Summary
It remains unclear how GPI-anchored proteins (GPIAPs), which lack cytoplasmic domains, transduce signals triggered by specific ligation. Such signal transduction has been speculated to require the ligated GPIAP to associate with membrane-spanning proteins that communicate with obligate cytoplasmic proteins. Transient anchorage of crosslinked proteins on the cell surface was previously characterized by single-particle tracking, and temporary association with the actin cytoskeleton was hypothesized to cause regulated anchorage. GPIAPs, such as Thy-1, require clustering, cholesterol and Src-family kinase (SFK) activity to become transiently anchored. By contrast, a transmembrane protein, the cystic fibrosis transmembrane conductance regulator (CFTR), which has a PDZ-binding motif in its cytoplasmic C-terminus that binds the ERM adaptor EBP50, exhibits anchorage that strictly requires EBP50 but has little dependence on cholesterol or SFK. We hypothesized that a transmembrane protein would be required to mediate the linkage between Thy-1 and the cytoskeleton. Here, we present evidence, obtained by shRNA knockdown, that the transmembrane protein Csk-binding protein (CBP) plays an obligatory role in the transient anchorage of Thy1. Furthermore, either a dominant-negative form of CBP that did not bind EBP50 or a dominant-negative EBP50 drastically reduced transient anchorage of Thy-1, indicating the involvement of this adaptor. Finally, we speculate on the role of phosphorylation in the regulation of transient anchorage.
Keywords: Lipid rafts, Signal transduction, Single-particle tracking, GPI-anchored proteins, CFTR, Thy-1, Cytoskeleton
Introduction
The issue of how crosslinking of membrane determinants induces linkage to the cytoskeleton dates back to the original patching and capping observations (Raff et al., 1970) and the ideas of Singer and colleagues (Holifield et al., 1990; Singer, 1977; Singer and Nicolson, 1972). The problem is more intriguing when we consider that even outer-leaflet lipids and glycosylphosphatidylinositol (GPI)-anchored proteins (GPIAPs), when crosslinked, undergo patching and capping (Holifield et al., 1990; Schroit and Pagano, 1981), implying a robust linkage of the cluster to the retrograde-flowing cytoskeleton under the plasma membrane. We know that ligated GPIAPs can signal across the plasma membrane (Rege and Hagood, 2006). For example, antibody binding to GPIAPs induces an association with Src-family kinases (SFKs) (Stefanova et al., 1991). Crosslinking Thy-1, a GPIAP on T cells, results in mitogenesis (Kroczek et al., 1986; Zhang et al., 1992) and group B coxsackieviruses initiate infection by binding to and clustering decay-accelerating factor (DAF), the GPIAP co-receptor, on the apical surface of epithelial cells (Coyne and Bergelson, 2006).
Previously, we showed that a novel feature of single-particle tracking (SPT) trajectories of crosslinked membrane proteins could be used as a nano-assay to dissect how the linkage of certain GPIAPs and transmembrane proteins to the cytoskeleton is regulated (Chen et al., 2006). In that study, 40-nm gold particles were used to form clusters of GPI-anchored proteins that were much smaller than the typical size of patched antigen observed by immunostaining (Holifield et al., 1990). The crosslinking induced by the gold particle produced a nanoscale phenotype in the SPT trajectories in which the particle seemed to stop for periods of milliseconds to seconds; we termed this signature transient anchorage (TA). We found that TA for GPIAPs depended on SFKs, PI3 kinase, cholesterol and caveolin-1. By contrast, a transmembrane protein, the cystic fibrosis transmembrane conductance regulator (CFTR), also exhibited TA but this was strictly dependent on its C-terminal PDZ-binding domain, and only slightly dependent on SFKs and cholesterol. This strong dependence of CFTR anchorage on linkage to the cytoskeleton was also reported by Verkman and colleagues (Haggie et al., 2006). Recently, Kusumi and co-workers reported a comprehensive study on the transient confinement of CD59, a GPIAP. This study concluded that the aggregated CD59 molecules can undergo a temporary confinement that requires cholesterol, an intact actin-filament network and Gαi2-dependent SFK activation (Suzuki et al., 2007b). For GPIAPs, the phenomena of TA and stimulation-induced temporary arrest of lateral diffusion (STALL) (Suzuki et al., 2007a; Suzuki et al., 2007b) bear marked similarities in their requirements for clustering, cholesterol and SFKs, although the SPT-detection algorithms are different so the relative abundances of each immobilization type might differ.
A key issue is how these clustered GPIAPs are linked to the cytoskeleton and how this linkage is regulated. Simons and Toomre suggested that crosslinking of receptors might be a key to effecting signal transduction by either altering partitioning into existing raft domains or bringing smaller rafts together (Simons and Toomre, 2000). The Kusumi group (Kusumi et al., 2004; Suzuki et al., 2007b) suggested that the mechanism involves the binding of GPIAP clusters to actin filaments indirectly through an unspecified transmembrane protein and/or through actin-associated membrane microdomains. The working hypothesis that we proposed (Chen et al., 2006) invoked the former alternative, but the key transmembrane protein was not identified. In this study, we tested that hypothesis and identified a candidate for the unknown transmembrane protein either acting alone or as component of a required transmembrane protein complex.
