Summary
Stimulation of Na+/K+-ATPase activity in alveolar epithelial cells by cAMP involves its recruitment from intracellular compartments to the plasma membrane. Here, we studied the role of the actin molecular motor myosin-V in this process. We provide evidence that, in alveolar epithelial cells, cAMP promotes Na+/K+-ATPase recruitment to the plasma membrane by increasing the average speed of Na+/K+-ATPase-containing vesicles moving to the cell periphery. We found that three isoforms of myosin-V are expressed in alveolar epithelial cells; however, only myosin-Va and Vc colocalized with the Na+/K+-ATPase in intracellular membrane fractions. Overexpression of dominant-negative myosin-Va or knockdown with specific shRNA increased the average speed and distance traveled by the Na+/K+-ATPase-containing vesicles, as well as the Na+/K+-ATPase activity and protein abundance at the plasma membrane to similar levels as those observed with cAMP stimulation. These data show that myosin-Va has a role in restraining Na+/K+-ATPase-containing vesicles within intracellular pools and that this restrain is released after stimulation by cAMP allowing the recruitment of the Na+/K+-ATPase to the plasma membrane and thus increased activity.
Keywords: Na+/K+-ATPase, Myosin-V, Traffic
Introduction
The Na+/K+-ATPase is an essential enzyme that generates the Na+ and K+ gradients required for maintaining membrane potentials, cell volume, and secondary transport of other solutes, such as transcellular transport in the intestine, kidneys and lungs (Gadsby, 2007; Jorgensen et al., 2003; Sznajder et al., 2002). In the lungs, the Na+/K+-ATPase located at the basolateral plasma membrane drives the vectorial Na+ transport across the alveolar epithelium necessary to keep the alveoli free of edema (Mutlu and Sznajder, 2005; Sznajder et al., 2002). Alveolar fluid reabsorption is increased after stimulation of Na+/K+-ATPase activity by G-protein-coupled-receptor (GPCR) agonists that activate the second messenger cAMP (Frank et al., 2000; Litvan et al., 2006; Saldias et al., 1998). cAMP increases the Na+/K+-ATPase function in alveolar epithelial cells by promoting its recruitment from intracellular vesicle pools into the plasma membrane (Bertorello et al., 1999; Lecuona et al., 2003).
Trafficking of vesicles from intracellular pools to the plasma membrane typically involves their transport along microtubules and actin filaments (Soldati and Schliwa, 2006). To accomplish this task, cells utilize molecular motors. Actin-based molecular motors constitute a superfamily of proteins, called myosins (Berg et al., 2001; Richards and Cavalier-Smith, 2005). They use the energy from cycles of ATP binding, hydrolysis, and product release to perform mechanical work along actin filaments (De La Cruz and Ostap, 2004). Within the myosin family, a major actin molecular motor involved in intracellular trafficking is myosin-V, which is involved in the transport of vesicles and organelles in several cell types (Langford, 2002; Mermall et al., 1998). Three genes encoding myosin-V (myosin-Va, Vb and Vc) have been described with specific distribution in different tissues (Espreafico et al., 1992; Rodriguez and Cheney, 2002; Zhao et al., 1996). Myosin-Va has been shown to be involved in the transport of many vesicular carriers, including dense core secretory vesicles (Ivarsson et al., 2005; Varadi et al., 2005), melanosomes (Wu et al., 1998; Wu et al., 1997) and GLUT4-containing vesicles (Yoshizaki et al., 2007), and a role for myosin-Va in tethering vesicles to the actin cytoskeleton has also been suggested (Wu et al., 1998).
We generated an alveolar epithelial cell line that expresses the Na+/K+-ATPase α1-subunit tagged with GFP and, using these cells, we reported that GPCR agonist stimulation increases the directional movement of ATPase-containing vesicles (Bertorello et al., 2003). In the present study, we investigated the role of myosin-V in the recruitment of the Na+/K+-ATPase to the plasma membrane. We provide evidence that myosin-Va has a role in restraining Na+/K+-ATPase-containing vesicles in the intracellular pool; furthermore, the inhibition of myosin-Va by overexpression of a dominant-negative mutant or knockdown with shRNA results in increased traffic and insertion of the Na+/K+-ATPase into the plasma membrane, which is not sensitive to further stimulation.
Results
cAMP increases Na+/K+-ATPase localization at the plasma membrane and the distance traveled by the Na+/K+-ATPase-containing vesicles in A549-GFPα1 cells
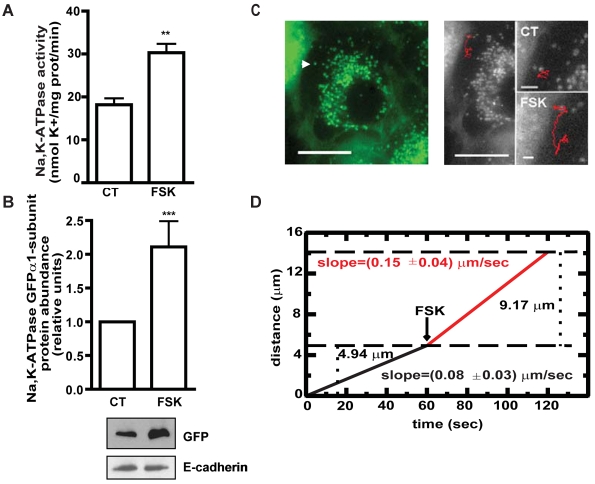
To establish whether the A549-GFPα1 cells are a good model to study the effects of cAMP on the Na+/K+-ATPase traffic, we incubated the cells for 10 minutes with 50 μM of the adenylyl cyclase activator forskolin (FSK) at 37°C. As shown in Fig. 1, incubation with FSK increased Na+/K+-ATPase activity (Fig. 1A) as well as the Na+/K+-ATPase protein abundance at the plasma membrane (Fig. 1B), suggesting that in response to FSK the Na+/K+-ATPase is recruited to the plasma membrane from intracellular pools as previously described (Bertorello et al., 1999; Lecuona et al., 2003).
