Abstract
Background
Müllerian inhibiting substance (MIS) is a member of the transforming growth factor β family of growth and differentiation factors that inhibits elongation and branching of mammary ducts and has been shown to inhibit mammary tumor growth in vitro and in animal models. The objective of this study was to determine whether serum MIS levels are associated with breast cancer risk.
Methods
We conducted a prospective case–control study of 309 participants who were registered in the Columbia, Missouri Serum Bank. Each of 105 in situ or invasive breast cancer case patients with prediagnostic serum collected before menopause was matched to two control subjects by age, date, menstrual cycle day, and time of day of blood collection. MIS was measured in serum by using an enzyme-linked immunosorbent assay, and estradiol and testosterone concentrations were quantified by using specific radioimmunoassays. Data were analyzed using conditional logistic regression. All tests of statistical significance were two-sided.
Results
The relative odds ratio of breast cancer for women in increasing MIS quartiles were 1, 2.8 (95% confidence interval [CI] = 1.0 to 7.4), 5.9 (95% CI = 2.4 to 14.6), and 9.8 (95% CI = 3.3 to 28.9, Ptrend < .001). The association of MIS with breast cancer was weaker in women who were not taking oral contraceptives at the time of blood collection, but adjustment for estradiol and testosterone levels did not materially alter results for these women. The association of MIS with breast cancer did not vary by age at blood collection but was stronger among women who were diagnosed with breast cancer at an older age than among those who were diagnosed at a younger age.
Conclusion
MIS may be a novel biomarker of increased breast cancer risk. Additional research including confirmatory epidemiological studies and mechanistic studies is needed.
CONTEXT AND CAVEATS
Prior knowledge
Müllerian inhibiting substance (MIS) inhibits mammary tumor growth in vitro and in mouse models of breast cancer.
Study design
Prospective case–control study to determine whether MIS serum concentrations from blood samples that were collected before diagnosis are associated with breast cancer risk.
Contributions
Increasing MIS serum concentrations were associated with increased breast cancer risk in this population. Adjustment for estrogen and testosterone levels did not change the results. The association did not vary with age at blood collection but was stronger among women who were diagnosed with breast cancer at an older age.
Implications
Increased serum concentrations of MIS may be associated with increased breast cancer risk.
Limitations
The association of increased serum MIS concentrations and increased risk of breast cancer is in contrast to previous findings in vitro and in animal models and what is known about the mechanisms of MIS on breast physiology. The patients in the population less frequently used oral contraceptives than the control subjects, which contrasts with previous data, and could indicate that the control population was not representative of the general population.
From the Editors
Müllerian inhibiting substance (MIS) is a member of the transforming growth factor β family of growth and differentiation factors and is primarily known for its role in regulating in utero sexual differentiation of boys (1). In girls, MIS is secreted by the ovaries beginning during the prepubertal period (2). Serum levels remain elevated during women's reproductive years but decline gradually when ovaries age, reaching undetectable levels at menopause (2–4). MIS plays a key role in regulating the normal development of the breast. In particular, MIS stimulates apoptosis of breast ductal epithelium and, in vivo, inhibits proliferation of mammary ducts (5). MIS has been reported to have direct inhibitory effects on breast tumor growth. It has been shown to inhibit growth of breast cancer cell lines in vitro via inhibition of cell cycle progression and induction of apoptosis (6). MIS also has been reported to suppress mammary tumor growth in mouse models (7). Thus, MIS potentially could decrease breast cancer risk via effects on breast morphology or via direct effects on tumor growth. Alternatively, MIS is a marker of ovarian reserves such that serum levels decline with ovarian aging and the approach of menopause (3,4). In premenopausal women at a median age of 40 years, a decline in serum MIS levels has been shown to be inversely associated with occurrence of the menopausal transition during the next 4 years (8). Thus, higher MIS levels in older premenopausal women may indicate an older age at menopause and, thereby, be associated with increased breast cancer risk. To clarify the association of serum MIS concentration with breast cancer risk, we conducted a prospective case–control study nested in the Columbia, Missouri (MO) Serum Bank.
Subjects and Methods
The Columbia, MO Serum Bank initially was established in 1977 as part of the National Cancer Institute's Biological Markers Project to identify serum markers for breast cancer. Participants were volunteers identified primarily through the Breast Cancer Detection Demonstration Project at the University of Missouri Hospital and the Ellis Fischel Cancer Center in Columbia. A total of 6915 women who initially were free of cancer, other than nonmelanoma skin cancer, donated blood to the bank on one or more occasions between December 1977 and June 1989. All women gave written informed consent before donating serum to the serum bank, and the Fox Chase Cancer Center Institutional Review Board approved the research reported herein.
Serum specimens were collected and clinical data, including age, height, weight, menstrual and reproductive histories, smoking, hormone replacement and oral contraceptive use, and family history of breast cancer, were obtained by self-report or medical record review. Approximately 30 mL of blood were collected from each woman using standard procedures. Blood was allowed to stand at room temperature for at least one-half hour or until it was thoroughly clotted and then refrigerated. Within 2 hours of collection, blood was centrifuged and serum was separated and aliquoted into 1.1-mL sterile glass vials. Vials were labeled, sealed with rubber stoppers, and stored at −70°C.