The Csk-binding protein (CBP), also known as PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains), is a ∼45 kDa (calculated weight, but which migrates on gels as a 70-85 kDa protein) transmembrane adaptor protein having a very small ectodomain and a large cytoplasmic domain that contains ten tyrosine residues (Horejsi et al., 2004). The protein binds a cytoplasmic protein tyrosine kinase (C-terminal Src kinase; CSK), which then negatively regulates SFKs (Brdickova et al., 2001; Brdicka et al., 2000). Photobleaching experiments on T cells suggest that the reduced mobility of GPIAP-enriched lipid rafts (microdomains), containing Fyn-phosphorylated CBP (Yasuda et al., 2002), is caused by the linkage of CBP-EBP50-ERM complexes to the cytoskeleton (Itoh et al., 2002). Moreover, CBP was previously shown to be associated with Thy-1 clusters upon phosphorylation and/or pervanadate treatment (Durrheim et al., 2001).
On the basis of these results, we hypothesized (Chen et al., 2006) that CBP might be the transmembrane phosphoprotein or part of a transmembrane complex that recognizes in some way clustered GPIAPs and permits linkage of the cluster to the underlying cytoskeleton. We also postulated that phosphatase activity would be required to release the TA state. To test this hypothesis, we examined the involvement of CBP in TA when Thy-1 molecules are clustered by quantum dots (Qdots) on the fibroblast membrane. We show that CBP is indispensable for the TA of Thy1; this observation suggests that CBP is at least part of a transmembrane `cluster sensor' of the `Protein X' type invoked by Singer more than 30 years ago (Singer, 1977). The involvement of CBP was further supported by the immunostaining experiments in which anchored GPIAP clusters labeled with Qdots, and trapped by pervanadate treatment, were always colocalized with CBP staining. Furthermore, the presence of dominant-negative forms of ERM-binding protein 50 (EBP50) – a cytoskeletal adaptor protein that is invoked to anchor GPIAP clusters – and a CBP that cannot bind EBP50 drastically reduced TA of Thy-1.
Results
In this study, we first show that Qdot-based SPT reproduces the results from gold-particle-based SPT; fluorescent Qdots were employed to make two-color colocalization studies feasible. Next, we show that CBP is an obligatory component for TA of Thy-1. Finally, we show that CBP binds to the adaptor EBP50 to achieve TA and contrast this behavior to that of CFTR, which also links to the cytoskeleton via EBP50.
Qdot-based SPT reproduces the results from gold-particle-based SPT
To explore the correlative relationship between TA and different putative components of the TA complex, it is important to have the capability to visualize signals from different components in the same experiment. For this reason we switched from using video-enhanced bright-field microscopy to visualize gold particles to fluorescence microscopy to visualize Qdots for SPT experiments.
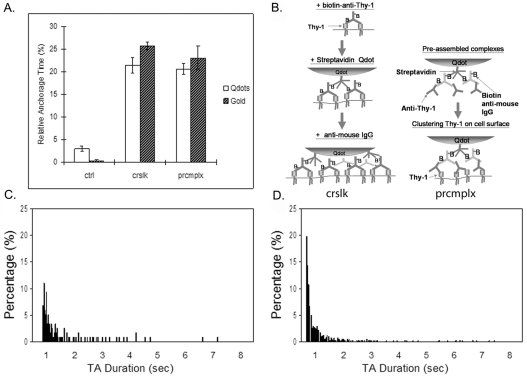
Therefore, previous experiments that used gold particles (Chen et al., 2006) were repeated using Qdots to cluster Thy-1. As shown in Fig. 1A, Thy-1 molecules, when clustered by the maximal crosslinking method (Fig. 1B, left) or by pre-assembled complexes (Fig. 1B right), exhibited similar degrees of TA; by contrast, little TA was observed when Thy-1 was not deliberately crosslinked. These results demonstrate that both Qdots and gold particles are effective in generating the TA phenotype. The diffusion coefficients from Qdot- and gold-particle-based SPT both were in the order of 5×10–2 μm2/second (supplementary material Table S1); note that these values are similar to those reported by (Suzuki et al., 2007a) for gold-mediated crosslinking of CD59, another GPIAP. Although the overall distribution of anchorage times was similar, the distribution of stopping times detected with Qdots was shifted towards the right so that the shortest anchorage times were longer for Qdots than gold particles (∼700 ms as compared with ∼300 ms; compare Fig. 1C with 1D). This effect might be explained by the stronger affinity between biotin and streptavidin (Kd∼10–15 M–1), the former labeling the antibody against surface molecules and latter coating the Qdot surface, as compared with the situation for gold-particle labeling. In the case of gold, the affinity between biotin-labeled antibody binding Thy-1 and anti-biotin antibody coating the gold surface is weaker (Kd∼10–10 M–1 or less) (Van Regenmortel, 1998) than the stronger affinity provided by Qdot-based pre-assembled complexes. Thus, the higher affinity of the Qdot label might contribute to slightly denser clustering of Thy-1, leading to longer minimum TA times. Additionally, the higher maximum valency between biotin and streptavidin (4:1) than between biotin and anti-biotin antibody (2:1) might also play a role.
Fig. 1.
Single-particle tracking (SPT) using quantum dots (Qdots) gives comparable results to gold-particle-based SPT. (A) TA of Thy-1 clusters labeled by Qdots (white bars) in C3H cells was induced by (B, left) the maximal crosslinking [crslk; see procedure 1 in Materials and Methods; the number of trajectories (n)=70] scheme or by (B, right) preassembled complexes (prcmlx; see procedure 2 in Materials and Methods; n=78), whereas the control observation was made in cells treated without crosslinking tertiary antibody (ctrl; n=60). The Qdot-based SPT results are similar to the results previously published for gold-particle-based SPT (hatched bars; n=69 for ctrl, n=90 for crslk and n=92 for prcmplx) (Chen et al., 2006). (C,D) Thy-1-cluster-bound Qdots underwent TA ranging from ∼700 ms to more than 7 seconds (C) and exhibited a bi-exponential decay in TA duration distribution, similar to the results previously obtained in gold-particle-based SPT (D).
