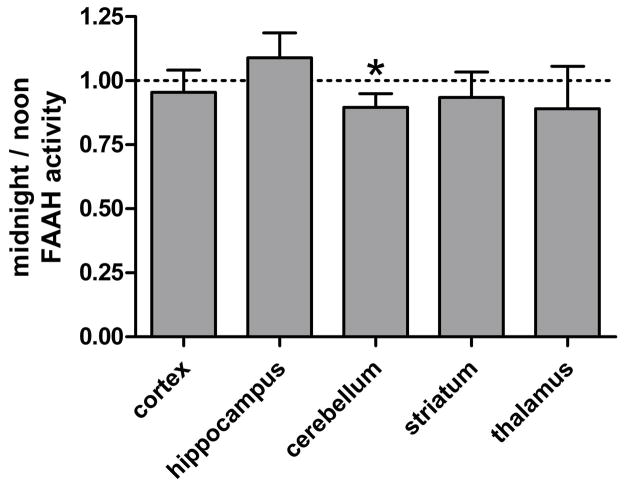
Fig. 2. Regional FAAH protein levels.
Following sample normalization against actin, FAAH protein levels were compared between noon and midnight conditions. Bars represent the regional mean + standard error. Significance was determined by paired 2-Tailed T-test. No significant (P>0.05) circadian changes were observed in cortical, hippocampal, cerebellar, striatal, or thalamic homogenates.
In vitro FAAH activity assays
While changes in protein levels were not detectable, it remained possible that FAAH activity levels differed between noon and midnight. Therefore, we incubated regional homogenates with [3H]anandamide and compared the in vitro generation of [3H]ethanolamine by noon and midnight samples (Fig. 3). No significant difference (P>0.05) was observed between noon and midnight cortical, hippocampal, striatial, and thalamic homogenates. However, a subtle, yet significant (P<0.05) decline in cerebellar FAAH activity was detected at midnight relative to noon samples.
Fig. 3. Regional FAAH activity assays.
Regional homogenates were generated at the midpoint of the light (“noon”) and dark (“midnight”) cycles, and in vitro FAAH activity assays were performed. Homogenates were incubated at 37°C in buffer containing BSA, 100μM AEA, and [3H]AEA for 15 min. The rate of [3H]ethanolamine production was compared between noon and midnight samples. Significance was determined by paired 2-Tailed T-test. Bars represent the regional mean + standard error. While most regional homogenates generated at noon and midnight exhibited similar rates (P>0.05) of [3H]AEA hydrolysis activity, midnight cerebellar homogenates exhibited a modest, yet significant (★ P<0.05), decline in hydrolysis activity relative to noon samples.
Ex vivo autoradiography
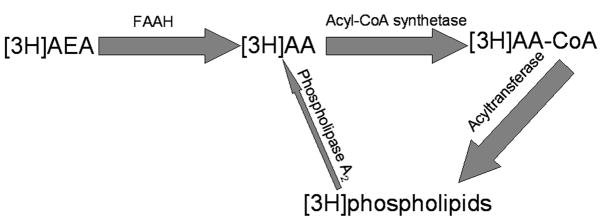
Regional FAAH activity was next analyzed by ex vivo imaging. For ex vivo imaging, mice were administered [3H]AEA+1mg/kgAEA at the midpoint of either the light (“noon”)or dark (“midnight”) cycle, and their brains isolated and fixed fifteen minutes later. Sections were generated and imaged in the BetaImager. Following tracer administration, [3H]AEA circulates through the blood and is either metabolized in the periphery or passes the blood brain barrier and enters the brain. As described in the trapping mechanism schematic (Fig. 4), [3H]AEA is subsequently taken-up by cells with FAAH and metabolized into [3H]arachidonic acid ([3H]AA). This is very similar to FAAH activity driving [3H]AEA uptake into cultured cells 5, 7. [3H]AA is subsequently incorporated into the membranes of the same cells that hydrolyzed the tracer. As a result, cells with the highest levels of [3H]AEA hydrolysis trap the most tritium in their membranes. In sharp contrast to WT mice, radiotracer administered to FAAH knockout mice remains unmetabolized. The intact [3H]AEA distributes in a homogeneous and diffuse manner 14, becuase the radiolabel escapes membrane entrapment.
Fig. 4. Trapping mechanism for ex vivo imaging of FAAH activity.