Follow-up of women who donated blood to the Columbia, MO Serum Bank was conducted in two phases. Initial follow-up continued for up to 12 years until December 1989. A questionnaire was mailed to participants annually to ascertain information on interim cancer diagnoses. Women who indicated that they had a breast biopsy or breast cancer were sent a consent form for permission to obtain medical records including pathology reports. For cancers at sites other than the breast, date of diagnosis was ascertained. An extended follow-up of Columbia, MO Serum Bank participants was conducted by the National Cancer Institute in 1999–2004. Cancer diagnoses were ascertained via self-report and by searching the Missouri Cancer Registry, Breast Cancer Detection Demonstration Project Cohort files, and National Death Index Plus. Of the 6720 women included in the extended follow-up because they had one or more vials of serum remaining, 6131 (91%) were located as either alive or dead, 589 (9%) were not able to be located, 59 (<1%) refused to participate, and 49 (<1%) were too ill to participate. A total of 1751 women (25%) were identified as deceased with confirmation of causes of death provided via National Death Index.
Case patients for the current study included women in the Columbia, MO Serum Bank who were free of cancer other than nonmelanoma skin cancer at the time they donated blood while they were premenopausal and who subsequently were diagnosed with in situ or invasive breast cancer that was confirmed by medical records, the Missouri Cancer Registry, or the National Death Index. Initially, potential case patients were selected if less than 1 year had elapsed since their last menses or if they were younger than 50 years at blood collection and were missing data on (ie, had not recorded) the date of their last menses. (Fifty years is the median age at menopause for naturally menopausal women in the Columbia, MO Serum Bank.) For each of 122 potential case patients that were identified, two potential control subjects were randomly selected who met these same criteria but remained free of cancer. They were matched to the case patient by age (±2 years), date (±1 year), menstrual cycle day (±2 days; if the potential case patient was missing data on the date of her last menses, a potential control subject with missing data on the date of her last menses was selected), and time of day (±2 hours) of blood collection. Matching criteria were relaxed by age for 14 potential control subjects (median = 4.4 years), by date for 18 potential control subjects (median = 2.2 years), by cycle day for 20 potential control subjects (median = 9 days), and by time for nine potential control subjects (median = 3 hours). Final determination of menopausal status for potential participants who were missing data on the date of their last menses was based on age at blood collection, use of exogenous estrogens, and serum follicle stimulating hormone (FSH) and estradiol concentrations. Because 44 years is the 10th percentile of age at menopause in naturally menopausal women in the Columbia, MO Serum Bank, women who were aged 44 years or younger at blood collection and missing data on the date of their last menses were presumed to be premenopausal. For women who were aged 45 years or older and missing data on the date of their last menses, serum FSH and estradiol were measured. Those with serum FSH concentrations greater than 25 mIU/mL and estradiol concentrations less than 25 pg/mL were considered to be postmenopausal and excluded from analysis. This is the standard biochemical definition of menopause used by the laboratory where the assays were performed. We also excluded women who were aged 45 years or older, missing data on the date of their last menses, and using exogenous estrogens at the time of blood collection because their menopausal status could not be determined. After exclusion of postmenopausal women and women with indeterminate menopausal status, 105 case patients and 204 matched control subjects remained and were included in analyses.
All laboratory analyses were performed at the Reproductive Endocrine Research Laboratory, University of Southern California Keck School of Medicine. Serum from each case patient and her matched control subjects was grouped together and matched sets were randomly organized within batches. The laboratory was blinded to which samples were from case patients and which were from control subjects. MIS was quantified using a commercially available enzyme-linked immunosorbent assay kit that uses a monoclonal antibody against MIS (Diagnostics Systems, Webster, TX). Estradiol and testosterone in serum could potentially confound associations of MIS with breast cancer risk. Thus, estradiol and testosterone were quantified by specific radioimmunoassays following extraction and Celite column partition chromatography as described previously (9,10). Sex hormone–binding globulin (SHBG) was measured by a chemiluminescent immunoassay on the Immulite analyzer (Siemens Medical Solutions, Los Angeles, CA) to allow calculation of bioavailable (free plus albumin-bound) estradiol and testosterone (11–13). FSH was similarly measured on the Immulite analyzer. The average coefficient of variation for MIS was 8.0% and its limit of detection was 0.06 ng/mL. As for other serum biomarkers, the average coefficients of variation were 8.5% for estradiol, 11.2% for testosterone, 7.0% for SHBG, and 6.9% for FSH.
Statistical Analysis
The association of serum MIS with breast cancer risk for matched sets was evaluated using conditional logistic regression. Women were stratified into quartiles of MIS, and a set of categorical (dummy) variables was included in the models. Models were fit using quartile ranks entered as a linear term to test for trend because the MIS values were extremely skewed. For women with MIS levels below the assay limit of detection, the value was imputed using 0.03 ng/mL, which is the midpoint between 0 and the assay limit of detection. Univariate associations of individual characteristics including age at blood collection (continuous), height (continuous), body mass index (BMI) (continuous), age at menarche (continuous), age at first pregnancy (continuous), number of full-term pregnancies (continuous), use of exogenous estrogens (referred to as oral contraceptives in the remainder of the article; yes or no), smoking (never, former, or current), history of breast cancer in a first-degree relative (yes or no), and days since last menses when blood was collected (categorized to approximate menstrual cycle phase [follicular: days 0–8, midcycle: days 9–14, luteal: days 15–33, long cycles: days 34+]) with breast cancer were evaluated using conditional logistic regression to retain the matching. Associations of these characteristics with serum MIS concentration in control subjects were evaluated by estimating medians and testing statistical significance of differences using the Wilcoxon rank sum or Kruskal–Wallis test for categorical variables and by Spearman correlations for continuous variables. Analyses to evaluate associations of total and bioavailable estradiol and testosterone concentrations with serum MIS and breast cancer risk were restricted to participants not using oral contraceptives. For two participants with estradiol concentrations below the assay limit of detection, the value was imputed to be 1.5 pg/mL, which is the midpoint between 0 and the limit of detection. Associations of total and bioavailable estradiol and testosterone with MIS concentration in control subjects were evaluated using Spearman correlations. Median concentrations for each hormone in case patients and control subjects were calculated and compared by testing the statistical significance of the trend of quartile ranks entered as a linear term in conditional logistic regression models. Characteristics associated with breast cancer risk and serum MIS concentration at P less than .10 in univariate analyses were deemed potential confounders. A forward stepwise approach was used to evaluate confounding; variables were considered to be confounders if they changed the estimate of the odds ratio (OR) for the highest quartile of MIS by 10% or more. In an age-stratified analysis, women were classified into tertiles of MIS because of small numbers in some cells with finer gradation. Effect modification was assessed by testing the statistical significance of cross-product terms in models. All tests of statistical significance were two-sided and a cutoff of P less than .05 was used to determine statistical significance. All analyses were performed using SAS 9.2 (Cary, NC).