Overall, the fact that TA can be induced by preassembled complexes illustrates that aggregation on this scale [less than 135 molecules (see Chen et al., 2006)] is enough to trigger a signal for TA to occur. Given the TA-inducing capacity of preassembled Qdot complexes, as well as the similarity of the behavior between gold particles and Qdots with respect to TA, we employed preassembled Qdot complexes throughout this study to cluster Thy-1 molecules for subsequent SPT experiments.
CBP plays a role in transiently anchoring Thy-1 clusters to the actin cytoskeleton
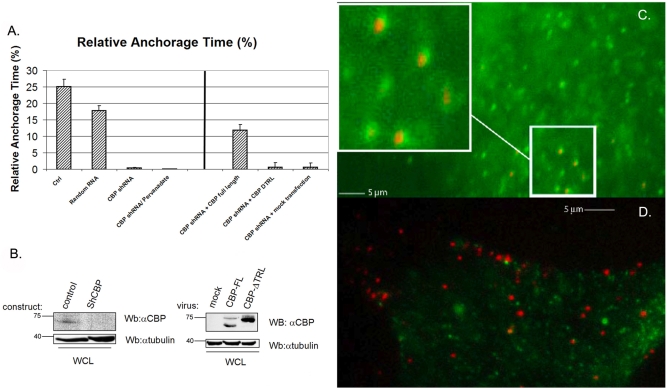
To test the role of CBP in TA of Thy-1 clusters, short hairpin RNA (shRNA) for CBP was introduced into murine 3T3 fibroblasts and successfully reduced expression of CBP in whole-cell lysates (Fig. 2B, left panel). Knockdown was estimated to be 70-80% based on analysis by ImageQuant software. In CBP-silenced cells (Fig. 2B), TA was significantly reduced compared with control experiments, in which either non-transfected 3T3 cells or 3T3 cells expressing a random DNA sequence were examined (Fig. 2A, left). Transfection with a random-sequence control shRNA did not affect CBP expression (supplementary material Fig. S1B). Moreover, CBP knockdown by shRNA could be partially rescued by transfecting knockdown mouse 3T3 cells with full-length human CBP (Fig. 2A, right panel; compare to leftmost bar in left panel of Fig. 2A, which serves as a control). By contrast, anchorage of CFTR, a transmembrane protein, was permanent for most CFTR-bound Qdots (Table 1) and was not affected by CBP abundance. Thus, these results indicate that CBP mediates TA of Thy-1 clusters but is not required for anchorage of CFTR.
Fig. 2.
The transmembrane protein CBP mediates TA of Thy-1. (A, left) After shRNA for CBP was introduced in 3T3 cells, TA of Thy-1 clusters was markedly reduced (n=57) compared with control 3T3 cells (n=40) or cells transfected with a random DNA sequence (n=79). Without CBP, there was minimal TA after pervanadate treatment (n=33). After CBP shRNA was introduced, 3T3 cells were infected with retroviral viruses coding for either full-length CBP (CBPFL, n=49) or a CBP construct with the TRL deletion (CBPDTRL, n=55), which does not bind EBP50 (right). (A, right) Compared with CBP knockdown cells, TA was partially rescued by full-length CBP, whereas almost no rescue was achieved with the TRL mutant. The negative control was mock transfection in the CBP-knockdown cells (n=30). (B) Blots show that CBP was knocked down in whole-cell lysates (WCL) and the effective restoration of CBPFL and CBPTRL in the shRNA-infected cells compared with the mock transfection. Molecular masses are indicated in kDa. (C) TA of Thy-1 clusters on C3H was prolonged by pervanadate treatment to over 1 minute. Every Thy-1-cluster-bound Qdot (red) was seen to be accompanied by proximate CBP staining (green). The inset in the upper left corner is the magnified view of the area surrounded by the white line. (D) In control C3H cells, in which not all Thy-1 clusters would be expected to be undergoing TA at the time of fixation, CBP staining (green) was not necessarily observed close to every Thy-1-cluster-bound Qdot (red). Scale bars: 5 μm.
Table 1.
CFTR is immobile when the ERM-binding capacity of EBP50 is intact and CFTR anchorage is independent of CBP
| shRNA-CBP (3T3) | Random sequence (3T3) | WT-EBP50 (C3H) | EGFP–DN-EBP50 (C3H) | |
|---|---|---|---|---|
| Degree of immobility* (%) | >99 | >99 | >99 | 2.0 |
The movement of CFTR-bound Qdots was recorded for 1 minute and the mobility was assessed by visual inspection and TA detection software if not completely immobile. In cells other than EGFP-DN-EBP50 transfectants, 99% or more CFTR-bound Qdots were visually immobile throughout the entire duration
We previously postulated that phosphatase activity would be required to release TA (Chen et al., 2006). To test this hypothesis, we examined Qdots and CBP immunofluorescence after treating C3H cells with pervanadate, a broad-spectrum membrane-permeable phosphatase inhibitor. On the basis of previous literature (Brdickova et al., 2001; Brdicka et al., 2000; Durrheim et al., 2001; Itoh et al., 2002; Yasuda et al., 2002), the rationale was that CBP and adaptors in the postulated linkage between clustered Thy-1 and the cytoskeleton, among other phosphoproteins, would remain phosphorylated, trapping the TA state. Indeed, pervanadate treatment, which preserves CBP phosphorylation (supplementary material Fig. S1A), resulted in prolonged anchorage, which lasted more than 1 minute (see supplementary material Movie 1A, in which two particles freely move before pervanadate treatment, and Movie 1B, in which one particle in the lower right is representative of the pervanadate-immobilized particles). Moreover, Qdots, which generated and bound to Thy-1 clusters, were found exclusively within regions containing phosphorylated CBP (Fig. 2C); by contrast, such a correlation was not observed in control cells (Fig. 2D). Among six cells examined after pervanadate treatment, 255 out of 279 Qdots were counted as being encompassed by CBP staining. In control C3H cells, in which not all Thy-1 clusters would be expected to be undergoing TA at the time of fixation, only 19 out of 276 Qdots were counted as being encompassed by CBP staining in the four cells examined. The importance of phosphorylation for CBP function in TA was also demonstrated by the fact that TA in CBP-knockdown cells could not be restored by pervanadate treatment (Fig. 2A, left panel); this suggests that TA requires phosphorylation of CBP and/or the phosphorylation of another protein(s) whose phosphorylation status regulates CBP function.