Following the i.v. administration of [3H]AEA, the radiotracer crosses the blood brain barrier. Upon entry into a cell that has active FAAH, [3H]AEA is quickly metabolized into [3H]AA, which is in turn promptly incorporated into membrane phospholipids of the same cell6, 40 (as represented by the wide dark grey arrows). This process is mediated by acyl CoA synthetase and acyl transferase and is the predominant metabolic pathway for AA in the rodent brain32. As a result, tritium accumulates in cells and brain regions with the most [3H]AEA metabolism14. After fifteen minutes, the majority of [3H]AEA hydrolysis and subsequent [3H]AA membrane incorporation is complete, yet the amount of tritium release (as represented by the thin light arrow) from cellular membranes remains negligible 13, 29, 31. For review of both major and alternate arachidonic acid metabolic pathways that must be considered while imaging arachidonic acid, see Rapoport (2003)30.
Ex vivo imaging is more sensitive technique than in vitro FAAH activity assays because use of the latter is limited to regions of a minimal size that one can efficiently isolated by dissection. In addition, becuase tissue is not disrupted during AEA hydrolysis, ex vivo imaging permits both the determination of the tissue’s capacity for AEA hydrolysis by FAAH and the capacity of AEA delivery mechanisms such as fatty acid binding proteins18. Because substrate delivery to FAAH affects the rate of AEA hydrolysis, ex vivo imaging provides a more accurate representation of regional FAAH capacity than in vitro activity assays. We utilized this technique to compare FAAH activity levels in eleven regions of noon and midnight brains (Fig. 5).
Fig. 5. Regions analyzed by ex vivo autoradiography.
A) Serial sections of mouse brains were imaged and the regions depicted in gray were analyzed. They include the 1) caudate putamen, 2) the piriform cortex (cx), 3) the somatosensory (somatosens) cx, 4) the hypothalamus, 5) the amygdala, 6) the thalamus, 7) the hippocampus, 8) the entorhinal cx, 9) the visual cx, 10) the periaqueductal gray (PAG), and 11) the cerebellum. (These images are modified with permission from The Mouse Brain in Stereotaxic Coordinates 28). B) Representative autoradiographs of noon mouse brains.
Similar to the in vitro FAAH activity data, tritium accumulation in most brain regions did not significantly vary (P>0.05) between noon and midnight brains (Fig. 6). In addition, ex vivo imaging confirmed a slight decrease (P<0.05) in cerebellar FAAH activity in midnight brains relative to noon samples. Ex vivo imaging also detected a significant decline in FAAH activity in the periaqueductal gray (PAG), a region too small to analyze by traditional in vitro methods.
Fig. 6. Ex vivo autoradiography assay of FAAH activity.
Mice were i.v. administered 1mg/kg AEA + 50μCi [3H]AEA at the midpoint of the light (“noon”) or dark (“midnight”) cycle. Fifteen minutes following tracer administration, brains were isolated, fixed, and processed for imaging in the BetaImager. Regional tritium accumulation (cpm/mm2) due to FAAH activity was quantified using BetaVision software. Regional tritium accumulation was normalized against tritium levels in the pontine nuclei, the brain region with the lowest tritium levels. Normalized regional tritium levels were compared between noon and midnight samples and significance determined by paired 2-Tailed T-test. Bars represent the regional mean + standard error. The dotted line represents the relative value of the pontine nuclei. Midnight cerebellar and PAG exhibited significantly (★ P<0.05) less tritium accumulation relative to noon samples. No other region significantly varied (P>0.05) between noon and midnight samples.
Analysis of brain samples by thin layer chromatography (run in parallel to these imaging experiments) indicated the bulk of tritium in these mouse brains was in the form of [3H] phospholipids (69% +/−5%). This is consistent with our prior publication (Glaser et al. 2006 JPET 316:1088–1097) and suggests the majority of tritium was trapped in regional membranes following [3H]AEA hydrolysis by FAAH.
Discussion
There has been little investigation of temporal changes of enzymes mediating endogenous AEA tone. Valenti et al. reported an increase in endogenous AEA levels in the nucleus acumbens, prefrontal cortex, striatum, and hippocampus of the rat brain in the dark phase. This increase in endogenous AEA tone complemented a decline in FAAH activity in the striatum and hippocampus 37. In further support of these observations, endogenous levels of the FAAH substrates AEA, palmitoylethanolamide, and oleoylethanolamide have been shown to vary with a circadian cycle in several rat brain regions and in cerebrospinal fluid 27.
This is the first comprehensive study of temporal changes in regional FAAH activity in the mouse brain. The novel imaging technique14 permitted the examination of brain regions too small to analyze by standard in vitro methods, thereby producing the most thorough characterization of regional temporal fluctuations in FAAH activity to date. In sharp contrast to prior rat studies37, both regional and temporal variations in mouse brain FAAH activity are more subtle, with only the mouse cerebellum and PAG exhibiting significantly reduced tritium accumulation in midnight brains (Fig. 6). No nighttime increases in tritium accumulation were observed in any brain region. In vitro FAAH activity assays confirmed that midnight cerebellar homogenates exhibited slightly reduced in vitro activity relative to noon samples (Fig. 3). Importantly, these experiments demonstrate the sensitivity of this novel autoradiography technique, and provide proof that similar results can be obtained with a traditional in vitro assay. As the difference in in vitro cerebellar FAAH activity was only 10%, it is not surprising that changes in protein levels were not detected by immunoblot (Fig. 2).