Results
Participant characteristics are summarized in Table 1. All women were premenopausal at the time of blood collection. One case patient and one control subject were African American, and the remainder were white. Case patients’ and control subjects’ ages averaged 44.6 years (range = 31.4–56.1 years) and 44.7 years (range = 33.3–54.7 years), respectively. Case patients did not differ from control subjects in terms of anthropometric or reproductive characteristics. Fewer case patients used oral contraceptives compared with control subjects (4.8% vs 12.2%; P = .05), and more case patients had a history of breast cancer in a first-degree relative compared with control subjects (18.1% vs 7.4%; P = .007). Data on date of last menses were missing for 15.2% of case patients and 13.2% of control subjects, and 8.6% of case patients and 6.9% of control subjects had blood collected 34 days or more after their last menses. The median number of days from last menses at blood collection for these participants was 51 days (range = 35–81 days). Case patients’ mean (±SD) age at breast cancer diagnosis was 58.7 ± 8.3 years (range = 37.7–74.0 years). The mean (±SD) time from blood collection to diagnosis was 14.1 ± 6.6 years.
Table 1.
Participant characteristics at blood collection*
| Characteristic | Case patients (n = 105) | Control subjects (n = 204) | |||
| Continuous variables | Mean ± SD | P† | |||
| Age, y | 44.6 ± 4.7 | 44.7 ± 4.4 | .10 | ||
| Height, cm | 163.3 ± 6.0 | 164.2 ± 5.9 | .26 | ||
| BMI, kg/m2 | 25.9 ± 5.5 | 25.0 ± 4.7 | .13 | ||
| Menarche, y | 12.4 ± 1.3 | 12.7 ± 1.4 | .12 | ||
| Age at first pregnancy, y‡ | 22.3 ± 4.1 | 22.0 ± 3.9 | .58 | ||
| No. of full-term pregnancies | 2.7 ± 1.6 | 2.8 ± 1.6 | .62 | ||
| Categorical variables | Frequency, No. (%) | P§ | |||
| Nulliparous | 9 (8.6%) | 14 (6.9%) | .71 | ||
| Oral contraceptive use‖ | 5 (4.8%) | 25 (12.2%) | .05 | ||
| Smoking status | .81 | ||||
| Never | 67 (63.8%) | 127 (62.2%) | |||
| Former | 19 (18.1%) | 34 (16.7%) | |||
| Current | 19 (18.1%) | 43 (21.1%) | |||
| History of breast cancer in first-degree relative | 19 (18.1%) | 15 (7.4%) | .007 | ||
| Menstrual cycle day at blood collection | .29 | ||||
| Days 0–8 | 23 (21.9%) | 47 (23.0%) | |||
| Days 9–14 | 19 (18.1%) | 36 (17.6%) | |||
| Days 15–33 | 38 (36.2%) | 80 (39.2%) | |||
| Days ≥34 | 9 (8.6%) | 14 (6.9%) | |||
| Unknown | 16 (15.2%) | 27 (13.2%) |
Participants are case patients and control subjects in the prospective case–control study of serum Müllerian inhibiting substance and breast cancer risk nested in the Columbia, Missouri Serum Bank. BMI = body mass index.
P values (two-sided) were calculated by using conditional logistic regression using a Wald test.
Includes 96 parous case patients and 190 parous control subjects.
P values (two-sided) were calculated by using conditional logistic regression using a Wald test for categorical variables with two categories or a likelihood ratio test for categorical variables with more than two categories.
Oral contraceptive use at time of blood collection.