The adaptor EBP50 facilitates TA by linking Thy-1 clusters to the actin cytoskeleton via CBP
In our previous paper (Chen et al., 2006), we suggested that an adaptor, such as EBP50, might be involved in TA, based on literature showing that it can bind to phosphorylated CBP (Brdickova et al., 2001; Itoh et al., 2002) and its demonstrated role in transiently anchoring CFTR. It is well known that CFTR and a number of other transmembrane proteins, such as the renal brush border membrane Na(+)-H+ exchanger (Weinman et al., 1995), the β2-adrenergic receptor (Hall et al., 1998), the G-protein-coupled receptor kinase-6A (Hall et al., 1999) and the Yes-associated protein 65 (Mohler et al., 1999), are anchored to the actin cytoskeleton through EBP50 (Short et al., 1998) and proteins of the ERM family. Recently, the B-cell receptor has been shown to undergo an ezrin-regulated linkage to the cytoskeleton (Gupta et al., 2006) that also seems to be mediated by CBP.
To explore this facet of TA, wild-type (EGFP–WT-EBP50) and a dominant-negative mutant (EGFP–DN-EBP50) were overexpressed to evaluate EBP50 involvement in TA. EGFP–DN-EBP50 competes with endogenous EBP50 to occupy the PDZ-binding motif of interacting proteins but cannot bind ERM proteins, thereby breaking the adaptor linkage (Fouassier et al., 2000; Reczek et al., 1997). As a result, any event relying on EBP50 would be disrupted by overexpression of EGFP–DN-EBP50. C3H cells transfected with EGFP–DN-EBP50 were selected with neomycin (0.5 mg/ml) for SPT (supplementary material Fig. S2). TA of Thy-1 clusters induced by preassembled complexes was significantly suppressed in EGFP–DN-EBP50-overexpressed cells compared with control cells or EGFP–WT-EBP50-overexpressed cells (Fig. 3; supplementary material Movie 2). It was observed that, in EGFP–WT-EBP50-overexpressed cells, TA was not increased significantly, implying that the limiting factor for TA was not the quantity of EBP50 molecules available.
Fig. 3.

The cytoskeletal adaptor, EBP50, is required for TA of both the GPIAP Thy-1 and the transmembrane protein CFTR. C3H cells were transfected with GFP-tagged wild-type EBP50 (EGFP–WT-EBP50, labeled as wt-EBP50) or dominant-negative EBP (EGFP–DN-EBP50, labeled as dn-EBP50), which fails to bind to ERM proteins. SPT using preassembled Qdot complexes showed that cells with overexpressed EGFP–DN-EBP50 had significantly reduced TA for both Thy-1 clusters (n=64) and CFTR molecules (n=52) compared with the control cells (n=78) and cells transfected with EGFP–WT-EBP50 (n=58). CFTR-bound Qdots were immobile when EGFP–DN-EBP was not present (see supplementary material Movie 2). This is depicted as 100% relative anchorage time.
The importance of the affinity of CBP for EBP50 was demonstrated using a mutant form of CBP, CBPDTRL, in which a C-terminal sequence (TRL) of CBP was deleted with the consequence that the binding to the N-terminal PDZ domain(s) of EBP50 to CBP was abrogated (Brdickova et al., 2001). In cells transfected with CBP shRNA, the expression of full-length human CBP could restore TA to a significant level as noted above (Fig. 2A); by contrast, if CBPDTRL was used instead, the TA level remained negligible (Fig. 2A, right panel). CBP silencing and CBPDTRL rescue were verified using western blots (Fig. 2B, right panel).
It is instructive to compare the differences in the lateral-mobility characteristics between a GPIAP and a transmembrane protein, CFTR. As previously demonstrated, CFTR, when its C-terminus PDZ-binding motif is present, exhibits spontaneous anchorage by linking to the cytoskeleton through ERM proteins even when the crosslinking tertiary antibody is absent on the particle employed for tracking (Chen et al., 2006). CFTR-bound Qdots showed anchorage in excess of 1 minute in EGFP–WT-EBP50-overexpressed cells or control C3H cells (supplementary material Movie 3), consistent with the observations by Haggie et al. (Haggie et al., 2006). However, overexpressed EGFP–DN-EBP50 interfered with the binding between CFTR and endogenous EBP50 so that the prolonged anchorage of CFTR-bound Qdots could not be detected and only sporadic anchorage was observed (Fig. 3), also consistent with the observations made by Haggie et al. (Haggie et al., 2006). It is notable that both Haggie et al. and we observed that, when Qdots were used as the labeling particle for CFTR, the anchorage was not transient but more or less permanent. By contrast, when gold particles were employed to label CFTR, TA was observed (Chen et al., 2006). The variation in CFTR expression level, as Haggie et al. have argued, or the intrinsic differences between Qdots and gold particles and hence the perturbation imposed by these two ligands, possibly explains mobility differences observed in these two studies.
