Abstract
Eukaryotes have evolved highly conserved vesicle transport machinery to deliver proteins to the vacuole. In this study we show that the filamentous fungus Aspergillus parasiticus employs this delivery system to perform new cellular functions, the synthesis, compartmentalization, and export of aflatoxin; this secondary metabolite is one of the most potent naturally occurring carcinogens known. Here we show that a highly pure vesicle-vacuole fraction isolated from A. parasiticus under aflatoxin-inducing conditions converts sterigmatocystin, a late intermediate in aflatoxin synthesis, to aflatoxin B1; these organelles also compartmentalize aflatoxin. The role of vesicles in aflatoxin biosynthesis and export was confirmed by blocking vesicle-vacuole fusion using 2 independent approaches. Disruption of A. parasiticus vb1 (encodes a protein homolog of AvaA, a small GTPase known to regulate vesicle fusion in A. nidulans) or treatment with Sortin3 (blocks Vps16 function, one protein in the class C tethering complex) increased aflatoxin synthesis and export but did not affect aflatoxin gene expression, demonstrating that vesicles and not vacuoles are primarily involved in toxin synthesis and export. We also observed that development of aflatoxigenic vesicles (aflatoxisomes) is strongly enhanced under aflatoxin-inducing growth conditions. Coordination of aflatoxisome development with aflatoxin gene expression is at least in part mediated by Velvet (VeA), a global regulator of Aspergillus secondary metabolism. We propose a unique 2-branch model to illustrate the proposed role for VeA in regulation of aflatoxisome development and aflatoxin gene expression.
Keywords: aflatoxin biosynthesis, aflatoxisomes, compartmentalization, VeA, vb1
Secondary metabolites, natural products generated by filamentous fungi, plants, bacteria, algae, and animals, have an enormous impact on humans due to their application in health, medicine, and agriculture. Many secondary metabolites are beneficial (antibiotics, statins, morphine, etc.), though phytotoxins (e.g., ricin, crotin, amygdalin) and fungal poisons called mycotoxins (e.g., aflatoxin, sterigmatocystin, fumonisin) are detrimental to humans and animals. To control or customize biosynthesis of these natural products we must understand how and where secondary metabolism is orchestrated within the cell.
Vacuoles and vesicles are known to sequester secondary metabolites to protect host cells from self-toxicity (1). Enzymes involved in secondary metabolism are often found in vesicles and vacuoles, including those for biosynthesis of alkaloids (e.g., berberine, sanguinarine, camptotecin, and morphine; reviewed in refs. 1 and 2) and flavonoids (e.g., aurone) (reviewed in refs. 1 and 3) in plants and the nonribosomal peptide cyclosporin (4), the β-lactam antibiotic penicillin (5) (localization of ACVS is still controversial), and the polyketide aflatoxin (6–8) in fungi. However, the functional role of these compartments in secondary metabolism was unclear because these organelles potentially could be involved in synthesis, storage, protein turnover, transport, or export of the end product or biosynthetic enzymes.
Aflatoxins are polyketide-derived furanocoumarins synthesized primarily by the filamentous fungi Aspergillus parasiticus and A. flavus (9, 10). Aflatoxin biosynthesis is one of the most highly characterized secondary metabolic pathways and provides a useful system to understand secondary metabolism in eukaryotes. Aflatoxin B1 (AFB1) is the most potent naturally occurring carcinogen known (10) and has significant health and economic impacts worldwide (10). Aflatoxin biosynthesis involves at least 17 enzyme activities encoded by 25 or more genes that are clustered in a 75-Kb region on one chromosome. The molecular mechanisms that regulate aflatoxin biosynthesis have been studied extensively by us and others (reviewed in refs. 10 and 11).
The intracellular site for synthesis of fungal polyketide-derived secondary metabolites was not known before our current work. Previous studies from our laboratory demonstrated that as aflatoxin synthesis increases, early (Nor-1), middle (Ver-1), and late pathway enzymes (OmtA) localize to 2 primary subcellular locations: (i) the cytoplasm (6–8) and (ii) vesicle and vacuole-like organelles (6–8) (we define organelles <2.5 μm in size as vesicles; we define organelles ≥2.5 μm as vacuoles).
As a first step to determine the functional role of these organelles in Aspergillus secondary metabolism, we developed a unique “high-density sucrose cushion” method for purification of a vesicle-vacuole fraction from A. parasiticus during aflatoxin synthesis (12). In the current study, we show the functional role of this fraction in aflatoxin synthesis, storage, and export. This fraction could convert sterigmatocystin (ST), a late intermediate in aflatoxin biosynthesis, to aflatoxin B1 and could compartmentalize aflatoxin. To differentiate the roles of vesicles and vacuoles, we blocked vesicle-vacuole fusion using 2 independent approaches; the data show that predominantly, vesicles catalyze the final 2 steps in aflatoxin biosynthesis and compartmentalize and export aflatoxin to the cell exterior. An increase in vesicle number [high vesicle number (HVN) phenotype] was positively correlated to aflatoxin-inducing growth conditions and aflatoxin accumulation/export; the HVN phenotype was inversely correlated with down-regulation of A. parasiticus avaA and vps16 gene expression. Finally we show that Velvet (13–15), a key global regulator of Aspergillus secondary metabolism, helps to mediate the observed changes in aflatoxin gene expression and vesicle number.