Fig. 1.
cAMP increases Na+/K+-ATPase activity, protein abundance at the plasma membrane and the distance traveled by the Na+/K+-ATPase-containing vesicles in A549-GFPα1 cells. (A) A549-GFPα1 cells were incubated in the absence (CT) or presence of 50 μM forskolin (FSK) for 10 minutes and the Na+/K+-ATPase activity was measured as 86Rb+ uptake. Graph represents mean ± s.e.m. of three experiments. (B) A549-GFPα1 cells were incubated as in A, and the Na+/K+-ATPase abundance at the basolateral plasma membrane was determined by western blot of the BLM fraction using a specific antibody against GFP. E-cadherin was used as a loading control. Graph represents mean ± s.e.m. of three experiments. A representative western blot is shown. (C) The movement of the GFP-labeled particles was recorded as Metamorph stacks and vesicle trajectories were obtained by single-particle tracking using Metamorph software. Vesicles were randomly selected from those that showed plus-end-directed displacement. Left panel shows a representative image of A549-GFPα1 cells. Arrowhead indicates the vesicle whose trajectory is shown in the right panel before (CT) and after FSK treatment (FSK). (D) Average contour length traveled by the vesicles as a function of time. The black line represents control vesicles; at 60 seconds, upon addition of FSK (red line), the vesicles move at a faster rate. The average contour length is determined by averaging over many trajectories as described in the Materials and Methods. **P<0.01; ***P<0.001. Scale bars: 10 μm and 2 μm (magnified images).
Live imaging of A549-GFPα1 cells showed that, after incubation with FSK for 1 minute, the average speed of the GFP-containing vesicles moving towards the cell periphery increased by ∼80% (Fig. 1C,D) (control, 0.08±0.03 μm/second; FSK, 0.15±0.04 μm/second; P<0.05).
Myosin-Va and myosin-Vc colocalize with the Na+/K+-ATPase in intracellular compartments
To study whether myosin-V has a role in Na+/K+-ATPase trafficking, we determined its expression in A549 cells. We found that the three isoforms of myosin-V (a, b and c) were detected at the mRNA level (Fig. 2A) and at the protein level (Fig. 2B). As shown in Fig. 2C,D, the three myosin-V isoforms distributed equally in fraction 6 of a broad sucrose gradient in which the Na+/K+-ATPase peaks, although the overall distribution of each isoform varied across different fractions.
Fig. 2.
The three isoforms of myosin-V are expressed in A549 cells. (A) RT-PCR using mRNA obtained from A549 and HeLa cells. Primers used for the amplification are described in supplementary material Table S6. (B) Cell lysates from A549 and HeLa cells were obtained and analyzed by western blot with specific antibodies against the three myosin-V isoforms. A representative western blot is shown. (C) The particulate fraction (100,000 g pellet) of A549-GFPα1 cells was loaded onto a flotation sucrose gradient and eight fractions were recovered. The distribution of the proteins of interest was analyzed by western blotting with specific antibodies. A representative western blot is shown. Rab5 and Rab7 are used as markers of early and late endosomes, respectively. (D) Gradients obtained in C were scanned and the marker content was digitally quantified as indicated. Results are expressed as percentage of the total amount of protein.
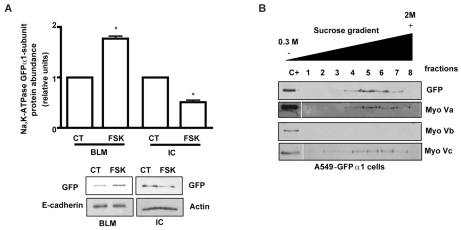
To elucidate which myosin-V isoform has a role in the traffic of Na+/K+-ATPase, we isolated the intracellular fraction enriched in the cAMP-responsive Na+/K+-ATPase vesicles (Fig. 3A) (Bertorello et al., 1999). This fraction was loaded on a broad sucrose gradient (2 M to 0.5 M) and analyzed for distribution of the Na+/K+-ATPase and myosin-V isoforms. Fig. 3B shows that Na+/K+-ATPase present in the intracellular compartment distributed mainly in fractions 4 to 7. Myosin-Va and myosin-Vc co-distributed in the same fractions, whereas myosin-Vb was not detectable.
Fig. 3.
Myosin-Va and myosin-Vc colocalize with Na+/K+-ATPase. (A) A549-GFPα1 cells were incubated in the absence or presence of 50 μM FSK for 10 minutes, basolateral membranes (BLM) and intracellular compartments (IC) were isolated and the Na+/K+-ATPase abundance was determined by western blot using a specific antibody against GFP. E-cadherin and actin were used as loading controls for the BLM and IC fractions, respectively. Graph represents mean ± s.e.m. of three experiments. A representative western blot is shown. (B) The IC fraction of A549-GFPα1 cells was loaded onto a flotation sucrose gradient and eight fractions were recovered. The distribution of the proteins of interest was analyzed by western blotting with specific antibodies. A representative western blot is shown. C+, positive control.
Myosin-Va is a negative regulator of the long-range movement of Na+/K+-ATPase-containing vesicles
To determine whether myosin-Va and/or myosin-Vc have a role in cAMP-induced Na+/K+-ATPase traffic, we analyzed by live-cell imaging A549-GFPα1 cells transiently transfected with dominant-negative (DN) myosin-Va (DN-Va) or with DN myosin-Vc (DN-Vc) containing an m-cherry tag. As shown in Fig. 4A,B, expression of m-cherry-DN-Va increased the basal motility of GFP-containing vesicles, motility that was not further increased by FSK. m-Cherry-DN-Vc did not have any effect on the baseline motility nor did it prevent cAMP-mediated stimulation (Fig. 4C,D). These data suggest a role for myosin-Va in the regulation of the basal motility of the GFP-containing vesicles (but not for myosin-Vc) and were confirmed in experiments using shRNA against myosin-Va and myosin-Vc (Fig. 5). We found increased basal motility of GFP-containing vesicles in cells transfected with the shRNA against myosin-Va (Fig. 5A,C) and no change in motility in the cells where myosin-Vc was knocked down (Fig. 5B,C). Knockdown of myosin-Va and myosin-Vc using these shRNA was ∼65% (Fig. 5D).
Fig. 4.
The average speed of Na+/K+-ATPase-containing vesicles moving towards the cell periphery is increased in cells expressing a myosin-Va stalk-tail. (A) Live imaging of A549-GFPα1 cells (green) transiently transfected with a dominant-negative myosin-Va that has a m-cherry-tag (red) (m-cherry-DN-Va). The movement of the GFP-labeled particles was recorded. Upper panels show a representative image of the transfected A549-GFPα1 cells. Lower panels show the tracking of the movement of one vesicle before (CT) and after forskolin treatment (FSK). (B) Average contour length traveled by the vesicles in A as a function of time. The black line represents the control vesicles; FSK was added at time 60 seconds and is represented as a red line. (C) Live imaging of A549-GFPα1 cells (green) transiently transfected with a dominant-negative myosin-Vc that has a m-cherry-tag (red) (m-cherry-DN-Vc). The movement of the GFP-labeled particles was recorded. Upper panels show a representative image of the transfected A549-GFPα1 cells. Lower panels show the tracking of the movement of one vesicle before (CT) and after forskolin treatment (FSK). (D) Average contour length traveled by the vesicles in C as a function of time. The black line represents control vesicles; FSK was added at 60 seconds and is represented as the red line. Scale bars: 10 μm and 2 μm (magnified images).