Correlations of serum MIS concentration with individual characteristics of control women are shown in Table 2. MIS concentration was statistically significantly inversely correlated with age at blood collection (Spearman r = −0.64; P < .001). MIS was not correlated with the anthropometric and reproductive characteristics that were examined, including number of pregnancies as a continuous variable. However, nulliparous women had lower MIS levels compared with parous women (P = .04); their median concentrations were 0.03 ng/mL (5–95 percentiles = 0.03–1.6 ng/mL) and 0.10 ng/mL (5–95 percentiles = 0.03–2.4 ng/mL), respectively. MIS levels did not differ by family history of breast cancer (in a first-degree relative) or by smoking status (data not shown). MIS levels also did not differ by the number of days since the start of the women's last menses when blood was collected during the follicular (days 0–8), midcycle (days 9–14), or luteal (days 15–33) phases of the cycle. The median concentration was 0.2 ng/mL (5–95 percentiles = 0.03–2.5 ng/mL). In contrast, all control subjects whose blood was collected at 34 days or more after last menses had MIS levels below the assay limit of detection (imputed value = 0.03 ng/mL), which was statistically significantly lower than for those whose blood was collected within 33 days of last menses (P < .001). The median MIS concentration for control subjects who were missing data on the date of last menses was 0.03 ng/mL (5–95 percentiles = 0.03–1.1 ng/mL), which was not statistically significantly different from the median MIS concentration for subjects with a known date of last menses overall or that for control subjects whose blood was collected within 33 days of last menses. Control subjects using oral contraceptives at the time of blood collection had statistically significantly lower MIS concentrations compared with those not using oral contraceptives (P = .006); median concentrations were 0.03 ng/mL (5–95 percentiles = 0.03–0.5 ng/mL) and 0.1 ng/mL (5–95 percentiles = 0.03–2.5 ng/mL), respectively.
Table 2.
Correlations of serum Müllerian inhibiting substance concentration with characteristics of control subjects (n = 204)*
| Characteristic | Spearman correlation | P† |
| Age, y | −.64 | <.001 |
| Height, cm | .01 | .87 |
| BMI, kg/m2 | .07 | .33 |
| Menarche, y | .07 | .34 |
| Age at first pregnancy, y‡ | −.05 | .47 |
| No. of full-term pregnancies | .08 | .28 |
Control subjects are from the prospective case–control study of serum Müllerian inhibiting substance and breast cancer risk nested in the Columbia, Missouri Serum Bank. All variables are continuous. BMI = body mass index.
P values (two-sided) were calculated by using a t-statistic transformation to test correlation = 0.
Includes 190 parous control subjects.
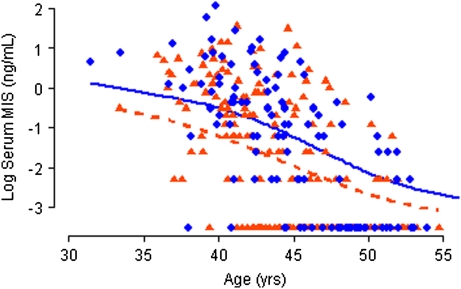
As shown in Figure 1, case patients had higher MIS concentrations than control subjects. In analysis that included all participants, case patients’ and control subjects’ median MIS values were 0.4 ng/mL and 0.1 ng/mL, respectively (P < .001). Similar results were obtained when the analysis was restricted to matched sets in which the case patient and at least one control subject were not using oral contraceptives (Table 3). Among participants who did not use oral contraceptives, total and bioavailable estradiol concentrations did not differ between the groups (Table 3). However, case patients had statistically significantly higher total testosterone and bioavailable testosterone concentrations than control subjects.
Figure 1.
Serum Müllerian inhibiting substance (MIS) by age in case patients and control subjects. Natural logarithm of serum MIS level plotted by age in case patients (blue circles) and control subjects (orange triangles) and smoothed lines fit with a kernel smoother for case patients (blue solid line) and control subjects (orange dashed line).
Table 3.
Median (5–95th percentiles) hormone concentrations in case patients and control subjects not using oral contraceptives*
| Case patients (n = 98) |
Control subjects (n = 169) |
||||
| Median | 5–95th percentiles | Median | 5–95th percentiles |
P† |
|
| Müllerian inhibiting substance, ng/mL | 0.30 | 0.03–3.4 | 0.10 | 0.03–2.5 | <.001 |
| Estradiol, pg/mL | 92.5 | 23.0–277.0 | 98.0 | 12.0–248.0 | .97 |
| Bioavailable estradiol, pg/mL | 56.5 | 12.2–153.2 | 54.8 | 8.6–146.1 | .84 |
| Testosterone, ng/dL | 27.5 | 16.0–49.6 | 25.7 | 13.0–44.3 | .01 |
| Bioavailable testosterone, ng/dL | 11.7 | 5.7–23.5 | 10.2 | 4.9–19.0 | .004 |
| SHBG, nmol/L | 57.5 | 27.3–114.0 | 60.7 | 26.1–117.0 | .24 |
Case patients and control subjects from matched sets where the case patient and at least one control subject were not using oral contraceptives at blood collection in the prospective case–control study of serum Müllerian inhibiting substance and breast cancer risk nested in the Columbia, Missouri Serum Bank. All variables are continuous. SHBG = sex hormone–binding globulin.
P values (two-sided) were calculated by using conditional logistic regression with quartile ranks entered as a linear term to test for trend using a Wald test.
Serum MIS concentration was strongly and statistically significantly positively associated with risk of developing breast cancer (Ptrend < .001; Table 4). Risk increased monotonically with increasing MIS concentration; the relative odds ratios for increasing quartiles of MIS were 1.0, 2.8 (95% confidence interval [CI] = 1.0 to 7.4), 5.9 (95% CI = 2.4 to 14.6), and 9.8 (95% CI = 3.3 to 28.9). Adjustment for potential confounders and BMI did not substantially change risk estimates (BMI-adjusted models, increasing MIS quartiles, OR = 1, OR = 2.6, 95% CI = 1.0 to 7.2; OR = 5.6, 95% CI = 2.3 to 13.9; and OR = 9.9, 95% CI = 3.3 to 29.2; Ptrend < .001). There also was no evidence of effect modification of the MIS breast cancer association by BMI (Pinteraction = .43 between BMI and MIS quartile rank when included in model).