Results
High Vesicle Number Phenotype Is Positively Correlated with Aflatoxin Synthesis.
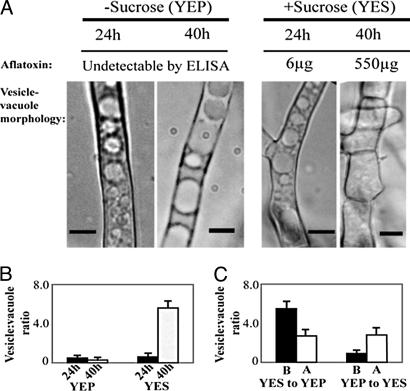
Yeast extract sucrose (YES) is an aflatoxin-inducing growth medium, and yeast extract peptone (YEP) is a noninducing medium; these media have the same composition except that peptone in YEP replaces sucrose as carbon source. Under standard growth conditions (100 mL YES liquid shake culture, batch fermentation) A. parasiticus initiates aflatoxin synthesis between 24 and 30 h, and by 40 h, synthesis occurs at peak levels (16). Aflatoxin enzymes and transcripts are also detected between 30 and 40 h (16) in roughly the same order as the genes in the aflatoxin cluster. Under standard aflatoxin-inducing conditions (YES), we observed a 5-fold increase in vesicle number (HVN) from 24 to 40 h in A. parasiticus SU-1 (Fig. 1 and Table 1) as aflatoxin increased up to 11-fold (16). In contrast, no change in vesicle number [low vesicle number (LVN) phenotype] was evident under standard aflatoxin-noninducing conditions (YEP) (Fig. 1). The fungus was then grown in YES or YEP for 36 h and transferred to the opposite medium (nutritional shift). Within 6 h after transfer from YES to YEP, we observed a 2-fold decrease in vesicle number that preceded a decline in aflatoxin accumulation; transfer from YEP to YES resulted in a 2-fold increase in vesicle number preceding an increase in aflatoxin accumulation (Fig. 1).
Fig. 1.
Positive correlation between aflatoxin biosynthesis and high vesicle number phenotype. A. parasiticus SU-1 was grown for 24 or 40 h in YES or YEP media; vesicle-vacuole morphology was analyzed by light microscopy and aflatoxin per flask was analyzed by ELISA (see Methods). (A) Bright-field microscopy of A. parasiticus grown in aflatoxin-inducing (+sucrose, YES) or -noninducing conditions (−sucrose, YEP). (Scale bar: 5 μm.) (B) Ratio of vesicle number to vacuole number (vesicle:vacuole ratio) calculated at 24 or 40 h in YEP or YES medium. (C) Nutritional shift: SU-1 was grown in YES or YEP growth medium for 36 h. Mycelia were harvested and transferred to the opposite medium, and vesicle:vacuole ratio was calculated before (B) and 6 h after (A) media shift.
Table 1.
Fungal stains, genotypes, and vesicle number under aflatoxin-inducing and -noninducing conditions
| Fungal strains and genotype | +Sucrose (YES) |
−Sucrose (YEP) |
||||
|---|---|---|---|---|---|---|
| v | V | v/V | v | V | v/V | |
| SU-1 (wild type; NRRL5862) | 69 ± 8 | 11 ± 5 | 8.3 ± 4.5 | 12 ± 3 | 21 ± 4 | 0.6 ± 0.2 |
| SU-1 treated with Sortin3 | 70 ± 5 | 12 ± 5 | 7.5 ± 3.5 | 56 ± 6 | 10 ± 3 | 6.4 ± 2.5 |
| AFS10 (aflR)a | 59 ± 6 | 14 ± 3 | 4.5 ± 2.5 | 14 ± 5 | 20 ± 1 | 0.7 ± 0.3 |
| ATCC 36595 (ver-1)b | 63 ± 6 | 15 ± 4 | 4.7 ± 1.6 | 12 ± 3 | 17 ± 4 | 0.8 ± 0.4 |
| ΔveA (ver-1 wh-1 veA)c | 19 ± 4 | 18 ± 1 | 1.1 ± 0.3 | 15 ± 3 | 21 ± 5 | 0.8 ± 0.3 |
| ΔveA treated with Sortin3 | 62 ± 5 | 9 ± 2 | 9.6 ± 2.2 | 65 ± 6 | 11 ± 2 | 6.2 ± 1.7 |
| TJYP1–22 (brn nor1 fadAG42R)d | 57 ± 4 | 11 ± 2 | 5.5 ± 1.3 | 21 ± 4 | 23 ± 5 | 1.0 ± 0.4 |
| NR1 (niaD)b | 60 ± 4 | 11 ± 3 | 6.0 ± 2.0 | 12 ± 4 | 22 ± 4 | 0.6 ± 0.3 |
| Δvb1e (this work)f | 73 ± 10 | 10 ± 4 | 9.1 ± 4.7 | 70 ± 8 | 7 ± 1 | 10.0 ± 2.6 |
| AC34 (niaD+) (this work) | 66 ± 7 | 14 ± 5 | 5.6 ± 2.5 | 13 ± 3 | 21 ± 5 | 0.7 ± 0.3 |
Fungal strains were grown for 40 h in either YES or YEP medium and then analyzed by light microscopy (see Methods). v, number of vesicles per 50 μm of mycelial length; V, number of vacuoles per 50 μm of mycelial length. Strains provided by aJeff Cary; bour laboratory; cAnna Calvo; dNancy Keller.