There is growing evidence that FAAH may represent an ideal pharmaceutical target for the design of novel analgesics. Unlike CB1R agonists that produce both beneficial effects and prominent psychotropic and motor effects, FAAH inhibitors provide analgesia and reduce anxiety without negative side-effects 19. As FAAH has great therapeutic potential for pain, temporal changes in PAG FAAH should be taken into account when optimizing treatment.
The observed temporal changes in mouse PAG FAAH activity support observations in prior stress-induced analgesia studies. Stress induced analgesia occurs when acute stress activates either opioid- or non-opioid signaling pathways 2, 38 in the brain that alleviate pain. While the opioid pathway is well studied 41, only recently were the endocannabinoids AEA and 2-arachidonoylglycerol shown to mediate the non-opioid component in the PAG. Non-opioid mediated stress-induced analgesia was potentiated by direct administration of inhibitors of endocannabinoid inactivation to the PAG and abolished by the CB1R antagonist Rimonabant 16, 33. Becuase the non-opioid-mediated component of stress-induced analgesia is known to exhibit a day-night rhythm with enhanced analgesia observed at night 20, it is expected that endocannabinoid levels would fluctuate with a temporal pattern within the PAG.
FAAH inhibition in the PAG following the microinjection of the selective FAAH inhibitor URB597 raises both endogenous AEA and 2-arachidonoylglycerol levels 24. Therefore, fluctuations in FAAH activity levels are sufficient to significantly alter endocannabinoid tone in this region. We observed [3H] accumulation levels (and therefore FAAH activity) significantly declined in the PAG of midnight brains relative to noon samples. The nocturnal decline in PAG FAAH activity observed in the current study may provide a molecular mechanism for the nocturnal increase of the non-opioid component of stress-induced analgesia.
The cerebellum is best known to mediate motor coordination. This brain region’s output, purkinje cells, are both FAAH and CB1R immunoreactive 9. Therefore, it was somewhat surprising that, unlike CB1R agonists, systemically administered FAAH inhibitors do not produce deficits in motor coordination 19, 22. It must be concluded that FAAH activity is not primarily responsible for regulating cerebellar endocannabinoid tone and motor coordination.
Becuase FAAH inhibition is insufficient to alter motor coordination in the cerebellum, the moderate decline (P<0.05) in in vitro FAAH activity and midnight cerebellar tritium accumulation (Figs 3 and 6) observed in this study is expected to have no negative effect upon motor coordination at night, when mice are most active. Instead, decreased cerebellar FAAH activity at midnight suggests FAAH is either mediating endocannabinoid hydrolysis remote from CB1R, or is mediating the tone of other substrates that have no effect upon CB1R stimulation in the cerebellum. It remains likely that either monoglyceride lipase alone, or FAAH and monoglyceride lipase in concert, mediate(s) endocannabinoid tone in the cerebellum 23, 34.
In conclusion, this is the first study to identify subtle temporal changes in regional mouse brain FAAH activity. Using traditional in vitro techniques, we determined FAAH activity in the cerebellum significantly declined in homogenates generated at midnight. Cortical, thalamic, and hippocampal homogenates exhibited no significant change in FAAH activity between noon and midnight samples. Using a novel autoradiography technique, we confirmed the significant reduction tritium accumulation (driven by [3H]AEA hydrolysis) in midnight cerebella following [3H]AEA administration. This sensitive method also permitted the identification of subtle reductions (P<0.05) in tritium accumulation in the PAG, a region too small to study by traditional in vitro methods. The temporal changes in PAG FAAH activity supports prior studies and suggests endocannabinoid involvement in circadian changes in non-opioid stress-induced analgesia. The observed changes in cerebellar FAAH activity are likely unrelated to any endocannabinoid-mediated declines in motor coordination at midnight in mice.
Acknowledgments
We would like to thank Dr. Stephen Yazulla for the use of his laboratory space and equipment while conducting this research. We thank Academic Press for the permission to reproduce images. We would like to acknowledge the NIH for their support of this study: NIH grant 1 K01 DA021806-01 (S.T.G.).