Discussion
TA of crosslinked proteins on the cell surface has been previously characterized by SPT, where temporary association with the actin cytoskeleton was hypothesized to cause regulated anchorage. GPIAPs such as Thy-1 require clustering, cholesterol and SFK activity to become transiently anchored (Chen et al., 2006). In this paper, we tested the hypothesis that a transmembrane protein would be required to mediate the linkage between clustered Thy-1 and the cytoskeleton. We concluded, on the basis of shRNA knockdown experiments, that, indeed, the transmembrane protein CBP plays an obligatory role in the TA of Thy-1. This conclusion was supported by the finding that, upon pervanadate treatment, which inhibited the release of TA, CPB was colocalized with Thy-1 clusters. Our results strongly suggest that TA occurs when CBP molecules and Thy-1 clusters are in close proximity. There are several possibilities for how this close proximity could come about. For example, recruitment of Qdot–Thy-1 complexes to CBP or vice versa could occur via random, diffusion-driven encounters between the crosslinked GPIAP and CBP clusters. Alternatively, formation of a crosslinked CBP aggregate over a CBP protein(s) could place CBP and Thy-1 in close proximity. We also found that, in addition to CBP, linkage of Thy-1 clusters to the actin cytoskeleton was mediated by the adaptor EBP50, because either a dominant-negative form of CBP that did not bind EBP50 or a dominant-negative EBP50 drastically reduced TA of clustered Thy-1.
Regulation of TA
The results of the pervanadate experiments, in which TA is converted to permanent anchorage, suggests a phosphorylation-dephosphorylation mechanism for CBP, and/or associated proteins, that regulates the linkage of Thy-1 clusters to cytoskeletal adaptors in a way that leads to the TA phenotype. Dephosphorylation of CBP, which coincides with its dissociation from lipid rafts (Yasuda et al., 2002) and from EBP50 (Itoh et al., 2002), could be the point at which Thy-1 clusters are released from their linkage to the cytoskeleton. These results are consistent with previous findings that SFK inhibition or removal reduces TA or STALL significantly (Chen et al., 2006; Suzuki et al., 2007b). Clearly, such a phosphorylation-dependent mechanism cannot be proved until the SFK tyrosine-phosphorylation sites on CBP are fully mapped and mutated.
Role of the cytoskeletal adaptor EBP50 in anchorage of membrane proteins
The SPT results for both Thy-1 clusters and CFTR suggest that EBP50 is responsible for anchoring these entities on the cell surface to the actin cytoskeleton, albeit with differing degrees of permanence. In this study, we found that EBP50 can anchor GPIAP clusters, provided that there is a transmembrane protein (CBP) available in very close proximity to the cluster for EBP50 to bind to. It also has been shown that EBP50, with its two PDZ-motif repeats, PDZ1 and PDZ2, is capable of oligomerization through PDZ-PDZ interactions forming dimers to tetramers (Fouassier et al., 2000), all of which could be observed in one cell type. EBP50 retains the ability to bind integral membrane proteins via its PDZ domains when it is oligomerized (Lau and Hall, 2001; Maudsley et al., 2000). Such a mechanism offers a plausible explanation for the discrepancy of the confinement sizes measured by different groups and in different cells. For example, the Kusumi group has shown that clusters of CD59, a GPIAP, undergo STALL [such events are also known as transient confinement zones (TCZs)], possibly through an actin-anchoring mechanism, but the size of STALL varies among different cell types (Suzuki et al., 2007a). It should be noted that we employed a smaller displacement range used to define TA in our studies as compared with that used by Suzuki et al. in defining TCZs and/or STALL. The length of the tethering agent, depending on the number of adaptor proteins involved, might determine the size of the confinement regions so that larger adaptor complexes containing multiple proteins would result in larger confinement zones. It is possible that EBP50 monomers, dimers, trimers and tetramers, with flexible oligomerization configurations, either through end-to-end (PDZ1-PDZ2) or lateral (PDZ1-PDZ1, PDZ2-PDZ2) binding (Fouassier et al., 2000), could interchangeably act as anchorage mediators in a cell. When the adaptor is a monomer, TA, which has the smallest confinement size, would be detected. As the number of the EBP50 proteins in the anchoring oligomer grows, larger confinement sizes would be detected.
Comparing the anchorage of clustered GPIAPs and CFTR: a working hypothesis
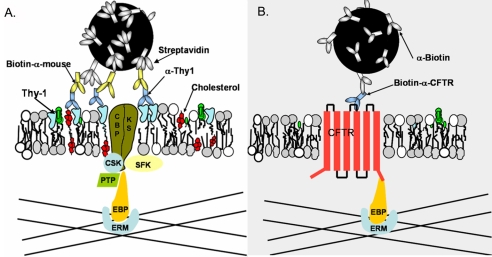
It seems that clustered GPIAPs – such as Thy-1 and CD73 (Chen et al., 2006) – and the non-clustered transmembrane protein CFTR utilize a common set of molecules for temporary or permanent anchorage via cytoskeletal association. EBP50 and ERM proteins are probably among the core components for anchorage (Fig. 4). To provide a more detailed working hypothesis than previously proposed (Chen et al., 2006), we postulate that CBP bridges GPIAP clusters on the outer leaflet of the plasma membrane with cytoplasmic adaptors. This linkage is hypothesized to be regulated by SFK phosphorylation of CBP itself and/or an associated protein(s), designated as KS in Fig. 4. A yet-to-be-identified phosphatase, whose substrate is CBP and/or an associated protein(s), could be responsible for reducing the ability of CBP to bind EBP50. In Qdot-based SPT, CFTR demonstrated much lengthier anchorage than Thy-1 clusters; this anchorage cannot be categorized as transient regardless of CBP expression level (Table 1). This indicates that the associations maintaining the entire adaptor linkage to CFTR are stable; by contrast, the linkage involving CBP is transient, perhaps reflecting the phosphorylation-dephosphorylation kinetics of CBP and/or associated proteins. It will be interesting to see whether CBP is a prototypical transmembrane cluster-recognition protein, acting either alone or as part of a complex, for outer-leaflet components.