eAC11 was used as a representative of the vb1 disruptant strains AC11, 5, and 7; this strain was labeled Δvb1 in the text and figures for convenience.
fSortin3 treatment of SU-1 produces a similar vesicle phenotype as observed in AC11, AC5, and AC7.
Vesicles and Vacuoles Are One Primary Site for the Late Steps in Aflatoxin Synthesis and Storage.
We previously observed that early (Nor-1), middle (Ver-1), and late (OmtA) aflatoxin enzymes localize in vesicles and vacuoles in A. parasiticus (6–8). To determine the functional role of these organelles in aflatoxin synthesis, a vesicle-vacuole fraction (V) was first isolated from A. parasiticus grown for 36 h in aflatoxin-inducing medium (YES) using a high-density sucrose cushion method.
Next, we performed feeding experiments (using a protocol described previously) (17) with protoplasts, the vesicle-vacuole fraction (V), and the nonvacuole fraction (NV) (contains the remaining cell materials) in vitro with a late pathway intermediate sterigmatocystin (ST) to test whether these could convert ST to aflatoxin B1 (AFB1).
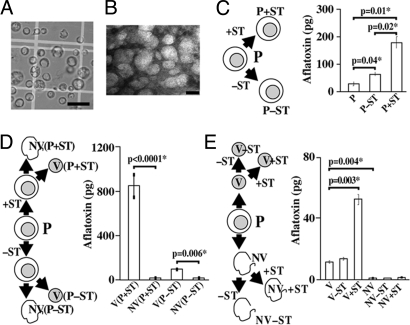
In protoplasts (Fig. 2A) fed ST, aflatoxin increased 6-fold; without added ST, aflatoxin increased 2-fold (Fig. 2C). Analogous feeding experiments were performed with protoplasts obtained from 2 strains impaired in aflatoxin synthesis, including AFS10 (gene disruption in a positive pathway regulator, aflR; no aflatoxin enzymes or aflatoxin are synthesized) and LW1432 (mutations in 2 pathway genes omtA and ver-1; accumulates the pathway intermediate versicolorin A). An increase in AFB1 was not observed in these strains, strongly suggesting that incorporation of ST into AFB1 in SU-1 was dependent on enzyme activities contained within the protoplast. The vesicle-vacuole fraction purified from protoplasts that had been fed ST carried the vast majority of the aflatoxin as compared with the nonvacuole fraction (Fig. 2D). ST fed directly to the vesicle-vacuole fraction increased aflatoxin more than 5-fold (Fig. 2E). In contrast, ST fed to the nonvacuole fraction resulted in nearly undetectable levels of aflatoxin. These data suggested that the vesicle-vacuole fraction carries functional aflatoxin enzymes and compartmentalizes aflatoxin.
Fig. 2.
Feeding experiments. A. parasiticus SU-1 was grown for 36 h in YES medium, and protoplasts (P), a vesicle-vacuole fraction (V), and a nonvesicle-vacuole (NV) fraction were purified (see Methods). ST was then fed to P, V, or NV overnight and the quantity of aflatoxin measured using ELISA (see Methods). (A) Bright-field microscopy of P fraction. (Scale bar: 25 μm.) (B) Transmission electron microscopy of V fraction. (Scale bar: 50 nm.) [Fig. 2 A and B reproduced with permission from Chanda et al. (12) (copyright 2009, Elsevier).] (C) Feeding ST to P fraction. P+ST and P-ST, protoplasts fed and not fed with ST, respectively. (D) Feeding ST to P followed by detection of aflatoxin in V and NV fractions. V(P+ST) and NV(P+ST), V and NV fractions obtained from P fed with ST; V(P–ST) and NV(P–ST), V and NV fractions obtained from protoplasts not fed with ST. (E) Feeding ST to fractions V and NV. V+ST and NV+ST, V and NV fractions fed with ST; V–ST and NV–ST, V and NV fractions not fed with ST. Two-tailed P values used to determine statistical significance were calculated using an unpaired t test with sample size of 3 (aflatoxin measurements in each experiment were conducted in triplicate). Feeding experiments were repeated 3 times with similar trends.
Blocking Vesicle-Vacuole Fusion Increases Aflatoxin Synthesis/Export and Aflatoxin Enzyme Accumulation but Does Not Affect Aflatoxin Gene Expression.