Fig. 5.
The average speed of Na+/K+-ATPase-containing vesicles moving towards the cell periphery is increased in cells expressing a shRNA against myosin-Va. (A) Live imaging of A549-GFPα1 cells (green) transiently transfected with a shRNA against myosin-Va that has a m-cherry-tag (red) (m-cherry-sh-Va). The movement of the GFP-labeled particles was recorded. Upper panels show a representative image of the transfected A549-GFPα1 cells. Lower panels show the tracking of the movement of two vesicles (arrowheads) under control (CT) conditions. (B) Live imaging of A549-GFPα1 cells (green) transiently transfected with a shRNA against myosin-Vc that has a m-cherry-tag (red) (m-cherry-shRNA-Vc). The movement of the GFP-labeled particles was recorded. Upper panels show a representative image of the transfected A549-GFPα1 cells. Lower panels show the tracking of the movement of two vesicles (arrowheads) under control (CT) conditions (C). Graph represents the average contour length traveled by the vesicles as a function of time, calculated as described in methods. The black line represents the m-cherry-sh-Va vesicles and the red line, the m-cherry-sh-Vc vesicles. (D) A549-GFPα1 cells were transfected with a shRNA against myosin-Va or myosin-Vc, cell lysates were isolated and the myosin-Va (left panel) or myosin-Vc (right panel) abundance was determined by western blot using specific antibodies. E-cadherin and tubulin were used as loading controls. Scale bars: 10 μm and 4 μm (magnified images).
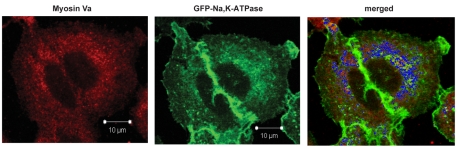
To study the role of myosin-Va in the recruitment of Na+/K+-ATPase-containing vesicles, we generated A549-GFPα1 cells clones permanently transfected with DN-Va or DN-Vc tagged with V5 (Fig. 6A). Cells were incubated with 50 μM FSK for 10 minutes, which increased the Na+/K+-ATPase activity (Fig. 6B) and protein abundance at the plasma membrane (Fig. 6C) in control cells and cells expressing DN-Vc. However, DN-Va cells had increased baseline levels of Na+/K+-ATPase activity and protein abundance at the plasma membrane and treatment with FSK did not result in further stimulation (Fig. 6B,C). These data are in agreement with the colocalization of the GFP-containing vesicles and myosin-Va observed by immunofluorescence (Fig. 7).
Fig. 6.
Dominant-negative myosin-Va mimics cAMP-mediated Na+/K+-ATPase increased activity and recruitment to the plasma membrane in A549-GFPα1 cells. (A) Stable clones expressing myosin-Va tail (DN-Va) and myosin-Vc tail (DN-Vc) were generated as described. Expression of the constructs in the permanent clones was analyzed by western blotting using and antibody against the V5 tag. A representative western blot is shown. (B) A549-GFPα1 cells (CT) and A549-GFPα1 cells permanently transfected with DN-Va and DN-Vc were incubated in the absence or presence of 50 μM FSK for 10 minutes and the Na+/K+-ATPase activity was measured as 86Rb+ uptake. Graph represents mean ± s.e.m. of three different experiments. (C) Control (CT), DN-Va and DN-Vc cells were incubated in the absence or presence of 50 μM FSK for 10 minutes and western blots of the basolateral membrane fraction were performed using a specific antibody against GFP. E-cadherin was used as loading control. A representative western blot is shown. *P<0.05; **P<0.01; n.s., not significant; u.s., unstimulated.
Fig. 7.
Myosin-Va and the Na+/K+-ATPase-containing vesicles colocalize. A549-GFPα1 cells were fixed, permeabilized and blocked. Myosin-Va was visualized by using an anti-myosin-Va antibody and a secondary antibody labeled with Alexa Fluor 568. GFP was directly visualized. Cellular distribution of Na+/K+-ATPase-GFPα1 and myosin-Va was analyzed using a Zeiss LSM 510 laser-scanning confocal microscope and colocalization (blue) was determined using the LSM 510 Meta software.
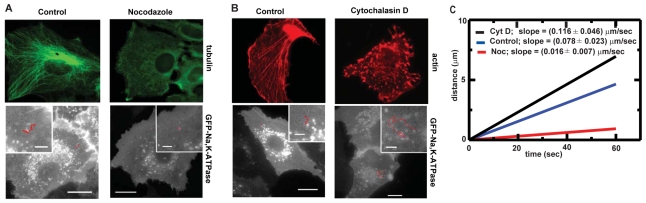
Taken together, these data suggest that myosin-Va has a restraining role on Na+/K+-ATPase-containing vesicles and that this restraint is released upon FSK stimulation. These observations are consistent with the finding that disruption of the microtubule network prevented Na+/K+-ATPase traffic (Fig. 8A,C), whereas depolymerization of the actin cytoskeleton increased it (Fig. 8B,C).
Fig. 8.
Microtubules and actin filaments are involved in Na+/K+-ATPase traffic. (A) Live imaging of A549 cells incubated with 10 μM nocodazole for 3 hours. The movement of the GFP-labeled particles was recorded as Metamorph stacks and vesicle trajectories were obtained by single-particle tracking using Metamorph software. Upper panels show a representative immunofluorescence of the microtubule cytoskeleton in control (left) and nocodazole (right) conditions. Lower panel shows the tracking of the movement of one vesicle in control (left) and nocodazole (right) conditions. (B) Live imaging of A549 cells incubated with 5 μM cytochalasin D (Cyto D) for 1 hour. The movement of the GFP-labeled particles was recorded as Metamorph stacks and vesicle trajectories were obtained by single-particle tracking using Metamorph software. Upper panels show a representative immunofluorescence of the actin cytoskeleton under control (left) and cytochalasin D (right) conditions. Lower panel shows the tracking of the movement of one vesicle in control (left) and cytochalasin D (right) conditions. (C) Average contour length traveled by the vesicles as a function of time. The blue line represents the control vesicles; the black line, cells treated with cytochalasin D and the red line, cells treated with nocodazole. Scale bars: 10 μm and 2 μm (inset images).