Fig. 4.
EBP50-ERM assembly is the common adaptor complex for both Thy-1 clusters and CFTR. (A) Thy-1 clusters are generated by tertiary complexes consisting of streptavidin-coated Qdots binding biotinylated mouse anti-IgG, which binds anti-Thy-1 mAb. CBP, alone or complexed with other unknown Src-family kinase substrates (KS), is either recruited into Thy-1 clusters or captured by Thy-1 clusters. Here, CBP and/or KS are phosphorylated by SFKs, enabling CBP to bind to actin filaments via the EBP50-ERM assembly. The anchorage can be terminated when either CBP or the adaptors are dephosphorylated by an unspecified phosphatase (PTP). (B) CFTR, containing its own PDZ-binding motif, binds to actin filaments via EBP50-ERM spontaneously without extensive crosslinking. This anchorage is not dependent on SFK phosphorylation.
Materials and Methods
Cells
C3H 10T1/2 murine fibroblasts (American Type Culture Collection, Rockville, MD) and NIH 3T3 murine fibroblasts with or without various transfections were maintained in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. At 2 to 4 days before an SPT experiment, fibroblasts were plated into 35-mm glass-bottom Petri dishes at an appropriate cell density that yielded single cells for SPT measurements. To suppress endogenous CBP expression, oligonucleotide 5′-gatccccGTCCAAGTCCACTTCTGCCttcaagagaGGCAGAAGTGGACTTGGACTtttttggaaa-3′ (lowercase bases represent sequences flanking the insert) was inserted into pSUPER. A pSUPER construct expressing a random siRNA was used as a control. Constructs were co-transfected with a neomycin-resistance plasmid into wild-type mouse 3T3 cells using Jet PEI. After 48 hours, transfected cells were selected with neomycin (1.8 mg/ml) for 5 days. Full-length CBP and TRL-deleted CBP were introduced into cells by retroviral infection as described previously (Collin et al., 2007). The silencing of full-length CBP and CBPDTRL-mutant expressions were validated by western blots (Fig. 2A, insets) on whole-cell lysates as previously described (Veracini et al., 2008). HA-tagged CFTR protein was expressed in C3H and 3T3 cells by Lipofectamine transfection. EGFP–WT-EBP50 and EGFP–DN-EBP50, generous gifts from Katherine Karlson (Dartmouth Medical School, Hanover, NH), were expressed in C3H cells by Lipofectamine transfection and neomycin (0.5 mg/ml) was used for 3 days to select cells with a high level of gene expression before SPT.
Qdot conjugation to cells
Procedure 1
Strepavidin-coated Qdot 605 particles (Invitrogen, CA) were diluted in HAMS F12 nutrition mixture (Life Technologies/Gibco-BRL) supplemented with 25 mM HEPES and 15% serum (HHS). Cells seeded in glass-bottom dishes were first incubated with biotinylated primary antibodies [monoclonal mouse anti-Thy-1 antibody (clone 19EX5, 0.2 mg/ml)] for 10 minutes, then washed three times with 1 ml HHS. 1 ml of the solution containing streptavidin-Qdots (0.1 nM) was added and incubated with cells for 10 minutes at 37°C. Unbound Qdots were removed by washing three times with 1 ml HH (HHS without serum). For maximal crosslinking, 100 μl of the solution containing tertiary antibodies (goat anti-mouse IgG from Zymed, CA, 0.02 mg/ml) were added to the cells. Cells were washed three times in HHS to remove unbound Qdots.
Procedure 2 (preassembled complexes)
Qdots conjugated with antibodies specific to proteins of interest were assembled before addition to the cells. Preassembled complexes against Thy-1 were made by mixing 10 μl streptavidin-Qdots (0.01 μM), 2 μl of biotinylated goat anti-mouse IgG antibodies (2 mg/ml) and 20 μl of mouse anti-Thy-1 antibodies (15.5 mg/ml) in HHS solution with a final volume of 200 μl, followed by incubating the mixture for 1 hour. Preassembled complexes against HA-CFTR were made by mixing, for 1 hour, 10 μl of streptavidin-Qdots (0.01 μM) and 10 μl of biotinylated mouse anti-HA antibody (clone 16B12, 0.1 mg/ml; from Covance, NJ) in HHS solution in a final volume of 200 μl. The preassembled complexes then were separated from free antibodies by centrifugation using 100K nanosep spin-filter (PALL Corporation, MI), resuspended into 500 μl of HHS and added (100 μl per treatment) to cells for SPT experiments as described above for procedure 1. On the basis of the number of streptavidin conjugated to Qdots (5-10 streptavidins/Qdot) as specified by the manufacturer (Invitrogen, CA), the added biotinylated goat-anti-mouse IgG antibodies (2.6×10–11 mole) were in 15-fold excess over the anti-biotin antibodies coated on Qdots, and mouse anti-Thy-1 antibodies (10–9 mole) added were in 40-fold excess over the goat anti-mouse IgG antibodies. For SPT of CFTR, biotinylated anti-HA antibodies (6.5×10–12 mole) were added in fourfold excess over the anti-biotin antibodies coated on Qdots. Particle trajectories were recorded for the following 30 minutes at 37°C in a temperature-controlled chamber on the microscope stage.