Table 4.
Relative odds ratios (ORs) and confidence intervals (CIs) of breast cancer by serum Müllerian inhibiting substance (MIS) concentration
| All participants* |
Excluding oral contraceptive users*† |
|||||
| MIS, ng/mL | No. of case patients/No. of control subjects | OR (95% CI) | P‡ | No. of case patients/No. of control subjects | OR (95% CI) | P‡ |
| <.001 | <.001 | |||||
| <0.06 | 28/90 | 1.0 | 28/70 | 1.0 | ||
| 0.1 | 10/20 | 2.8 (1.0 to 7.4) | 10/16 | 2.3 (0.9 to 6.2) | ||
| 0.2–0.8 | 35/51 | 5.9 (2.4 to 14.6) | 31/45 | 4.2 (1.6 to 10.9) | ||
| 0.9–8.0 | 32/43 | 9.8 (3.3 to 28.9) | 29/38 | 7.3 (2.3 to 23.6) | ||
Case patients and control subjects are from the prospective case–control study of serum MIS and breast cancer risk nested in the Columbia, Missouri Serum Bank. Case patients and control subjects were matched on age, date, hour, and days since last menses at blood collection. MIS cut points define quartiles of MIS estimated from all participants.
Includes only matched sets where the case patient and at least one control subject were not using oral contraceptives at blood collection.
P values (two-sided) were calculated by using conditional logistic regression with quartile ranks entered as a linear term to test for trend using a Wald test.
The association of serum MIS with breast cancer was weaker but remained statistically significant in women not using oral contraceptives at the time of blood collection (Ptrend < .001; Table 4). In an analysis restricted to 98 matched sets in which the case patient and at least one control subject were not using oral contraceptives, the odds ratios for increasing quartiles of MIS were 1.0, 2.3 (95% CI = 0.9 to 6.2), 4.2 (95% CI = 1.6 to 10.9), and 7.3 (95% CI = 2.3 to 23.6). Because only five case patients used oral contraceptives at the time of blood collection, associations of MIS with breast cancer among oral contraceptive users were not reported.
Of the 105 women diagnosed with breast cancer, 12 had in situ cancers and 93 had invasive cancers. Results of an analysis restricted to invasive breast cancer were similar to those for in situ and invasive cancers combined that is shown above (for all invasive breast cancer, increasing MIS quartiles of MIS, OR = 1, OR = 3.0, 95% CI = 1.1 to 8.5; OR = 5.8, 95% CI = 2.2 to 14.9; and OR = 9.1, 95% CI = 3.0 to 28.1; Ptrend < .001). The association was weaker in the 86 matched sets of women who did not use oral contraceptives at the time of blood collection (OR = 1; OR = 2.5, 95% CI = 0.9 to 7.1; OR = 4.0, 95% CI = 1.5 to 10.7; and OR = 6.6, 95% CI = 2.0 to 22.1; Ptrend = .001).
Among control subjects not using oral contraceptives, serum MIS concentrations were not correlated with total estradiol (Spearman r = .12; P = .10) or total testosterone (Spearman r = .14; P = .07) concentrations. MIS concentrations, however, were correlated with concentrations of the bioavailable fractions of these hormones (for non–SHBG-bound estradiol, Spearman r = .16, P = .03; for non–SHBG-bound testosterone, Spearman r = .21, P = .005).
Adjustment for total and non–SHBG-bound estradiol and testosterone did not substantially change risk estimates for MIS among women who were not using oral contraceptives at the time of blood collection (highest vs lowest MIS quartile, adjusted for total estradiol, OR = 6.9, 95% CI = 2.1 to 22.6; adjusted for non–SHBG-bound estradiol, OR = 7.4, 95% CI = 2.3 to 24.0; adjusted for total testosterone, OR = 8.1, 95% CI = 2.4 to 27.9; adjusted for non–SHBG-bound testosterone, OR = 6.9, 95% CI = 2.1 to 22.6). The association of MIS with breast cancer risk remained statistically significant after adjustment for each of these hormones (Ptrend < .002). Tests for interaction between MIS and total or bioavailable estradiol and testosterone in relationship to breast cancer risk were not statistically significant.
To explore the association of MIS with breast cancer by age at blood collection, we conducted analyses stratified by age less than 45 years or 45 years and older at time of blood collection (Table 5). In 100 matched sets, the case patient and at least one control subject were either less than 45 years old (56 sets) or 45 years and older (44 sets) at blood collection. MIS was positively associated with breast cancer risk in both age strata. The association was similar among younger women (highest vs lowest MIS tertile, OR = 7.5, 95% CI = 1.9 to 29.8; Ptrend = .002) and among older women (highest vs lowest MIS tertile, OR = 6.9, 95% CI = 1.1 to 42.0; Ptrend = .01). The test for interaction between MIS and age at blood collection was not statistically significant. (Lower risk estimates from this stratified analysis compared with the unstratified analysis shown above is a result of using tertiles vs quartiles of MIS.)
Table 5.