Discussion
Short-term regulation of Na+/K+-ATPase activity can result from its traffic between intracellular compartments and the plasma membrane (Bertorello and Sznajder, 2005; Clausen, 2003; Dunbar and Caplan, 2001; Therien and Blostein, 2000). β-adrenergic receptor agonists have been shown to upregulate the Na+/K+-ATPase function via cAMP by promoting its recruitment from intracellular compartments into the plasma membrane in alveolar epithelial cells, which results in increased Na+ transport and enhanced lung edema clearance (Bertorello et al., 1999; Lecuona et al., 2003; Saldias et al., 1998). Recruitment of Na+/K+-ATPase-containing vesicles into the plasma membrane is known to involve both microtubules and the actin network (Bertorello et al., 2003; Bertorello et al., 1999; Saldias et al., 1998). Here, we provide the first evidence for the role of an actin-based motor, myosin-Va, in the modulation of the microtubule-dependent movement of the Na+/K+-ATPase in alveolar epithelial cells.
We have generated a human alveolar epithelial cell line that expresses GFP-tagged Na+/K+-ATPase α1-subunit. This cell line has been utilized for the study of trafficking of the Na+/K+-ATPase-containing vesicles (Bertorello et al., 2003). These cells responded to stimulation by cAMP by increasing Na+/K+-ATPase protein abundance and activity at the plasma membrane, in parallel with an increase in the distance traveled (see Fig. 1).
We focused on the role of the myosin-V family of molecular motors, because there is much evidence implicating them in organelle movement (Mermall et al., 1998). Moreover, the presence of myosin-V has been described in organelles that associate with both microtubules and actin (Kuznetsov et al., 1992; Rogers and Gelfand, 1998). We analyzed the presence of the three myosin-V motors, myosin-Va, myosin-Vb and myosin-Vc (Espreafico et al., 1992; Rodriguez and Cheney, 2002; Zhao et al., 1996), and found that the three isoforms were expressed both at the mRNA and at the protein levels, although only myosin-Va and myosin-Vc were found in the intracellular compartment from which the Na+/K+-ATPase moves towards the plasma membrane upon stimulation by cAMP (Bertorello et al., 1999). These findings were somewhat surprising because myosin-Vb has been shown to be involved in the traffic of proteins known to be stimulated by cAMP, such as aquaporin-2 in renal cells (Nedvetsky et al., 2007).
A powerful tool to study the role of myosin-V in the traffic of organelles/vesicles is the overexpression of its tail domain, which competes for binding to the cargo, but lacks the actin-interacting head and thus, acts as a dominant-negative inhibitor (Rodriguez and Cheney, 2002; Rogers et al., 1999). This technique has been useful to study myosin-Va in the traffic of intracellular organelles and vesicles, such as secretory granules in PC12 cells (Rudolf et al., 2003), small synaptic vesicles (Prekeris and Terrian, 1997) and melanosomes (Rogers et al., 1999; Wu et al., 1997). Time-lapse imaging of live cells showed that in basal conditions, the movement of Na+/K+-ATPase-containing vesicles followed a `random walk' pattern in agreement with a previous report (Bertorello et al., 2003). More significantly, we found that cAMP increased the average speed of the vesicles moving towards the cell periphery in control non-transfected cells and in cells transfected with DN-Vc. Cells expressing DN-Va experienced rapid microtubule-dependent vesicle movement to the periphery, independently of cAMP, similar to that observed in melanocytes lacking myosin-Va (Wu et al., 1998). Since a dominant-negative strategy has its pitfalls, such as non-specific effects on related proteins (Roos et al., 1999), we confirmed the role of myosin-Va in the regulation of the basal movement of the Na+/K+-ATPase by specifically knocking down the protein using an shRNA strategy.
Myosin-Va has a role in different aspects of the traffic of several vesicular carriers (Desnos et al., 2007; Eichler et al., 2006). It has been shown to be important in retaining these vesicular carriers (melanosomes, secretory granules) in the F-actin-rich cortex (Rudolf et al., 2003; Wu et al., 1998), and recently a role in fusion and exocytosis has also been reported via the interaction of myosin-Va with syntaxin-1A (Watanabe et al., 2005). Our data suggest that under basal conditions, microtubule-dependent transport of Na+/K+-ATPase-containing vesicles is restrained by myosin-Va. Experiments in cells overexpressing the DN-Va revealed increased Na+/K+-ATPase traffic and insertion into the plasma membrane that was similar to the effects of treating the cells with GPCR agonists.
Intracellular transport is a complex process, driven by at least three types of motors: kinesins and dyneins involved in bidirectional transport along microtubules and myosins that move along actin filaments (King, 2000; Mermall et al., 1998; Woehlke and Schliwa, 2000). Recent work suggests that switching between the two types of cytoskeletal tracks is based on a continuous `tug-of-war' between the two transport systems, where microtubule motors and myosins are simultaneously active on the same organelle and the choice of tracks is determined by competition between these motors (Gross et al., 2002).
Phosphorylation of myosin-Va has been reported to regulate its function (Karcher et al., 2001; Yoshizaki et al., 2007). Interestingly, phosphorylation at Ser1650 by CaMKII releases myosin-Va from melanosomes (Karcher et al., 2001). The consensus sequence for CaMKII (R-x-x-S/T) is very similar to the sequence for PKA (the main effector of cAMP) (Gronborg et al., 2002; O'Flaherty et al., 2004; Taylor et al., 2008), raising the possibility for phosphorylation of this residue in our system, which warrants further studies.
Accordingly, we provide the first evidence that myosin-Va regulates traffic of Na+/K+-ATPase containing-vesicles and that it has a key role in holding the Na+/K+-ATPase-containing vesicles in basal conditions, restraining its recruitment to the plasma membrane; this restraint is released upon stimulation with cAMP agonists.