In A. parasiticus, the delivery of OmtA to vacuoles appears to occur through fusion of vesicles containing OmtA to vacuoles (8); this fusion event represents an important late step in vacuole biogenesis (18). To clarify which compartments (vesicles or vacuoles) associate functionally with aflatoxin synthesis, we targeted the class C Vps tethering complex (Fig. 3A) that mediates fusion of vesicles and other prevacuolar compartments to vacuoles (18). We disrupted A. parasiticus vb1 that encodes a homolog of Ypt7 (one member of the tethering complex) in yeast and AvaA in Aspergillus nidulans that are necessary for vacuole biogenesis; ypt7 and avaA disruption results in fragmented vacuoles (19, 20). Vb1 contains 205 aa and is 70% identical to yeast Ypt7, 74% identical to mammalian Rab7 GTPases, and 94% identical to A. nidulans AvaA.
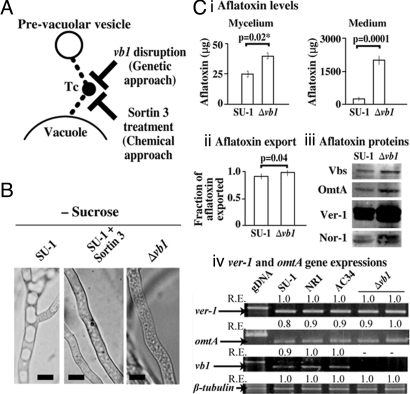
Fig. 3.
Effect of blocking vesicle-vacuole fusion in A. parasiticus. (A) Schematic representation of the experiment. Tc, the class C Vps tethering complex. Fusion of prevacuolar vesicles was blocked either by disruption of vb1 or by inhibition with Sortin3 treatment. (B) Fungal strains were grown for 40 h in −sucrose (YEP), and vesicles were analyzed by light microscopy. (Scale bar: 5 μm.) (C) Effect of vb1 disruption on aflatoxin biosynthesis. (i) Aflatoxin in mycelium and medium per 100 mL of culture. (ii) Aflatoxin export (calculated as ratio of aflatoxin in the medium to aflatoxin in medium plus mycelium in a 100 mL culture). (iii) Accumulation of early (Nor-1), middle (Vbs, Ver-1), and late (OmtA) aflatoxin enzymes. (iv) Relative expression (RE) of ver-1, omtA, vb1, and β-tubulin. Two-tailed P values for (i) and (ii) were calculated using an unpaired t test with sample size of 3 (aflatoxin measurements were conducted in triplicate). gDNA, genomic DNA from SU-1. See Table 2 for description of RE.
Disruption of vb1 (supporting information (SI) Fig. S1) in A. parasiticus strain AC11 and 2 other genetically identical isolates (AC5 and AC7) generated a fragmented vacuole morphology analogous to that seen with disruption of avaA in A. nidulans. We also observed a HVN phenotype even under aflatoxin-noninducing growth conditions (YEP; Fig. 3B); however, AC5, AC7, and AC11 did not accumulate aflatoxin in YEP. We confirmed the presence of the vb1 gene disruption at the level of DNA (Fig. S1) and mRNA (Fig. 3C iv). Under aflatoxin-inducing growth conditions (YES), AC11 and AC5 synthesized 7-fold more total aflatoxin and exported aflatoxin to the cell exterior at significantly higher levels than SU-1 (Fig. 3C i and ii). AC11 also accumulated higher quantities of aflatoxin enzymes than SU-1 (Fig. 3C iii); Vbs and OmtA increased 2-fold; Ver-1, 3-fold; and Nor-1, 2.5-fold as determined by densitometry. The increase in Ver-1 and OmtA proteins occurred in the absence of a significant change in gene expression at the mRNA level (Fig. 3C iv). In contrast to these data, the quantities of aflatoxin and vesicles were not statistically different from SU-1 in AC34, a control transformant in which vb1 was not disrupted [confirmed at the DNA (Fig. S1) and mRNA level (Fig. 3C iv)]. These data confirm that the process of transformation was not responsible for the changes in phenotype observed in AC11, AC7, and AC5.
Sortin3 blocks the activity of Vps16 (21), another protein in the class C Vps tethering complex in yeast (18) (Fig. 3A). Sortin3 generates a fragmented vacuole morphology reminiscent of Ypt7 disruption and causes enhanced export of cellular hydrolases (like carboxypeptidase Y, CPY) to the cell exterior (21). Treatment of SU-1 with Sortin3 resulted in a high vesicle number phenotype under aflatoxin-noninducing growth conditions (YEP) in agreement with vb1 disruption (Fig. 3A). Sortin3 treatment of SU-1 grown for 40 h in YES increased aflatoxin accumulation 5-fold (Fig. S2A) and also increased accumulation of the aflatoxin enzymes Vbs (1.4-fold), Ver-1 (3.5-fold), and OmtA (1.5-fold) as compared with untreated cells (Fig. S2B). Based on these observations, we conclude that vesicles represent one primary site for the late steps in aflatoxin synthesis, compartmentalization, and export to the cell exterior; in contrast, vacuoles appear to play only a minor role in these processes. We hypothesize that vacuoles predominantly participate in aflatoxin enzyme turnover; this is supported by the observation that blocking vesicle-vacuole fusion increases the quantity of aflatoxin enzymes (in the absence of increased transcription), possibly by preventing them from reaching the vacuole for degradation.