Relative odds ratios (ORs) and confidence Intervals (CIs) of breast cancer by serum Müllerian inhibiting substance (MIS) concentration stratified by age at blood collection
| Age <45 y*† |
Age ≥45 y*‡ |
|||||
| MIS, ng/mL | No. of case patients/No. of control subjects | OR (95% CI) | P§ | No. of case patients/No. of control subjects | OR (95% CI) | P§ |
| .002 | .01 | |||||
| <0.06 | 3/21 | 1.0 | 25/60 | 1.0 | ||
| 0.1–0.5 | 15/38 | 3.0 (0.8 to 11.5) | 13/15 | 3.2 (1.1 to 9.4) | ||
| 0.6–8.0 | 38/49 | 7.5 (1.9 to 29.8) | 6/4 | 6.9 (1.1 to 42.0) | ||
Case patients and control subjects are from the prospective case–control study of serum MIS and breast cancer risk nested in the Columbia, Missouri Serum Bank. Case patients and control subjects were matched on age, date, hour, and days since last menses at blood collection. MIS cut points define tertiles of MIS estimated from all participants.
Includes only matched sets where the case patient and at least one control subject were less than 45 years old at blood collection.
Includes only matched sets where the case patient and at least one control subject were 45 years or older at blood collection.
P values (two-sided) were calculated by using conditional logistic regression with tertile ranks entered as a linear term to test for trend using a Wald test.
Because MIS varies only slightly over the menstrual cycle (14), we included women with missing data on the date of their last menses at blood collection if they met our criteria for being premenopausal. Because 44 years was the 10th percentile of age at natural menopause in the cohort, women who were 44 years old or younger at blood collection were eligible even though some with missing data on the date of their last menses could have been postmenopausal. In an analysis that excluded these women, the association of MIS with breast cancer was stronger (highest vs lowest MIS quartile, OR = 13.6, 95% CI = 3.8 to 48.2; P < .001). Results were similar in an analysis that excluded all women with missing data on date of their last menses or for women whose blood was collected more than 33 days after their last menses (highest vs lowest MIS quartile, OR = 12.5, 95% CI = 3.5 to 44.3; Ptrend < .001).
Because we did not have data on eventual age at menopause, we could not perform an analysis stratified by menopausal status at diagnosis. As a proxy, we conducted an analysis stratified by age at diagnosis (Table 6). Thirty-two case patients were younger than 55 years at breast cancer diagnosis. When we restricted the analysis to these case patients and their matched control subjects, serum MIS concentration remained positively associated with breast cancer (highest vs lowest MIS tertile, OR = 3.9, 95% CI = 0.9 to 16.3; Ptrend = .03). In a similar analysis for the 73 matched sets in which the case patient was aged 55 years or older at diagnosis, the positive association of MIS with breast cancer risk was stronger (highest vs lowest MIS tertile, OR = 9.6, 95% CI = 2.8 to 33.3; Ptrend < .001).
Table 6.
Relative odds ratios (ORs) and confidence intervals (CIs) of breast cancer by serum Müllerian inhibiting substance (MIS) concentration stratified by age at diagnosis
| Age <55 y at diagnosis* |
Age ≥55 y at diagnosis* |
|||||
| MIS, ng/mL | No. of case patients/No. of control subjects | OR (95% CI) | P† | No. of case patients/No. of control subjects | OR (95% CI) | P† |
| .03 | <.001 | |||||
| <0.06 | 6/16 | 1.0 | 22/74 | 1.0 | ||
| 0.1–0.5 | 5/19 | 1.2 (0.3 to 5.4) | 27/36 | 5.7 (2.1 to 15.5) | ||
| 0.6–8.0 | 21/26 | 3.9 (0.9 to 16.3) | 24/33 | 9.6 (2.8 to 33.3) | ||
Case patients and control subjects are from the prospective case–control study of serum MIS and breast cancer risk. Case patients and control subjects were matched on age, date, hour, and days since last menses at blood collection. MIS cut points define tertiles of MIS estimated from all participants.
P values (two-sided) were calculated by using conditional logistic regression with tertile ranks entered as a linear term to test for trend using a Wald test.
The time from blood collection to diagnosis ranged from 2 months to 23 years. When we repeated analyses restricted to the 68 case patients whose blood was collected at least 12 years before diagnosis and their matched control subjects, results were similar to those from analyses that included all case patients (shown in Table 4) (highest vs lowest MIS quartile, OR = 9.0, 95% CI = 2.3 to 35.5; Ptrend = .001).
Discussion
To our knowledge, this is the first prospective study of premenopausal serum MIS concentration and breast cancer risk. We observed a strong positive association of MIS concentration with breast cancer (highest quartile of MIS vs lowest quartile, OR = 9.8, 95% CI = 3.3 to 28.9). The association was somewhat weaker in women who were not using oral contraceptives at the time of blood collection (highest MIS quartile vs lowest, OR = 7.3, 95% CI = 2.3 to 23.6). Adjustment for serum estradiol and testosterone concentrations in data from women who did not use oral contraceptives did not materially alter the association of MIS with breast cancer. The association of MIS with breast cancer did not vary by age at blood collection but was stronger among women who were diagnosed with breast cancer at an older age than among those who were diagnosed at a younger age. These results suggest that MIS may be a novel biomarker of breast cancer risk.
Data from in vitro studies and animal models support a protective effect of MIS for breast cancer (6,7). However, in this first study to evaluate the association of serum MIS with breast cancer risk in humans, we found little evidence for an inverse association between serum MIS concentration and breast cancer. By contrast, MIS concentration was statistically significantly positively related to breast cancer risk. MIS acts by binding to its receptor (MISRII), and MISRII mRNA is expressed by normal breast epithelial cells, fibroadenomas, and ductal carcinomas (6). Thus, MIS could potentially act directly on normal or abnormal breast tissue to stimulate carcinogenesis. Although at high doses MIS has been shown to inhibit mammary tumor growth in vitro and in animal models (6,7), its effect on carcinogenesis at physiological concentrations is unknown.