Materials and Methods
Reagents
All cell culture reagents were from Mediatech (Herndon, VA). 86Rb+ was purchased from Perkin Elmer (Billerica, MA). Ouabain was purchased from ICN Biomedicals (Aurora, OH). Percoll was from Amersham Biosciences (Uppsala, Sweden). Forskolin, nocodazole and cytochalasin D were from Calbiochem (La Jolla, CA). Restriction enzymes were purchased from Promega (Madison, WI). Rhodamine phalloidin was from Molecular Probes (Eugene, OR). Normal goat serum was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Antibody against α-tubulin and all other chemicals were purchased from Sigma (St Louis, MO). Mouse anti-GFP (B-2), rabbit anti-E-cadherin, rabbit anti-Rab5a, rabbit anti-Rab7, and Protein A/G PLUS-Agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-V5 antibody and secondary goat-anti-mouse Alexa Fluor 488 and 568 were from Invitrogen (Carlsbad, CA). Secondary goat anti-mouse-HRP and goat anti-rabbit-HRP were from Bio-Rad (Hercules, CA). Antibody against myosin-Va has been previously described (Rogers et al., 1999). Antibody against myosin-Vb was a gift from Alaa El-Husseini (University of British Columbia, Vancouver, Canada) and antibody against myosin-Vc was a gift from Richard Cheney (University of North Carolina at Chapel Hill, NC).
Cell culture
Human A549 (ATCC CCL 185) and HeLa cells (ATCC CCL 2) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 20 mM HEPES. A549 cells expressing the GFP-rat Na+/K+-ATPase-α1-subunit (A549-GFPα1) (Bertorello et al., 2003) were grown in the same conditions as above but 3 μM ouabain was added to the medium to suppress the endogenous Na+/K+-ATPase α1-subunit. Cells were incubated in a humidified atmosphere of 5% CO2, 95% air at 37°C.
86Rb+ uptake
The assay was run at 37°C in a reciprocating bath at 100 r.p.m. Cells were pre-incubated in serum-free DMEM, containing HEPES with or without 5 μM ouabain and 50 μM FSK for 5 minutes. Then, the medium was removed and fresh medium containing 1 μCi/ml 86Rb+, with or without 5 μM ouabain and 50 μM FSK was added. Following incubation for 5 minutes, uptake was terminated by aspirating the assay medium and washing the plates in 150 mM MgCl2 (4°C). Cells were air-dried and extracted with 0.1% NaOH. 86Rb+ influx was quantified by liquid scintillation counting. Initial influx, expressed as micromoles of K+/μg of protein per minute, was calculated as: Influx = (c.p.m./μg protein/5)/SAex, where SAex is the specific activity of the extracellular phase (c.p.m./μmol K+). The ouabain-inhibitable fraction of the Na+/K+-ATPase was measured as 86Rb+ uptake in the presence of 5 μM ouabain.
Preparation of basolateral membranes
A549-GFPα1 cells were incubated for 10 minutes with 50 μM FSK at 37°C, washed twice with ice-cold phosphate-buffered saline (PBS), and basolateral membranes were purified according to Hammond and Verroust by using a 16% Percoll gradient (Hammond et al., 1994).
Western blot analysis
Protein was quantified by Bradford assay (Bradford, 1976) (Bio-Rad) and resolved in 10% SDS-PAGE. Thereafter, proteins were transferred onto nitrocellulose membranes (Optitran, Schleider & Schuell, Keene, NH) using a semi-dry transfer apparatus (Bio-Rad). Incubation with specific antibodies was performed overnight at 4°C. Blots were developed with a chemiluminescence detection kit (PerkinElmer Life Sciences, Boston, MA) used as recommended by the manufacturer. The bands were scanned and quantified by ImageJ 1.41a (National Institutes of Health, USA).
Live-cell imaging
Time-lapse epifluorescent microscopy of A549-GFPα1 cells was performed using a Nikon TE2000 (Nikon Instruments, Melville, NY) equipped with an environmental control system chamber (FCS2 system, Bioptechs, Butler, PA) and a Planapo ×60/1.4 NA objective (Nikon Instruments, Melville, NY). During imaging, the chamber was perfused with DMEM saturated with gas mixture containing 5% CO2 and 21% O2. Images were acquired every second with an exposure time of 0.5 seconds and collected with a Cascade EMCCD camera with on-chip multiplication gain (Photometrics, Tucson, AZ) driven by MetaMorph Software v6.5 (Molecular Devices, Downingtown, PA). To decrease phototoxic effects, a 0.25 neutral density filter was used. In some experiments, during imaging process cells were treated with 5 μM FSK by addition of the drug into the perfusion medium.
Calculations
Vesicle trajectories were obtained by single-particle tracking using Metamorph software v6.5. 20 vesicles from five different experiments were randomly selected among those which showed plus-end-directed displacement (vesicles that display movement towards the cell periphery) and that were able to be measured for up to 120 frames. Their uninterrupted movement was analyzed one by one for each condition.
The trajectories were analyzed in the following way. For each trajectory we obtained the contour length of the trajectory as a function of time, and they showed a linear dependence. Thus, we performed a linear fit for each trajectory and found that the coefficient of determination R2, was close to unity in all cases. Supplementary material Table S1 shows the values of the slopes obtained from the linear regressions as well as the R2 values for the control and FSK-treated cases. All other analysis are in supplementary material Tables S2-S6. The straight lines plotted in the figures were obtained by using the average slope in the construction of the lines, i.e. the displacement = contour length = slope × (t+a). For the control cases, we take a=0 and for the treated vesicles the origin corresponds to the last point of the control contour length.
In order to assure that the differences between the slopes were statistically significant, a two-sample t-test was performed for each system of interest. The resulting P-values can be found in supplementary material Tables S1-S6.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
The reverse transcriptase (RT) reaction was performed using the Superscript first-strand synthesis system for RT-PCR by Invitrogen (Carlsbad, CA) following the manufacturer's instructions. 1 μg total RNA (RNeasy, Qiagen, Santa Clarita, CA) was converted into cDNA after denaturing at 70°C for 15 minutes, by incubation with a buffer containing oligo-dT primers, the RT enzyme and deoxynucleoside triphosphate (dNTP) mix for 50 minutes at 42°C. The RT enzyme was then inactivated by incubation at 70°C for 15 minutes. The resultant cDNAs were amplified by polymerase chain reaction (PCR) using myosin-V-specific primers (supplementary material Table S7), then analyzed by 1.5% agarose gel electrophoresis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a positive control.
Cell lysate
Cells were washed twice with ice-cold phosphate-buffered saline (PBS), and lysed in modified RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% NP-40 and 1% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 100 μg/ml N-tosyl-L-phenylalanine chloromethyl ketone (TPCK), 10 μg/ml leupeptin), and centrifuged 10 minutes at 20,000 g. Supernatant was considered the cell lysate.