A HVN Phenotype Is Inversely Correlated with Downregulation of vb1 and vps16 Expression.
What drives the shift to a HVN phenotype during aflatoxin biosynthesis on sucrose? We hypothesized that one possible mechanism is the down-regulation of vesicle-vacuole fusion via decreased activity of the class C tethering complex. We analyzed vb1 and vps16 expression in A. parasiticus SU-1 grown for 24, 30, and 40 h under aflatoxin-inducing (YES) and -noninducing (YEP) growth conditions. vb1 and vps16 transcript levels remained nearly constant in YEP. However, in YES, expression of both genes declined more than 2-fold from 24 to 40 h (Table 2 and Fig. S3) in parallel with the observed increase in aflatoxin accumulation (16) and in vesicle number (Fig. 1). We hypothesize therefore that the observed shift to a HVN phenotype is functionally linked to the down-regulation of vb1 and vps16 expression.
Table 2.
Relative expression (RE) of vb1, vps16, and ver-1 in A. parasiticus grown in aflatoxin-inducing (+sucrose) or -noninducing (−sucrose) growth media for 24, 30, or 40 h
| Gene | +Sucrose (YES) |
−Sucrose (YEP) |
||||
|---|---|---|---|---|---|---|
| 24 h | 30 h | 40 h | 24 h | 30 h | 40 h | |
| vb1 | 1.0 | 0.6 | 0.3 | 0.7 | 0.7 | 0.6 |
| vps16 | 0.8 | 0.5 | 0.3 | 1.0 | 0.9 | 0.9 |
| ver-1 | 0.1 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 |
Relative expression (RE) was determined by RT-PCR (see Methods). RE data for vb1, vps16, and β-tubulin are provided. RE measures the relative signal intensity (RI) of RT-PCR products resolved by agarose gel electrophoresis (see Figs. S3 and S5 for agarose gel images). RI is calculated using the ratio of absolute intensity for a RT-PCR product generated from one time point as compared with the highest absolute intensity recorded for any time point in which that gene was analyzed. As RE values decrease, gene expression declines (and vice versa). Absolute intensity values were measured by densitometry using Adobe Photoshop software.
VeA Coregulates the Onset of Aflatoxin Gene Expression and the Shift to a HVN Phenotype.
What are the molecular factors that participate in coregulation of aflatoxin gene expression and the shift to a HVN phenotype? AFS10 (ΔaflR, see above) does not synthesize aflatoxin or aflatoxin enzymes. Constitutive activation of FadA, an alpha subunit of a heterotrimeric G protein in A. parasiticus TJYP1–22, exerts a negative regulatory influence on aflatoxin synthesis and conidiation (22). Velvet protein, encoded by veA, is a global regulator of secondary metabolism and development in Aspergilli in response to light (13–15); the gene is expressed and its gene product is active predominantly in the dark. Disruption of veA in A. parasiticus (ΔveA) blocks synthesis of aflatoxin pathway intermediates and aflatoxin, blocks sclerotia development, and impairs conidiation in the light and dark (15). Aflatoxin synthesis is also blocked in A. parasiticus ATCC36537 that carries a mutation in the pathway gene, ver-1 (15) (accumulates the pathway intermediate versicolorin A). Although each of the 4 strains— AFS10, TJYP1–22, ΔveA, and ATCC36537—is impaired in aflatoxin gene expression and/or aflatoxin enzyme accumulation, only ΔveA failed to shift to a HVN phenotype under aflatoxin-inducing conditions (YES) at 48 h in the dark (Table 1 and Fig. S4). Wild-type SU-1 and the 3 other mutant strains did undergo this shift. However, Sortin3 treatment restored the HVN shift in ΔveA in the presence or absence of sucrose (Table 1 and Fig. S4). Our observations strongly suggest that veA helps to coordinate the shift to the HVN phenotype and the synthesis of aflatoxin enzymes. The data also suggest that in addition to global regulation by VeA, the LVN to HVN shift and induction of aflatoxin gene expression can be regulated independently.
How does VeA mediate the LVN-to-HVN shift? We analyzed Vb1 and Vps16 expression at the RNA level in ΔveA. We observed a decrease in vb1 and vps16 expression in controls (SU-1 and ATCC36537) from 24 to 72 h under aflatoxin-inducing conditions, whereas expression of these genes in ΔveA remained nearly constant (Table 3; and Fig. S5). These data suggest that VeA regulates class C Vps complex activity by modulating expression of tethering complex proteins.
Table 3.
Relative expression (RE) of vb1, vps16, and β-tubulin in SU-1, 36537, and ΔveA grown in YES for 24, 40, and 72 h
| Fungal strain |
vb1 |
vps16 |
β-tubulin |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 40 h | 72 h | 24 h | 40 h | 72 h | 24 h | 40 h | 72 h | |
| SU-1 | 1.0 | 0.5 | 0.4 | 1.0 | 0.4 | 0.3 | 0.9 | 1.0 | 1.0 |
| ATCC36537 | 1.0 | 0.3 | 0.3 | 1.0 | 0.4 | 0.6 | 1.0 | 1.0 | 1.0 |
| ΔveA | 0.9 | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 | 0.9 |
See footnote for Table 2 for description of RE.