Abbreviations
- AA
arachidonic acid
- AEA
anandamide
- CB1R
cannabinoid receptor 1
- cx
cortex
- EDTA
ethylenediaminetetraacetic acid
- FAAH
fatty acid amide hydrolase
- PAG
periaqueductal gray
- PBST
phosphate buffered saline + Tween
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aviello G, Romano B, Izzo AA. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharmacol Sci. 2008;12(Suppl 1):81–93. [PubMed] [Google Scholar]
- 2.Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982;243:315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- 3.Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res. 2008;36:135–145. doi: 10.1159/000115362. [DOI] [PubMed] [Google Scholar]
- 4.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol Pharmacol. 2001;59:1369–1375. doi: 10.1124/mol.59.6.1369. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, Abumrad N. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- 8.Egertova M, Cravatt BF, Elphick MR. Fatty acid amide hydrolase expression in rat choroid plexus: possible role in regulation of the sleep-inducing action of oleamide. Neurosci Lett. 2000;282:13–16. doi: 10.1016/s0304-3940(00)00841-7. [DOI] [PubMed] [Google Scholar]
- 9.Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 10.Fowler CJ, Stenstrom A, Tiger G. Ibuprofen inhibits the metabolism of the endogenous cannabimimetic agent anandamide. Pharmacol Toxicol. 1997;80:103–107. doi: 10.1111/j.1600-0773.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 11.Fride E. Endocannabinoids in the central nervous system: from neuronal networks to behavior. Curr Drug Targets CNS Neurol Disord. 2005;4:633–642. doi: 10.2174/156800705774933069. [DOI] [PubMed] [Google Scholar]
- 12.Fride E. Multiple roles for the endocannabinoid system during the earliest stages of life: pre- and postnatal development. J Neuroendocrinol. 2008;20(Suppl 1):75–81. doi: 10.1111/j.1365-2826.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 13.Giovacchini G, Chang MC, Channing MA, Toczek M, Mason A, Bokde AL, Connolly C, Vuong BK, Ma Y, Der MG, Doudet DJ, Herscovitch P, Eckelman WC, Rapoport SI, Carson RE. Brain incorporation of [11C]arachidonic acid in young healthy humans measured with positron emission tomography. J Cereb Blood Flow Metab. 2002;22:1453–1462. doi: 10.1097/01.WCB.0000033209.60867.7A. [DOI] [PubMed] [Google Scholar]
- 14.Glaser ST, Gatley SJ, Gifford AN. Ex vivo imaging of fatty acid amide hydrolase activity and its inhibition in the mouse brain. J Pharmacol Exp Ther. 2006;316:1088–1097. doi: 10.1124/jpet.105.094748. [DOI] [PubMed] [Google Scholar]
- 15.Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999;57:417–423. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- 16.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 17.Hosking RD, Zajicek JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth. 2008;101:59–68. doi: 10.1093/bja/aen119. [DOI] [PubMed] [Google Scholar]
- 18.Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proceedings of the National Academy of Sciences of the United States of America. 2009 doi: 10.1073/pnas.0901515106. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 20.Kavaliers M, Ossenkopp KP. Day-night rhythms of opioid and non-opioid stress-induced analgesia: differential inhibitory effects of exposure to magnetic fields. Pain. 1988;32:223–229. doi: 10.1016/0304-3959(88)90071-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- 22.Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- 23.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, Di Marzo V. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- 25.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 26.Murillo-Rodriguez E. The role of the CB1 receptor in the regulation of sleep. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1420–1427. doi: 10.1016/j.pnpbp.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Murillo-Rodriguez E, Desarnaud F, Prospero-Garcia O. Diurnal variation of arachidonoylethanolamine, palmitoylethanolamide and oleoylethanolamide in the brain of the rat. Life Sci. 2006;79:30–37. doi: 10.1016/j.lfs.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, California: 2001. [Google Scholar]
- 29.Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to signal transduction and membrane remodeling. Neurochem Res. 1999;24:1403–1415. doi: 10.1023/a:1022584707352. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr. 2003;143:S26–34. doi: 10.1067/s0022-3476(03)00399-8. [DOI] [PubMed] [Google Scholar]
- 31.Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 32.Sun GY. Metabolism of arachidonate and stearate injected simultaneously into the mouse brain. Lipids. 1977;12:661–665. doi: 10.1007/BF02533761. [DOI] [PubMed] [Google Scholar]
- 33.Suplita RL, 2nd, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49:1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda N, Yamanaka K, Yamamoto S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem. 2001;276:35552–35557. doi: 10.1074/jbc.M106261200. [DOI] [PubMed] [Google Scholar]
- 36.Ullrich O, Merker K, Timm J, Tauber S. Immune control by endocannabinoids - new mechanisms of neuroprotection? J Neuroimmunol. 2007;184:127–135. doi: 10.1016/j.jneuroim.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci. 2004;61:945–950. doi: 10.1007/s00018-003-3453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- 39.Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- 40.Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther. 1997;282:243–247. [PubMed] [Google Scholar]
- 41.Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- 42.Yazulla S. Endocannabinoids in the retina: from marijuana to neuroprotection. Prog Retin Eye Res. 2008;27:501–526. doi: 10.1016/j.preteyeres.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]