Sucrose gradient fractionation
A549-GFPα1 cells were placed on ice, washed twice with ice-cold PBS, scraped off the plate and collected by centrifugation. Pellet was resuspended in subcellular fractionation buffer (0.3 M sucrose, 5 mM EDTA 10 mM Tris-HCl, pH 7.4, 1 mM PMSF, 100 μg/ml TPCK, 10 μg/ml leupeptin) and subcellular fractionation by sucrose gradient was performed as previously described (Rose et al., 2003). Briefly, cells were gently homogenized by using a Dounce homogenizer, and the samples were subjected to a 5 minute centrifugation (4°C, 500 g). Supernatant was centrifuged at 100,000 g and pellet was resuspended in homogenization buffer and loaded on top of a sucrose gradient. Gradient consisted of seven 0.5 ml steps with sucrose concentration increasing from 0.5 M to 1.7 M, plus 1 ml of 2 M sucrose cushion at the bottom. Gradient was centrifuged at 200,000 g for 1 hour and 30 minutes and eight different fractions were collected from the interfaces between the sucrose steps. In some experiments, intracellular compartments obtained as described below were subjected to sucrose gradient fractionation. Briefly, intracellular fractions were centrifuged for 1 hour at 200,000 g, the pellet was resuspended in subcellular fractionation buffer and loaded on top of a sucrose gradient as described above.
Preparation of intracellular compartments
A549-GFPα1 cells were incubated for 10 minutes with 50 μM FSK at 37°C, placed on ice, washed twice with ice-cold PBS, scraped in PBS, centrifuged and resuspended in homogenization buffer (250 mM sucrose, 3 mM imidazole, 2 mM EGTA, 1 mM PMSF, 100 μg/ml TPCK, and 10 μg/ml leupeptin, pH 7.4). Cells were gently homogenized (15-20 strokes) using a Dounce homogenizer, and the samples were subjected to a 5 minute centrifugation (4°C, 500 g). Intracellular compartments were fractionated on a flotation gradient as described (Bertorello et al., 1999; Ridge et al., 2002) by using essentially the technique of Gorvel and colleagues (Gorvel et al., 1991).
Construction of m-cherry-tagged myosin-Va short tail (MST) and m-cherry-tagged myosin-Vc tail (DN-Vc)
pcDNA3-m-cherry-MST contains the C-terminal 601 amino acids of the mouse myosin-Va gene fused to the C-terminus of the m-cherry tag (Shaner et al., 2004). The following PCR primers were used to amplify the m-cherry DNA from the construct pm-cherry-C1: 5′-TTTAAGCTTATGGTGAGCAAGGG-3′ and 5′-TTTTTGGATCCTCTTGTACAGCTC-3′. The PCR product was digested with HindIII and BamHI, and cloned into the vector pcDNA3-Myc-MST (Rogers et al., 1999). To construct lenti-m-cherry-DN-Vc, the following PCR primers were used: 5′-AAAGGATCCATGGTGAGCAAGGGCGAGGA-3′ and 5′-GCTGGATCCTTCTTGTACAGCTCGTCCATGGC-3′ to amplify the m-cherry tag. The PCR product was digested with BamHI, and cloned into the lenti-DN-Vc (described below).
Small-hairpin RNA against myosin-Va and myosin-Vc
shRNAs against myosin-Va and myosin-Vc were generated using the m-cherry version of the pG-SUPER vector (Brummelkamp et al., 2002; Kojima et al., 2004) (a gift from Shin Kojima, Northwestern University, Chicago, IL). This vector co-expresses m-cherry fluorescent protein (under the SRα promoter) and shRNA, (under the human H1 promoter) simultaneously so that the knockdown of the target proteins could be analyzed at the level of individual cells as well as by western blot. The targeting sequences were as follows: MyoVa (NM_000259), 5′-TAAGATGCTACCAGAACTA-3′ and MyoVc (NM_018728), 5′-GGACATACATCGAGTTCTA-3′.
Transient transfection
A549-GFPα1 cells were grown on 40 mm round coverslips (Bioptech; Butler, PA) at a density of 1×104 cells per coverslip. m-cherry-MST and m-cherry-DN-Vc were transfected using jetPEI™ (Polyplus transfection; Illkirch, France) as indicated by the manufacturers. Cells were used 24 hours after transfection. Plasmids containing shRNAs constructs were transfected using Lipofectamine LTX (Invitrogen) following recommended protocols. Cells were harvested after 72 hours.
Generation of stable cell lines expressing the myosin-Va and myosin-Vc tails
The myosin tail domains (the last 909 amino acids for myosin-Va and the last 835 amino acids for myosin-Vc) were amplified from A549 cells using sequence specific primers (Myosin-Va, 5′-CACCATGGAGAAACTAACCAATCTG-3′ and 5′-GACCCGTGAAATAAAGCCAGG-3′; Myosin-Vc, 5′-CACCATGGTGGAGAAGCTGACTAGCCTG-3′ and 5′-TAACCTATTCAGAAAGCCTAG-3′. The gel-purified product was then used in TOPO cloning reaction (pLenti6/V5 D-TOPO, Invitrogen). Lentivirus was packaged in 293FT cells (Invitrogen). The supernatant containing the virus was harvested and used directly to infect A549-GFPα1 cells. Stable clones expressing myosin-Va tail (DN-Va) and Myosin-Vc tail (DN-Vc) were selected by adding 4 μg/ml Blastacidin (Invitrogen; Carlsbad, CA). Expression of the constructs in the permanent clones was analyzed by western blotting using an antibody against the V5 tag (Fig. 5A).
Immunocytochemistry
A549-GFPα1 cells were fixed for 10 minutes in 3.7% formaldehyde, permeabilized with 0.1% Triton X-100 and blocked in 1 μg/μl normal goat serum with 2% BSA. Myosin-Va was visualized by using an anti-myosin-Va antibody (1:75) and a secondary antibody labeled with Alexa Fluor 568 (1:300). GFP was directly visualized. Cellular distribution of Na+/K+-ATPase-GFPα1 and myosin-Va was analyzed by direct fluorescence using a Zeiss LSM 510 laser-scanning confocal microscope (objective Plan Apochromat, ×63/1.4 NA oil-immersion lens). Cross-sections were generated with a 0.2 μm motor step. Contrast and brightness settings were adjusted so that all pixels were in the linear range. Colocalization was established using the LSM 510 Meta software.