Discussion
Our studies provide direct demonstration of the functional significance for vesicles and vacuoles in fungal polyketide biosynthesis and export. The current study shows that at least the last 2 enzymatic steps in aflatoxin biosynthesis are completed in vesicles, and these organelles also participate in compartmentalization and export of the end product, aflatoxin, into the growth medium. We hypothesize that aflatoxin enzymes likely continue to make aflatoxin until they are eventually turned over in vacuoles.
Our data strongly suggest that the development of aflatoxin-synthesizing vesicles (aflatoxisomes) is functionally linked to down-regulation of vb1 and vps16 genes. VeA plays a novel and critical dual role in this process by helping to coordinate regulation of aflatoxin gene expression and the shift to aflatoxisome formation. Based on previous and current work, we propose a 2-branch model for coordination of subcellular compartmentalization, gene regulation, and carbon flow associated with aflatoxin biosynthesis in A. parasiticus (Fig. 4; see detailed description in figure legend). This model represents a logical expansion of a previous model (16) focused on carbon source regulation of aflatoxin synthesis. Understanding the mechanisms that coregulate the activation of aflatoxin synthesis and the LVN-to-HVN shift to form aflatoxisomes is a key to development of efficient strategies to manipulate secondary metabolism in general and aflatoxin synthesis specifically. Our studies also tend to support the idea that Aspergilli, like other organisms with small genomes, use existing conserved cellular machinery (vesicle trafficking) to conduct new cellular functions (aflatoxin synthesis).
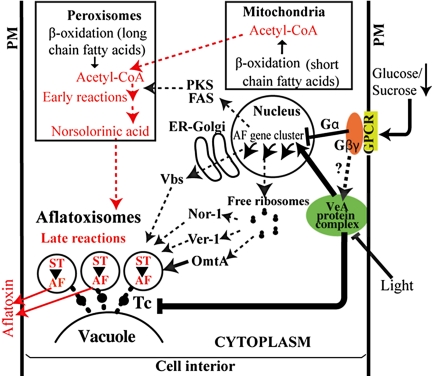
Fig. 4.
Two-branch model: regulation of subcellular compartmentalization, aflatoxin gene expression, and carbon flow. Compartmentalization (branch 1): the model proposes that Nor-1 (early), Ver-1 (middle), and OmtA (late) are synthesized on free ribosomes in the cytoplasm, packaged into transport vesicles (6–8), and transported to vacuoles via the cytoplasm-to-vacuole targeting (Cvt) pathway (6, 8). In contrast Vbs localizes in the cytoplasm and in structures thought to be Golgi (29), suggesting it is transported to vacuoles via the classical secretory pathway; VBS is glycolsylated in support of this notion. Gene regulation (branch 2): sensing of glucose concentration or glucose metabolism initiates a FadA/cAMP/PKA signaling pathway (16); details of mechanism for sensing or metabolism of sucrose are not known. Carbon flow highlighted in red. Mitochondria and peroxisomes supply acetyl-CoA (24) to polyketide synthesis. The occurrence of early steps in aflatoxin synthesis (acetyl-CoA→norsolorinic acid) in peroxisomes was proposed by others (24). In the current study, we show the function of late pathway enzymes in aflatoxigenic vesicles (aflatoxisomes) that also participate in aflatoxin export. Our data also suggest the presence and activity of early and middle pathway enzymes in aflatoxisomes. Coordination of 2 branches (current study). At least 2 separate signals (carbon source and light) trigger VeA activity that coordinates regulation of the 2 branches involved in aflatoxin synthesis/export (denoted by thick black arrows). One branch up-regulates gene transcription via activation of general and specific transcription factors (reviewed in ref. 10). The second branch down-regulates class C Vps tethering complex (Tc) activity (current study), resulting in accumulation of transport vesicles. When both branches are operational, aflatoxin enzymes accumulate in aflatoxisomes where they carry out aflatoxin synthesis and export the toxin to the cell exterior. Hypothesized pathways denoted by dashed lines; known pathways denoted by solid lines. PM, plasma membrane.
We know that VeA activity is light sensitive; the protein is expressed at highest levels and localizes to the nucleus in the dark (13, 14, 15). VeA is also reported to form a complex with several proteins and may play a role in chromatin remodeling (13). We therefore hypothesize that on the soil surface, little VeA is expressed or active, and low levels of aflatoxin accumulate. However, deeper in the soil where light does not penetrate, VeA mediates an increase in aflatoxin production. This in turn protects the colony and sclerotia from predation by worms and insects [building on recent studies by Rohlfs et al. (23)] and hence helps A. parasiticus to survive in its ecological niche.