MIS is a biomarker of ovarian function with properties that may make it particularly informative in epidemiological research. Although serum concentrations decline with age after 29 years (3), women's relative concentrations track over time such that age-adjusted differences from mean concentrations in serum samples collected 4 years apart are correlated (r = .66; P < .01) (4). MIS concentrations do not vary markedly by day of the menstrual cycle (14–16). MIS also is stable between menstrual cycles; the intraclass correlation for MIS in serum from three consecutive menstrual cycles was .89 (17). In an analysis based on the Columbia, MO Serum Bank, we similarly observed an intraclass correlation coefficient of .87 for MIS levels in serum samples collected from the same women at least 1 year apart (18).
In women, MIS is produced by ovarian granulosa cells of preantral and small antral follicles (19). Within the ovary, MIS suppresses follicle maturation by inhibiting the recruitment of primordial follicles into the pool of growing follicles and by decreasing the responsiveness of growing follicles to FSH (19). Production of estradiol in granulosa cells is critical for follicle maturation. MIS has been reported to inhibit FSH-stimulated aromatase activity in granulosa cells, resulting in decreased intracellular estradiol levels and suppressed follicle maturation (20). However, similar to our study, most investigations do not find an association between total serum estradiol and MIS levels in healthy women (3,16,21–23). We observed a weak positive correlation between serum MIS and non–SHBG-bound estradiol that, to our knowledge, has not been reported previously and that could be related to the older age of the participants and to the inclusion of women who were not regularly menstruating.
Serum MIS concentration is associated with the number of small antral follicles (3,23,24), and serum levels decline with ovarian aging, reaching undetectable levels at menopause when the pool of follicles is depleted (3,4). This decline in MIS levels begins at a young age, many years before the menopausal transition. In a longitudinal analysis, serum MIS declined 38% for a period of 2.6 years in women whose mean age was 29 years at their first measurement (3). However, even though MIS concentrations decline with aging, women often maintain the same relative concentration for their age over time (4). Thus, premenopausal women of the same age with higher MIS levels may experience menopause at a later age, which could contribute to increased breast cancer risk. However, the odds ratio for breast cancer increases about 4% for each additional year of age at menopause (25), which translates into an odds ratio of approximately 1.48 for a 10-year difference. In comparison, we observed an odds ratio between 7 and 10 for women with elevated MIS levels depending on oral contraceptive use. Therefore, later age at menopause would not explain most of the increased risk of breast cancer associated with elevated serum MIS levels.
MIS was positively related to breast cancer risk among women who were diagnosed with breast cancer when younger than 55 years, but the association was stronger among women diagnosed at an older age. MIS is not detectable in serum after menopause (3), and its continued association with breast cancer after menopause suggests two hypotheses. Exposure to higher MIS levels before menopause could permanently alter the breast to increase long-term risk of developing breast cancer. Alternatively, serum MIS could be a marker of underlying differences in ovarian physiology that encompass multiple endocrine and/or metabolic alterations, some of which may persist after menopause. MIS levels are elevated in women with polycystic ovarian disease (21,23), but specific MIS-related hormonal alterations that could lead to an increased risk of breast cancer have yet to be clarified.
Some data indicate that the ovaries continue to produce testosterone after menopause (26,27). MIS concentration was previously reported to be positively associated with testosterone in healthy women (22), but results are not consistent across studies (23). In our analysis, MIS and testosterone were positively correlated, but adjustment for total and non–SHBG-bound testosterone did not substantially alter associations of MIS with breast cancer in women not using oral contraceptives. Thus, although serum testosterone is related to breast cancer risk in postmenopausal women (28), it is unlikely to be the sole explanation for the continued strong association of MIS with breast cancer long after MIS is undetectable in serum, and other hormones and growth factors could also be involved.
Our study had many strengths. Most notably, MIS was measured in serum that was collected many years before the case patients’ breast cancer diagnoses, cohort follow-up was 91% complete, and the number of breast cancer case patients was large enough to draw meaningful conclusions.
It also had several potential limitations. These limitations included small numbers for some stratified analyses and lack of data on age at menopause. In addition, although the case patients and control subjects in this study differed as expected on some characteristics such as family history of breast cancer, the lower frequency of oral contraceptive use in case patients compared with control subjects is at odds with most data (29). The use of oral contraceptives as a whole was lower than expected, and these results could reflect the underlying distribution in the cohort; alternatively, the results could suggest that control subjects in the study are not representative of unaffected women in the cohort. If the latter is true, the observed associations between serum MIS and breast cancer could be biased. The positive association of serum MIS with breast cancer risk that we observed is opposite of what would be predicted based on the limited in vitro and in vivo results from animal studies on the association of MIS with mammary tumorigenesis (6,7). However, more preclinical data support a protective effect of MIS for ovarian cancer (30), and our pilot data that show an inverse association of MIS with ovarian cancer risk in this cohort are consistent with that (data not shown). Thus, the positive association that we observed between serum MIS and breast cancer risk, although unexpected based on earlier findings, could be real.
