Abstract
Objective
To test the hypothesis that diabetes is independently associated with reduced lung function, both cross-sectionally and longitudinally.
Methods
We conducted cross-sectional and prospective analyses of diabetes status and lung function decline using baseline and 3-year follow-up data on 1,100 diabetic and 10,162 non-diabetic middle-aged adults from the Atherosclerosis Risk in Communities (ARIC) Study. Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were measured at baseline and 3-year follow-up using standard spirometry.
Results
At baseline, adults with diabetes had significantly lower predicted FVC (96% vs. 103%, p< 0.001) and predicted FEV1 (92% vs. 96%, p < 0.001) than those without diabetes. These differences remained significant after adjustment for demographic characteristics, adiposity, smoking, physical activity index, education, and ARIC field center. Graded, inverse associations were observed between hyperglycemia, diabetes severity (i.e. duration of diabetes and types of anti-diabetes medications) and FVC and FEV1 (all p for trend < 0.001). In prospective analyses, FVC declined faster in diabetic adults than in their non-diabetic counterparts (64 vs. 58 ml/year, p= 0.01). Diabetes severity as indicated by intensity of anti-diabetic treatment also showed graded relationships with rate of FVC decline (p< 0.01).
Conclusions
These data support the notion that the lung is a target organ for diabetic injury. Additional research is required to identify pathophysiologic mechanisms and to determine clinical significance.
Keywords: Type 2 diabetes mellitus, Spirometry, Cohort study, Risk factors
Impaired lung function has attracted growing interest as a potential complication of diabetes mellitus (1–8, 16, 27). Cross-sectional studies have consistently shown that adults with diabetes have lower vital capacity than their non-diabetic counterparts (1–5, 7), but such studies cannot establish the temporal sequence of events. We therefore analyzed longitudinal data from the Atherosclerosis Risk in Communities (ARIC) Study, a biracial, community-based cohort of adults aged 45–64, to test the hypothesis that diabetes is associated with reduced lung function independently of known risk factors.
RESEARCH DESIGN AND METHODS
The ARIC Study is a prospective cohort study of 15,792 adults from the following four US communities: Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. The design and conduct of the ARIC Study has been previously described (10). The present analysis was based on approximately 3 years of follow-up, which included the baseline examination (1987 through 1989), and a follow-up clinic visit at year 3 (1990 to 1992). The follow-up for individuals still alive at the time of the second visit was 93 percent.
For the current analysis, the following criteria were used for excluding individuals: ethnicity other than black or white Americans (n = 48), missing data on spirometry or diabetes status at baseline (n= 2,014), self-reported asthma or chronic lung disease or use of medications for the conditions at baseline (n = 1,233), use of heart failure medications (n = 101), and missing data on relevant covariates (n = 250). We also excluded individuals in the upper or lower 1% of FVC, FEV1, or FEV1 to FVC ratio at baseline or 3-year follow-up visit who were presumed to represent outliers (n = 884). The final study sample consisted of 11,262 individuals including 1,100 adults with diabetes at baseline and 10,162 adults who were free of diabetes at baseline.
Spirometry
At the baseline and 3-year follow-up visits, measurement of forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were performed based on recommendations from the Epidemiology Standardization Project (11) and the American Thoracic Society (12). Methodology was standardized across the four field centers. Quality control and reproducibility were coordinated by a centralized pulmonary function reading center (Johns Hopkins School of Public Health, Baltimore, MD).
Type 2 Diabetes Mellitus
Individuals were classified as having diabetes if any of the following criteria, adapted from 1997 American Diabetes Association criteria, were met: fasting glucose level of at least 7.0 mmol/L (126 mg/dL); nonfasting glucose level of at least 11.1 mmol/L (200 mg/dL); current use of anti-diabetic medication; or a positive response to the question “Has a doctor ever told you that you had diabetes (sugar in the blood)?”
Other Variables
The definitions and methods used for other baseline measurements (age, race, education level, cigarette smoking status and pack-years, height, body-mass index [BMI], waist and hip circumferences, sport activity index, glucose, hypertension, white blood cell count, and fibrinogen) have been previously reported (13).
Hemoglobin A1c (HbA1c) was measured as part of a previous study (14) and was only available for all 1,637 prevalent cases of type 2 diabetes at the second ARIC visit and a subgroup of 598 randomly selected (stratified by age, sex, and ethnic origin) non-diabetic individuals from the ARIC visit 2 cohort (excluding prevalent and incident cases of CHD, diabetes, and TIA/stroke) which was used as a comparison group in our analysis of the HbA1c data.