For microtubule staining, cells were fixed for 5 minutes in cold methanol (–20°C) and 15 minutes in 3.7% formaldehyde (4°C); for F-actin staining, cells were fixed 10 minutes in 3.7% formaldehyde at room temperature. After fixation, cells were permeabilized and blocked as described above. Microtubules were visualized by using an anti-α-tubulin antibody (1:100) and a secondary antibody labeled with Alexa Fluor 488 (1:200). Actin was visualized by incubating with Rhodamine-phalloidin (1:60). Images were collected using a Nikon TE2000 epifluorescent microscope and a ×60/1.40 NA Planapo lens (Nikon Instruments, Melville, NY) using a Cascade II EMCCD (Photometrics, Tucson, AZ).
Statistical analysis
Data are represented as means ± s.e.m. Comparisons between two groups of values were evaluated by Student's t-test. Results were considered significant when P<0.05.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/21/3915/DC1
The authors acknowledge Kui Zhu for the generation of dominant-negative Va and Vc, and Alejandro Bertorello for the GFPα1 plasmid. This research was partially supported by NIH grants HL48129, HL71643, HL076139, GM52111 and RB1-2506-PU-03. D.G. is a recipient of a National Science Foundation Graduate Research Fellowship. Deposited in PMC for release after 12 months.
References
- Berg, J. S., Powell, B. C. and Cheney, R. E. (2001). A millennial myosin census. Mol. Biol. Cell 12, 780-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello, A. and Sznajder, J. I. (2005). The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am. J. Respir. Cell Mol. Biol. 33, 432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello, A. M., Ridge, K. M., Chibalin, A. V., Katz, A. I. and Sznajder, J. I. (1999). Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am. J. Physiol. 276, L20-L27. [DOI] [PubMed] [Google Scholar]
- Bertorello, A. M., Komarova, Y., Smith, K., Leibiger, I. B., Efendiev, R., Pedemonte, C. H., Borisy, G. and Sznajder, J. I. (2003). Analysis of Na+/K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell 14, 1149-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. [DOI] [PubMed] [Google Scholar]
- Brummelkamp, T. R., Bernards, R. and Agami, R. (2002). Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2, 243-247. [DOI] [PubMed] [Google Scholar]
- Clausen, T. (2003). Na+-K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 83, 1269-1324. [DOI] [PubMed] [Google Scholar]
- De La, Cruz, E. M. and Ostap, E. M. (2004). Relating biochemistry and function in the myosin superfamily. Curr. Opin. Cell Biol. 16, 61-67. [DOI] [PubMed] [Google Scholar]
- Desnos, C., Huet, S. and Darchen, F. (2007). “Should I stay or should I go?”: myosin V function in organelle trafficking. Biol. Cell 99, 411-423. [DOI] [PubMed] [Google Scholar]
- Dunbar, L. A. and Caplan, M. J. (2001). Ion pumps in polarized cells: sorting and regulation of the Na+/K+- and H+/K+-ATPases. J. Biol. Chem. 276, 29617-29620. [DOI] [PubMed] [Google Scholar]
- Eichler, T. W., Kögel, T., Bukoreshtliev, N. V. and Gerdes, H. H. (2006). The role of myosin Va in secretory granule trafficking and exocytosis. Biochem. Soc. Trans. 34, 671-674. [DOI] [PubMed] [Google Scholar]
- Espreafico, E., Cheney, R., Matteoli, M., Nascimento, A., De Camilli, P., Larson, R. and Mooseker, M. (1992). Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J. Cell Biol. 119, 1541-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, J. A., Wang, Y., Osorio, O. and Matthay, M. A. (2000). beta -Adrenergic agonist therapy accelerates the resolution of hydrostatic pulmonary edema in sheep and rats. J. Appl. Physiol. 89, 1255-1265. [DOI] [PubMed] [Google Scholar]
- Gadsby, D. C. (2007). Structural biology: ion pumps made crystal clear. Nature 450, 957-959. [DOI] [PubMed] [Google Scholar]
- Gorvel, J. P., Chavrier, P., Zerial, M. and Gruenberg, J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- Gronborg, M., Kristiansen, T. Z., Stensballe, A., Andersen, J. S., Ohara, O., Mann, M., Jensen, O. N. and Pandey, A. (2002). A mass spectrometry-based proteomic approach for identification of serine/threonine-phosphorylated proteins by enrichment with phospho-specific antibodies: identification of a novel protein, Frigg, as a protein kinase a substrate. Mol. Cell. Proteomics 1, 517-527. [DOI] [PubMed] [Google Scholar]
- Gross, S. P., Tuma, M. C., Deacon, S. W., Serpinskaya, A. S., Reilein, A. R. and Gelfand, V. I. (2002). Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156, 855-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. G., Verroust, P. J., Majewski, R. R., Muse, K. E. and Oberley, T. D. (1994). Heavy endosomes isolated from the rat cortex show attributes of intermicrovillar clefts. Am. J. Physiol. 267, F516-F527. [DOI] [PubMed] [Google Scholar]
- Ivarsson, R., Jing, X., Waselle, L., Regazzi, R. and Renström, E. (2005). Myosin 5a controls insulin granule recruitment during late-phase secretion. Traffic 6, 1027-1035. [DOI] [PubMed] [Google Scholar]
- Jorgensen, P. L., Hakansson, K. O. and Karlish, S. J. (2003). Structure and mechanism of Na/K-ATPase: Functional Sites and Their Interactions. Annu. Rev. Physiol. 65, 817-849. [DOI] [PubMed] [Google Scholar]
- Karcher, R. L., Roland, J. T., Zappacosta, F., Huddleston, M. J., Annan, R. S., Carr, S. A. and Gelfand, V. I. (2001). Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science 293, 1317-1320. [DOI] [PubMed] [Google Scholar]
- King, S. M. (2000). The dynein microtubule motor. Biochim. Biophys. Acta 1496, 60-75. [DOI] [PubMed] [Google Scholar]
- Kojima, S., Vignjevic, D. and Borisy, G. (2004). Improved silencing vector co-expressing GFP and small hairpin RNA. Biotechniques 36, 74-79. [DOI] [PubMed] [Google Scholar]
- Kuznetsov, S. A., Langford, G. M. and Weiss, D. G. (1992). Actin-dependent organelle movement in squid axoplasm. Nature 356, 722-725. [DOI] [PubMed] [Google Scholar]