Few cancer risk factors are as strongly associated with breast cancer as that we observed (OR = 9.8) for women in the highest vs lowest quartile of MIS. However, we previously reported odds ratios of 5.2 and 6.2 for breast cancer associated with elevated non–SHBG-bound estradiol and testosterone in this cohort (31). Premenopausal women included in the MIS analysis were distinct from those in our previous analysis of hormones and breast cancer risk that only included women who were postmenopausal at blood collection. However, the very strong association with endogenous hormone levels in both groups could reflect the same underlying, but as yet unidentified, cause. In the pooled analysis of prospective studies on serum hormones and breast cancer, the odds ratios for postmenopausal women in the highest quintiles of non–SHBG-bound estradiol and testosterone compared with the lowest were 2.39 and 2.22, respectively (28). The stronger associations in the Columbia, MO Serum Bank, in part, may have been related to the limited use of exogenous hormones in this cohort. None of the participants in the pooled analysis were using hormone replacement therapy at the time of blood collection, but some had used it previously. When these women were excluded, associations of endogenous hormones with breast cancer were stronger. Women in the cohort who develop breast cancer potentially also could be particularly susceptible to hormonal influences on risk. If this is the case, risk estimates would be expected to be smaller in other cohorts. Nonetheless, our results, if confirmed, could suggest that underlying differences in ovarian folliculogenesis associated with elevated serum MIS contribute to breast cancer risk.
In conclusion, MIS may be a biomarker of ovarian function that is associated with increased breast cancer risk. Additional research is needed, including confirmatory epidemiological studies on the association of serum MIS with breast cancer and studies aimed at identifying the biological mechanism underlying the association.
Funding
Department of Defense (BC062367 to J.F.D.), National Institutes of Health (P30CA006927), and National Institutes of Health intramural research program.
Footnotes
The sponsors had no role in the study design, analysis, collection and interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Present address: Clinical and Translational Medicine, Wyeth Research, Collegeville, PA (C. S. Spittle).
We thank all of the participants in the Columbia, Missouri Serum Bank.
References
- 1.Teixeira J, Maheswaran S, Donahoe PK. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22(5):657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- 2.Lee MM, Donahoe PK, Hasegawa T, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81(2):571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 3.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 4.van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Segev DL, Hoshiya Y, Stephen AE, et al. Mullerian inhibiting substance regulates NFkappaB signaling and growth of mammary epithelial cells in vivo. J Biol Chem. 2001;276(29):26799–26806. doi: 10.1074/jbc.M103092200. [DOI] [PubMed] [Google Scholar]
- 6.Segev DL, Ha TU, Tran TT, et al. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000;275(37):28371–28379. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 7.Gupta V, Carey JL, Kawakubo H, et al. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci U S A. 2005;102(9):3219–3224. doi: 10.1073/pnas.0409709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooij IA, Tonkelaar I, Broekmans FJ, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6, pt 1):601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 9.Probst-Hensch NM, Ingles SA, Diep AT, et al. Aromatase and breast cancer susceptibility. Endocr Relat Cancer. 1999;6(2):165–173. doi: 10.1677/erc.0.0060165. [DOI] [PubMed] [Google Scholar]
- 10.Goebelsmann U, Arce JJ, Thorneycroft IH, Mishell DR., Jr Serum testosterone concentrations in women throughout the menstrual cycle and following HCG administration. Am J Obstet Gynecol. 1974;119(4):445–452. doi: 10.1016/0002-9378(74)90199-9. [DOI] [PubMed] [Google Scholar]
- 11.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 13.Rinaldi S, Geay A, Dechaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11(10, pt 1):1065–1071. [PubMed] [Google Scholar]
- 14.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 15.Cook CL, Siow Y, Taylor S, Fallat ME. Serum mullerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73(4):859–861. doi: 10.1016/s0015-0282(99)00639-1. [DOI] [PubMed] [Google Scholar]
- 16.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21(12):3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 17.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20(4):923–927. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 18.Dorgan JF, Spittle CS, Egleston BL, Shaw CM, Kahle LL, Brinton LA. Assay reproducibility and within-person variation of mullerian inhibiting substance. Fertil Steril. doi: 10.1016/j.fertnstert.2009.03.032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124(5):601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 20.Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89(suppl 5):1364–1370. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 21.Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum mullerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77(1):141–146. doi: 10.1016/s0015-0282(01)02944-2. [DOI] [PubMed] [Google Scholar]
- 22.Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20(7):1820–1826. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- 23.Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 24.Pastor CL, Vanderhoof VH, Lim LC, et al. Pilot study investigating the age-related decline in ovarian function of regularly menstruating normal women. Fertil Steril. 2005;84(5):1462–1469. doi: 10.1016/j.fertnstert.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Kvale G, Heuch I. Menstrual factors and breast cancer risk. Cancer. 1988;62(8):1625–1631. doi: 10.1002/1097-0142(19881015)62:8<1625::aid-cncr2820620828>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Aiman J, Forney JP, Parker CR., Jr Secretion of androgens and estrogens by normal and neoplastic ovaries in postmenopausal women. Obstet Gynecol. 1986;68(1):1–5. [PubMed] [Google Scholar]
- 27.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92(8):3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 28.The Endogenous Hormones and Breast Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein L. The risk of breast, endometrial and ovarian cancer in users of hormonal preparations. Basic Clin Pharmacol Toxicol. 2006;98(3):288–296. doi: 10.1111/j.1742-7843.2006.pto_277.x. [DOI] [PubMed] [Google Scholar]
- 30.La Marca A, Volpe A. The anti-Mullerian hormone and ovarian cancer. Hum Reprod. 2007;13(3):265–273. doi: 10.1093/humupd/dml060. [DOI] [PubMed] [Google Scholar]
- 31.Dorgan JF, Longcope C, Stephenson HE, Jr, et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(7):533–539. [PubMed] [Google Scholar]