Data Analysis
Predicted FVC and FEV1 were calculated by the ARIC Data Coordinating Center using the equations developed by Crapo et al in non-smokers that included age, sex, height and race (15). Baseline differences between characteristics of diabetic and non-diabetic individuals were compared using t tests for continuous variables and χ2 tests for categorical variables.
Multiple linear regression models were fitted to describe the cross-sectional association between lung function and fasting glucose levels, duration of diabetes, and type of anti-diabetic medications at baseline (1987 to 1989) after adjustment for potential confounding variables. Additional multivariable analyses were performed to investigate the roles of the inflammatory markers as potential confounders.
Weighted-univariate and multivariable analyses were used to assess the cross-sectional relationship between HbA1c and lung function in the diabetes cases and subgroup of individuals without diabetes for which HbA1c were available at ARIC Visit 2 (1990 to 1992), accounting for the stratified random sampling design.
In the prospective analyses, multiple linear regressions were used to model the changes of FVC, FEV1, FVC (% predicted), FEV1 (% predicted), and the FEV1 to FVC ratio per year from baseline to ARIC visit 2 (3-year follow- up) in relation to the baseline diabetes status, fasting glucose level, diabetes duration, and use of anti-diabetes medications after adjusting for covariates.
In all multivariable models, tests for interactions with diabetes status were conducted with gender, race, waist circumference, and smoking status. No interactions were detected (all p>0.05), and therefore only pooled results were presented. Diabetes-status-specific categories were included as an ordinal variable in the linear model to test for linear trend. All tests of significance were two-tailed, with an alpha level of 0.05. All analyses were performed using SAS (Cary, NC) statistical software package (Version 9.1).
RESULTS
Baseline Characteristics
Table 1 presents baseline characteristics of the sample by diabetes status. Compared to their non-diabetic counterparts, adults with diabetic were significantly more likely to be male, to be African American, older, and less educated; furthermore, among ever smokers, diabetic subjects smoked more cigarettes; were less physically active; had higher BMI and higher waist-to-hip ratio; and were noted to have higher prevalence of hypertension and have higher white blood cell counts and fibrinogen levels. Adults with diabetes also had significantly lower FVC, FEV1, FVC (% predicted), and FEV1 (% predicted) at baseline compared to adults without diabetes. In contrast, the ratio of FEV1 to FVC was significantly higher in adults with diabetes than without diabetes.
Table 1.
Baseline characteristics of 11,262 middle-aged adults according diabetes status.
| Diabetes (N = 1,100) |
No Diabetes (N = 10,162) |
P values | |
|---|---|---|---|
| Male (%) | 48 | 44 | 0.01 |
| African American, % | 37 | 21 | < 0.001 |
| Age (yrs) | 55 ± 5.6 | 54 ± 5.6 | < 0.001 |
| Education < 12 yrs, % | 32 | 19 | < 0.001 |
| Smoking status, % | 0.03 | ||
| Current | 19 | 23 | |
| Former | 33 | 33 | |
| Never | 48 | 44 | |
| Pack-years in ever smokers | 28± 21 | 25± 20 | 0.0004 |
| Sport index | 2.3± 0.74 | 2.5± 0.80 | < 0.001 |
| BMI (kg/m2) | 30.9± 5.7 | 27.2± 4.8 | < 0.001 |
| Waist circumference (cm) | 105.7± 13.6 | 95.4± 12.9 | < 0.001 |
| White blood count (x 109/L) | 6.5± 1.0 | 6.0± 1.9 | < 0.001 |
| Fibrinogen(g/L) | 3.2± 0.7 | 3.0± 0.6 | < 0.001 |
| Hypertension, % | 65 | 37 | < 0.001 |
| FVC (L) | |||
| Men | 4.2± 0.8 | 4.6± 0.7 | < 0.001 |
| Women | 3.0± 0.5 | 3.3± 0.6 | < 0.001 |
| FEV1 (L) | |||
| Men | 3.2± 0.6 | 3.4± 0.7 | < 0.001 |
| Women | 2.3± 0.5 | 2.5± 0.5 | < 0.001 |
| FEV1/FVC, % | 76.7± 6.3 | 75.1± 6.6 | < 0.001 |
| FVC % predicted | 96.5± 13.2 | 103.1± 13.6 | < 0.001 |
| FEV1 % Predicted | 92.5± 14.1 | 96.4± 14.6 | < 0.001 |
Results shown as percentage or mean ± standard deviation
Cross-Sectional Analyses
As shown in Table 2, compared to their non-diabetic counterparts, in the models adjusting for age, gender, race, BMI, waist circumference, height, pack-years of smoking, sport activity index, educational level, and ARIC field center, adults with diabetes had FVC, FEV1, FVC (% predicted), FEV1 (% predicted) lowered by 133ml, 72ml, 3.6%, and 2.4%, respectively (all p<0.001). In the subsequent analysis, we stratified individuals with diabetes according to the level of fasting glucose (Table 2). In diabetic adults, higher fasting glucose was significantly associated with further reductions in FVC and FVC (% predicted) (all p for trend < 0.001). A similar, but less pronounced, graded inverse relationship was observed between fasting glucose and FEV1 (P for trend <0.001), resulting in a graded increase in the ratio of FEV1 to FVC with increasing hyperglycemia.