Immunofluorescence
Colocalization of crosslinked GPIAPs with CBP after pervanadate treatment
Crosslinking of Thy-1 molecules was accomplished by incubating cells with biotinylated anti-Thy-1, streptavidin-Qdots and goat anti-mouse-IgG sequentially as in procedure 1 above. Cells then were treated with pervanadate (50 μM) for 10 minutes. After washing with PBS, cells were fixed with 4% paraformaldehyde for 10 minutes, washed three times with PBS and permeabilized with 0.5% Tween 20. Mouse anti-mouse CBP antibody (Exbio, Czech) and Alexa-Fluor-488-conjugated goat anti-mouse IgG antibody (Invitrogen, CA) were used to stain CBP inside the cell. Following extensive washing in PBS, cells were observed using an Olympus IX-81 fluorescence microscope with a 100× objective and images recorded using a cooled digital 12-bit CCD camera (SensiCam, Cooke, OR). As a negative control, cells were pre-fixed with 4% paraformaldehyde without pervanadate treatment.
Time-lapse Qdot SPT
Qdots bound to cells were imaged using a filter set with an excitation band centered at 430 nm and an emission band centered at 610 nm (Chroma, VT) using an Olympus IX-81 fluorescence microscope equipped with a 100× objective. Movies of moving particles then were recorded with an intensified CCD camera (XG0010, Stanford Photonics, CA) at video rate (30 frames/second). Recordings typically lasted for 40 seconds and the image stacks were saved in tiff format for later analysis. Tiff stacks were analyzed employing in-house software incorporating a tracking algorithm written by Daniel Blair and Eric Dufresne (http://www.seas.harvard.edu/%20projects/weitzlab/matlab/) that identifies relative changes of Qdot positions on the cell surface with a precision of ±20 nm. All trajectories were visually inspected to ensure correct tracking of Qdots.
Data analysis
Trajectories of Qdots obtained from SPT movies were analyzed for TA detection based on the algorithm developed by Chen et al. (Chen et al., 2006). The analysis software was designed to detect regions of trajectories where no displacement within experimental error occurred – as defined by all aspects of measurement stability. By recording movies of Qdots firmly glued on coverslips using FluorSave reagent (Calbiochem) and then detecting particle centroids over the time recorded, it was determined that 95% of centroids fell within ±40 nm along both the x and y axes. A program was developed to recognize portions from a trajectory as TA events when the displacement in five successive frames was less than 40 nm in both the x and y dimensions. Note that TA differs from complete immobilization in which the particle does not move during the experimental observation time of a particular cell. To further characterize TA, we defined the relative anchorage time as: sum of durations in which a single particle is transiently anchored/total time of trajectory.
Image-acquisition descriptions
In Fig. 2C,D and supplementary material Fig. S2, we used a microscope (IX-81; Olympus) with a 100× NA 1.25 oil-immersion objective (Olympus). FluorSave reagent (Calbiochem) was used as the imaging medium. EGFP was the fluorochrome in Fig. 2C,D and Alexa Fluor 488 was employed as the fluorochrome in supplementary material Fig. S2. Images were captured with a cooled CCD camera (SensiCam, Cooke, OR) and MetaMorph imaging software (Molecular Devices, CA). MetaMorph was also used to crop the whole-cell images into small regions and overlay images from green and red channels for the colocalization assay.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/21/3966/DC1
This work was supported by NIH GM 41402 and the NIH Cell Migration Consortium (NIH GM 64346). We thank Bing Yang for excellent technical assistance. We also thank Vaclav Horejsi for CBP constructs and antibodies. Deposited in PMC for release after 12 months.
References
- Brdickova, N., Brdicka, T., Andera, L., Spicka, J., Angelisov, P., Milgram, S. L. and Horejsi, V. (2001). Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Letters 507, 133-136. [DOI] [PubMed] [Google Scholar]
- Brdicka, T., Pavlitova, D., Leo, A., Bruyns, E., Koinek, V., Angelisova, P., Scherer, J., Shevchenko, A., Shevchenko, A., Hilgert, I. et al. (2000). Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase Csk and is involved in regulation of T cell activation. J. Exp. Med. 191, 1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Thelin, W. R., Yang, B., Milgram, S. L. and Jacobson, K. (2006). Transient anchorage of cross-linked glycosyl-phosphatidylinositol-anchored proteins depends on cholesterol, Src family kinases, caveolin, and phosphoinositides. J. Cell Biol. 175, 169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, G., Franco, M., Simon, V., Benistant, C. and Roche, S. (2007). The Tom1L1-clathrin heavy chain complex regulates membrane partitioning of the tyrosine kinase Src required for mitogenic and transforming activities. Mol. Cell. Biol. 27, 7631-7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, C. B. and Bergelson, J. M. (2006). Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124, 119-131. [DOI] [PubMed] [Google Scholar]
- Durrheim, G. A., Garnett, D., Dennehy, K. M. and Beyers, A. D. (2001). Thy-1 associated pp85-90 is a potential docking site for SH2 domain-containing signal transduction molecules. Cell Biol. Int. 25, 33-42. [DOI] [PubMed] [Google Scholar]
- Fouassier, L., Yun, C. C., Fitz, J. G. and Doctor, R. B. (2000). Evidence for Ezrin-Radixin-Moesin-binding Phosphoprotein 50 (EBP50) Self-association through PDZ-PDZ Interactions. J. Biol. Chem. 275, 25039-25045. [DOI] [PubMed] [Google Scholar]