The use of an endomembrane system to compartmentalize proteins, substrates, intermediates, and products appears to be a common feature in eukaryotic secondary metabolism (1–8, 24, 25). Another illustration of compartmentalization of pathway enzymes in fungal secondary metabolism is the biosynthesis of penicillin (an amino acid-derived secondary metabolite) in Penicillium chrysogenum [reviewed recently by Evers et al. (25)] and A. nidulans (28). Although it is clear that the penicillin pathway enzymes localize to unique subcellular compartments [Golgi-derived vesicles (26), vacuoles (5), cytosol (25), and peroxisomes (25, 27, 28)], the data do not directly demonstrate the functional role of these compartments in penicillin biosynthesis, storage, or export. An interesting feature of penicillin biosynthesis is that carbon (α-aminoadipate) flows from vacuoles to the cytoplasm to the peroxisome before exiting the cell. In contrast, carbon (acetyl-CoA) appears to flow in aflatoxin biosynthesis from peroxisome to the vesicle and then to the cell exterior (Fig. 4). Maggio-Hall et al. (24) reported that peroxisomes supply at least part of the acetyl-CoA required for aflatoxin synthesis and demonstrated that norsolorinic acid (NA) accumulates in peroxisomes in a nor-1 mutant strain. These data suggest that the early steps in aflatoxin synthesis (before averantin) may occur in this location. The increased accumulation of Nor-1, Ver-1, Vbs, and OmtA levels that results from a block in the fusion of vesicles with vacuoles (current study) implies that all subsequent steps occur in aflatoxisomes. Previous data from our lab and others strongly suggest that peroxisomes are not part of the purified vesicle-vacuole fraction (12).
During the course of these studies, we generated vb1 gene disruption mutant strains to analyze the role of this gene and the class C tethering complex in vesicle fusion and aflatoxin synthesis. A. parasiticus Vb1 exhibits 94% identity with A. nidulans AvaA at the amino acid level, and the phenotypes generated upon disruption of avaA and vb1 are very similar. We hypothesize that vb1 and avaA are homologous members of the tethering complex; we also propose to rename vb1, avaA.
We recently conducted preliminary analysis of the vesicle-vacuole proteome (the same fraction used for feeding experiments in the current study) using multidimensional protein identification technology (MudPIT) and detected ≈200 proteins, including 9 aflatoxin enzymes, suggesting that a large portion of the aflatoxin pathway is present in this organelle. In support of this notion, Fas1, which catalyzes the first step in the pathway (synthesis of hexanoyl CoA), was among these 9 enzymes. Details of how vesicles and vacuoles undergo morphological, chemical, and functional development as cells switch to secondary metabolism will be elucidated in our future studies using proteome analysis.
Methods
Strains, Media, and Growth Conditions.
The strains used in this study are listed in Table 1 (see SI Methods for generation of Δvb1 strains). YES liquid medium [contains 2% yeast extract and 6% sucrose (pH 5.8)] was used as an aflatoxin-inducing growth medium, and YEP liquid medium [contains 2% yeast extract and 6% peptone (pH 5.8)] was used as an aflatoxin-noninducing medium. Sterigmatocystin (ST) used for feeding experiments was obtained from Sigma. Sortin3 was obtained from DIVERSetE (Chembridge). Sortin3 treatment was conducted with established dosages (21). Fungal growth was conducted in the dark.
Feeding Experiments.
Feeding experiments (see SI Text) were conducted 3 times with similar trends. Aflatoxin was measured by ELISA using standard methods (16), and the values were normalized to total protein in the fraction.
Proteins and RT-PCR Analysis.
Protein measurements, Western blot analysis, and RT-PCR analysis of gene expression were performed as described previously (16).
Microscopy.
For bright-field microscopy, we used forceps to remove hyphal filaments from a mycelial pellet harvested from the growth medium. These filaments were placed on a microscope slide and observed using a Nikon Eclipse E600 microscope. Counting of vesicles and statistical analysis was done as described in SI Text. Methods for bright-field microscopy of protoplasts (Fig. 2A) and electron microscopy of vesicles-vacuoles fraction in (Fig. 2B) are described in ref. 12.
Supplementary Material
Acknowledgments.
We thank Dr. Nancy Keller (Medical Microbiology and Immunology, University of Wisconsin, Madison, WI) for sharing TJYP1–22, and Dr. Jeff Cary (Southern Regional Research Center, USDA Agricultural Research Service, New Orleans) for sharing AFS10. This work was supported by National Institutes of Health Grant CA52003–18 (to J.E.L. and L.V.R.) and the Michigan Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The nucleotide sequence for Aspergillus parasiticus vb1 (avaA) has been deposited in GenBank (accession no. AY52045).
This article contains supporting information online at www.pnas.org/cgi/content/full/0907416106/DCSupplemental.