- Langford, G. M. (2002). Myosin-V, a versatile motor for short-range vesicle transport. Traffic 3, 859-865. [DOI] [PubMed] [Google Scholar]
- Lecuona, E., Ridge, K., Pesce, L., Batlle, D. and Sznajder, J. I. (2003). The GTP-binding protein RhoA mediates Na+/K+-ATPase exocytosis in alveolar epithelial cells. Mol. Biol. Cell 14, 3888-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan, J., Briva, A., Wilson, M. S., Budinger, G. R. S., Sznajder, J. I. and Ridge, K. M. (2006). beta-Adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na+/K+-ATPase and alveolar fluid reabsorption. J. Biol. Chem. 281, 19892-19898. [DOI] [PubMed] [Google Scholar]
- Mermall, V., Post, P. L. and Mooseker, M. S. (1998). Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279, 527-533. [DOI] [PubMed] [Google Scholar]
- Mutlu, G. M. and Sznajder, J. I. (2005). Mechanisms of pulmonary edema clearance. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L685-L695. [DOI] [PubMed] [Google Scholar]
- Nedvetsky, P. I., Stefan, E., Frische, S., Santamaria, K., Wiesner, B., Valenti, G., Hammer, J. A., Nielsen, S., Goldenring, J. R., Rosenthal, W. et al. (2007). A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8, 110-123. [DOI] [PubMed] [Google Scholar]
- O'Flaherty, C., de Lamirande, E. and Gagnon, C. (2004). Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol. Hum. Reprod. 10, 355-363. [DOI] [PubMed] [Google Scholar]
- Prekeris, R. and Terrian, D. M. (1997). Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex. J. Cell Biol. 137, 1589-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, T. A. and Cavalier-Smith, T. (2005). Myosin domain evolution and the primary divergence of eukaryotes. Nature 436, 1113-1118. [DOI] [PubMed] [Google Scholar]
- Ridge, K. M., Dada, L., Lecuona, E., Bertorello, A. M., Katz, A. I., Mochly-Rosen, D. and Sznajder, J. I. (2002). Dopamine-induced exocytosis of Na+/K+-ATPase is dependent on activation of protein kinase C-epsilon and -delta Mol. Biol. Cell 13, 1381-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, O. C. and Cheney, R. E. (2002). Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 115, 991-1004. [DOI] [PubMed] [Google Scholar]
- Rogers, S. L. and Gelfand, V. I. (1998). Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 8, 161-164. [DOI] [PubMed] [Google Scholar]
- Rogers, S. L., Karcher, R. L., Roland, J. T., Minin, A. A., Steffen, W. and Gelfand, V. I. (1999). Regulation of melanosome movement in the cell cycle by reversible association with myosin V. J. Cell Biol. 146, 1265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, E., Soede, R., Stroeken, P. and Driessens, M. (1999). Advances in molecular genetic technology to be used in experimental metastasis research. In Intermolecular Cross-Talk in Tumor Metastasis (ed. G. G. Skouteris and G. L. Nicolson). Washington, DC: IOS Press.
- Rose, S. D., Lejen, T., Casaletti, L., Larson, R. E., Pene, T. D. and Trifaro, J. M. (2003). Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J. Neurochem. 85, 287-298. [DOI] [PubMed] [Google Scholar]
- Rudolf, R., Kogel, T., Kuznetsov, S. A., Salm, T., Schlicker, O., Hellwig, A., Hammer, J. A., 3rd and Gerdes, H. H. (2003). Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J. Cell Sci. 116, 1339-1348. [DOI] [PubMed] [Google Scholar]
- Saldias, F., Lecuona, E., Friedman, E., Barnard, M. L., Ridge, K. M. and Sznajder, J. I. (1998). Modulation of lung liquid clearance by isoproterenol in rat lungs. Am. J. Physiol. 274, L694-L701. [DOI] [PubMed] [Google Scholar]
- Shaner, N. C., Campbell, R. E., Steinbach, P. A., Giepmans, B. N. G., Palmer, A. E. and Tsien, R. Y. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567-1572. [DOI] [PubMed] [Google Scholar]
- Soldati, T. and Schliwa, M. (2006). Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell. Biol. 7, 897-908. [DOI] [PubMed] [Google Scholar]
- Sznajder, J. I., Factor, P. and Ingbar, D. H. (2002). Lung edema clearance: role of Na+-K+-ATPase. J. Appl. Physiol. 93, 1860-1866. [DOI] [PubMed] [Google Scholar]
- Taylor, S. S., Kim, C., Cheng, C. Y., Brown, S. H. J., Wu, J. and Kannan, N. (2008). Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim. Biophy. Act 1784, 16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien, A. G. and Blostein, R. (2000). Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 279, C541-C566. [DOI] [PubMed] [Google Scholar]
- Varadi, A., Tsuboi, T. and Rutter, G. A. (2005). Myosin Va transports dense core secretory vesicles in pancreatic MIN6 {beta}-cells. Mol. Biol. Cell 16, 2670-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M., Nomura, K., Ohyama, A., Ishikawa, R., Komiya, Y., Hosaka, K., Yamauchi, E., Taniguchi, H., Sasakawa, N., Kumakura, K. et al. (2005). Myosin-Va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol. Biol. Cell 16, 4519-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehlke, G. and Schliwa, M. (2000). Walking on two heads: the many talents of kinesin. Nat. Rev. Mol. Cell Biol. 1, 50-58. [DOI] [PubMed] [Google Scholar]
- Wu, X., Bowers, B., Wei, Q., Kocher, B. and Hammer, J. (1997). Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J. Cell Sci. 110, 847-859. [DOI] [PubMed] [Google Scholar]
- Wu, X., Bowers, B., Rao, K., Wei, Q. and Hammer, J. A., 3rd (1998). Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 143, 1899-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki, T., Imamura, T., Babendure, J. L., Lu, J.-C., Sonoda, N. and Olefsky, J. M. (2007). Myosin 5a is an insulin-stimulated Akt2 (protein kinase B{beta}) substrate modulating GLUT4 vesicle translocation. Mol. Cell. Biol. 27, 5172-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. P., Koslovsky, J. S., Reinhard, J., Bahler, M., Witt, A. E., Provance, D. W., Jr and Mercer, J. A. (1996). Cloning and characterization of myr 6,an unconventional myosin of the dilute/myosin-V family. Proc. Natl. Acad. Sci. USA 93, 10826-10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.