- Gupta, N., Wollscheid, B., Watts, J. D., Scheer, B., Aebersold, R. and DeFranco, A. L. (2006). Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat. Immunol. 7, 625-633. [DOI] [PubMed] [Google Scholar]
- Haggie, P. M., Kim, J. K., Lukacs, G. L. and Verkman, A. S. (2006). Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol. Biol. Cell 17, 4937-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, R. A., Ostedgaard, L. S., Premont, R. T., Blitzer, J. T., Rahman, N., Welsh, M. J. and Lefkowitz, R. J. (1998). A C-terminal motif found in the beta 2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. PNAS 95, 8496-8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, R. A., Spurney, R. F., Premont, R. T., Rahman, N., Blitzer, J. T., Pitcher, J. A. and Lefkowitz, R. J. (1999). G protein-coupled receptor kinase 6A phosphorylates the Na+/H+ exchanger regulatory factor via a PDZ domain-mediated interaction. J. Biol. Chem. 274, 24328-24334. [DOI] [PubMed] [Google Scholar]
- Holifield, B. F., Ishihara, A. and Jacobson, K. (1990). Comparative behavior of membrane protein-antibody complexes on motile fibroblasts: implications for a mechanism of capping. J. Cell Biol. 111, 2499-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsi, V., Zhang, W. and Schraven, B. (2004). Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol. 4, 603-616. [DOI] [PubMed] [Google Scholar]
- Itoh, K., Sakakibara, M., Yamasaki, S., Takeuchi, A., Arase, H., Miyazaki, M., Nakajima, N., Okada, M. and Saito, T. (2002). Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol. 168, 541-544. [DOI] [PubMed] [Google Scholar]
- Kroczek, R. A., Gunter, K. C., Seligmann, B. and Shevach, E. M. (1986). Induction of T cell activation by monoclonal anti-Thy-1 antibodies. J. Immunol. 136, 4379-4384. [PubMed] [Google Scholar]
- Kusumi, A., Koyama-Honda, I. and Suzuki, K. (2004). Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 5, 213-230. [DOI] [PubMed] [Google Scholar]
- Lau, A. G. and Hall, R. A. (2001). Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl-termini and by phosphorylation. Biochemistry 40, 8572-8580. [DOI] [PubMed] [Google Scholar]
- Maudsley, S., Zamah, A. M., Rahman, N., Blitzer, J. T., Luttrell, L. M., Lefkowitz, R. J. and Hall, R. A. (2000). Platelet-derived growth factor receptor association with Na(+)/H(+) exchanger regulatory factor potentiates receptor activity. Mol. Cell. Biol. 20, 8352-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler, P. J., Kreda, S. M., Boucher, R. C., Sudol, M., Stutts, M. J. and Milgram, S. L. (1999). Yes-associated protein 65 localizes p62c-Yes to the apical compartment of airway epithelia by association with EBP50. J. Cell Biol. 147, 879-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff, M. C., Sternberg, M. and Taylor, R. B. (1970). Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature 225, 553-554. [DOI] [PubMed] [Google Scholar]
- Reczek, D., Berryman, M. and Bretscher, A. (1997). Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the Ezrin-Radixin-Moesin family. J. Cell Biol.. 139, 169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege, T. A. and Hagood, J. S. (2006). Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys. Acta. 1763, 991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroit, A. J. and Pagano, R. E. (1981). Capping of a phospholipid analog in the plasma membrane of lymphocytes. Cell 23, 105-112. [DOI] [PubMed] [Google Scholar]
- Short, D. B., Trotter, K. W., Reczek, D., Kreda, S. M., Bretscher, A., Boucher, R. C., Stutts, M. J. and Milgram, S. L. (1998). An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273, 19797-19801. [DOI] [PubMed] [Google Scholar]
- Simons, K. and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1, 31-39. [DOI] [PubMed] [Google Scholar]
- Singer, S. J. (1977). Thermodynamics, the structure of integral membrane proteins, and transport. J. Supramol. Struct. 6, 313-323. [DOI] [PubMed] [Google Scholar]
- Singer, S. J. and Nicolson, G. L. (1972). The fluid mosaic model of the structure of cell membranes. Science 175, 720-731. [DOI] [PubMed] [Google Scholar]
- Stefanova, I., Horejsi, V., Ansotegui, I. J., Knapp, W. and Stockinger, H. (1991). GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254, 1016-1019. [DOI] [PubMed] [Google Scholar]
- Suzuki, K. G., Fujiwara, T. K., Edidin, M. and Kusumi, A. (2007a). Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J. Cell Biol. 177, 731-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K. G., Fujiwara, T. K., Sanematsu, F., Iino, R., Edidin, M. and Kusumi, A. (2007b). GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177, 717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel, M. H. (1998). Thermodynamic parameters in immunoassay. Clin. Chem. Lab. Med. 36, 353-354. [DOI] [PubMed] [Google Scholar]
- Veracini, L., Simon, V., Richard, V., Schraven, B., Horejsi, V., Roche, S. and Benistant, C. (2008). The Csk-binding protein PAG regulates PDGF-induced Src mitogenic signaling via GM1. J. Cell Biol. 182, 603-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman, E. J., Steplock, D., Wang, Y. and Shenolikar, S. (1995). Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J. Clin. Invest. 95, 2143-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, K., Nagafuku, M., Shima, T., Okada, M., Yagi, T., Yamada, T., Minaki, Y., Kato, A., Tani-Ichi, S., Hamaoka, T. et al. (2002). Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 169, 2813-2817. [DOI] [PubMed] [Google Scholar]
- Zhang, F., Schmidt, W. G., Hou, Y., Williams, A. F. and Jacobson, K. (1992). Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc. Natl. Acad. Sci. USA 89, 5231-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.