References
- 1.Sirikantaramas S, Yamazaki M, Saito K. Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochem Rev. 2008;7:467–477. [Google Scholar]
- 2.Ziegler J, Facchini PJ. Alkaloid biosynthesis: Metabolism and trafficking. Annu Rev Plant Biol. 2008;59:735–769. doi: 10.1146/annurev.arplant.59.032607.092730. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008;54:733–749. doi: 10.1111/j.1365-313X.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoppert M, Gentzsch C, Schorgendorfer K. Structure and localization of cyclosporin synthetase, the key enzyme of cyclosporin biosynthesis in Tolypocladium inflatum. Arch Microbiol. 2001;176:285–293. doi: 10.1007/s002030100324. [DOI] [PubMed] [Google Scholar]
- 5.Lendenfeld T, Ghali D, Wolschek M, Kubicek-Pranz EM, Kubicek CP. Subcellular compartmentation of penicillin biosynthesis in Penicillium chrysogenum. The amino acid precursors are derived from the vacuole. J Biol Chem. 1993;268:665–671. [PubMed] [Google Scholar]
- 6.Hong SY, Linz JE. Functional expression and subcellular localization of the aflatoxin pathway enzyme Ver-1 fused to enhanced green fluorescent protein. Appl Environ Microbiol. 2008;74:6385–6396. doi: 10.1128/AEM.01185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong SY, Linz JE. Functional expression and sub-cellular localization of the early aflatoxin pathway enzyme Nor-1 in Aspergillus parasiticus. Mycol Res. 2009;113:591–601. doi: 10.1016/j.mycres.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee LW, Chiou CH, Klomparens KL, Cary JW, Linz JE. Subcellular localization of aflatoxin biosynthetic enzymes Nor-1, Ver-1, and OmtA in time-dependent fractionated colonies of Aspergillus parasiticus. Arch Microbiol. 2004;181:204–214. doi: 10.1007/s00203-003-0643-3. [DOI] [PubMed] [Google Scholar]
- 9.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MJ, Linz JE. Genetic mechanisms involved in regulation of mycotoxin biosynthesis. In: Kalidas Shetty GP, Pometto A, Levin RE, editors. Food Biotechnology. 2nd Ed. Taylor & Francis, New York: CRC; 2006. Chapter 3.09. [Google Scholar]
- 11.Keller NP, Turner G, Bennett JW. Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 12.Chanda A, Roze LV, Pastor A, Frame MK, Linz JE. Purification of a vesicle-vacuole fraction functionally linked to aflatoxin synthesis in Aspergillus parasiticus. J Microbiol Methods. 2009;78:28–33. doi: 10.1016/j.mimet.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayram O, et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 14.Calvo AM. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet Biol. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Calvo AM, Bok J, Brooks W, Keller NP. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roze LV, Arthur AE, Hong SY, Chanda A, Linz JE. The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol Microbiol. 2007;66:713–726. doi: 10.1111/j.1365-2958.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee LW, Chiou CH, Linz JE. Function of native OmtA in vivo and expression and distribution of this protein in colonies of Aspergillus parasiticus. Appl Environ Microbiol. 2002;68:5718–5727. doi: 10.1128/AEM.68.11.5718-5727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 19.Ohsumi K, Arioka M, Nakajima H, Kitamoto K. Cloning and characterization of a gene (avaA) from Aspergillus nidulans encoding a small GTPase involved in vacuolar biogenesis. Gene. 2002;291:77–84. doi: 10.1016/s0378-1119(02)00626-1. [DOI] [PubMed] [Google Scholar]
- 20.Wichmann H, Hengst L, Gallwitz D. Endocytosis in yeast: Evidence for the involvement of a small GTP-binding protein (Ypt7p) Cell. 1992;71:1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- 21.Zouhar J, Hicks GR, Raikhel NV. Sorting inhibitors (Sortins): Chemical compounds to study vacuolar sorting in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:9497–9501. doi: 10.1073/pnas.0402121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks JK, Yu JH, Keller NP, Adams TH. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 1997;16:4916–4923. doi: 10.1093/emboj/16.16.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohlfs M, Albert M, Keller NP, Kempken F. Secondary chemicals protect mould from fungivory. Biol Lett. 2007;3:523–525. doi: 10.1098/rsbl.2007.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggio-Hall LA, Wilson RA, Keller NP. Fundamental contribution of beta-oxidation to polyketide mycotoxin production in planta. Mol Plant Microbe Interact. 2005;18:783–793. doi: 10.1094/MPMI-18-0783. [DOI] [PubMed] [Google Scholar]
- 25.Evers ME, Trip H, van den Berg MA, Bovenberg RA, Driessen AJ. Compartmentalization and transport in beta-lactam antibiotics biosynthesis. Adv Biochem Eng Biotechnol. 2004;88:111–135. doi: 10.1007/b99259. [DOI] [PubMed] [Google Scholar]
- 26.Kurylowicz W, Kurzatkowski W, Kurzatkowski J. Biosynthesis of benzylpenicillin by Penicillium chrysogenum and its Golgi apparatus. Arch Immunol Ther Exp (Warsz) 1987;35:699–724. [PubMed] [Google Scholar]
- 27.Muller WH, et al. Involvement of microbodies in penicillin biosynthesis. Biochim Biophys Acta. 1992;1116:210–213. doi: 10.1016/0304-4165(92)90118-e. [DOI] [PubMed] [Google Scholar]
- 28.Sprote P, Brakhage AA, Hynes MJ. Contribution of peroxisomes to penicillin biosynthesis in Aspergillus nidulans. Eukaryot Cell. 2009;8:421–423. doi: 10.1128/EC.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiou CH, et al. Distribution and sub-cellular localization of the aflatoxin enzyme versicolorin B synthase in time-fractionated colonies of Aspergillus parasiticus. Arch Microbiol. 2004;182:67–79. doi: 10.1007/s00203-004-0700-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.