Table 2.
Baseline spirometry by diabetes status and adjusted* mean differences [95% confidence intervals] in adults with diabetes vs. without diabetes at baseline.
| FVC (ml) | FEV1 (ml) | FVC (% pred) | FEV1 (% pred) | FEV1/FVC (%) | |
|---|---|---|---|---|---|
| No Diabetes | 3,873 [3,863 – 3,882] | 2,911 [2,904 – 2,919] | 102.8 [102.6– 103.1] | 96.5 [96.3 – 96.8] | 75.4 [75.3–75.5] |
| Diabetes | 3,740 [3,711 – 3,769] | 2,839 [2,856 – 2,863] | 98.3 [98.5 – 100.1] | 94.1 [93.3 – 94.9] | 76.2 [75.8–76.5] |
| Diabetes vs. No diabetes | −133 [−163 to −103] | −72 [−97 to −47] | −3.6 [−4.4 to −2.7] | −2.4 [−3.2 to −1.6] | 0.8 [0.4 to 1.1] |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Diabetes, by fasting glucose (mg/dL) | |||||
| < 140 vs. No diabetes | −109 [−155 to −63] | −66 [−105 to −29] | −2.9 [−4.1 to −1.7] | −2.3 [−3.5 to −1.0] | 0.4 [−0.1 to 0.9] |
| 140 – 199 vs. No diabetes | −147 [−202 to −93] | −81 [−127 to −36] | −3.8 [−5.3 to −2.4] | −2.6 [−4.1 to −1.1] | 0.9 [−0.2 to 0.1] |
| 200+ vs. No diabetes | −155 [−216 to −94] | −69 [−120 to −19] | −4.2 [−5.7 to −2.6] | −2.4 [−4.1 to −0.8] | 1.3 [0.6 to 1.9] |
| P for trend† | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Diabetes, by duration (years) | |||||
| <=5 vs. No diabetes | −105 [−166 to −43] | −27 [−78 to 24] | −2.8 [−4.5 to −1.2] | −1.1 [−2.7 to 0.6] | 1.3 [0.7 to 2.0] |
| 6 – 9 vs. No diabetes | −153 [−249 to −56] | −77 [−158 to 3] | −3.9 [−6.4 to −1.4] | −2.5 [−5.1 to 0.1] | 1.0 [−0.02 to 2.1] |
| >=10 vs. No diabetes | −155 [−224 to −86] | −86 [−144 to −29] | −4.0 [−5.8 to −2.2] | −2.8 [−4.7 to −0.9] | 0.8 [0.01 to 1.5] |
| Unknown vs. No diabetes | −135 [−175 to −95] | −84 [−118 to −51] | −3.7 [−4.7 to −2.6] | −2.8 [−3.9 to −1.8] | 0.5 [0.05 to 0.94] |
| P for trend in known duration† | <0.0001 | 0.0005 | <0.0001 | 0.0005 | 0.0002 |
| Diabetes, by medications | |||||
| No medication vs. No diabetes | −112 [−154 to −77] | −70 [−100 to −36] | −3.0 [−4.0 to −2.0] | −2.2 [−3.2 to −1.1] | 0.5 [0.1 to 1.0] |
| Oral agents vs. No diabetes | −141 [−198 to −84] | −56 [−103 to −9] | −3.7 [−5.2 to −2.2] | −2.0 [−3.5 to −0.4] | 1.3 [0.7 to 1.9] |
| Insulin (alone or with oral) vs. No diabetes | −187 [−257 to −117] | −112 [−170 to −53] | −5.3 [−7.1 to −3.4] | −4.0 [−5.9 to −2.1] | 0.9 [0.1 to 1.6] |
| P for trend† | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Adjusted for baseline age, gender, race, height, BMI, waist circumference, pack-years of smoking, sport activity index, education level, and ARIC center.
P values correspond to tests for linear trend across categories.
To further explore the relationship between diabetes severity and lung function at baseline, we conducted two additional analyses: one stratified by diabetes duration, the other by type of anti-diabetic medication currently used. Compared to their non-diabetes counterparts, diabetic adults who reported longer duration of diabetes had further reductions in FVC, FEV1, FVC (% predicted), and FEV1 (% predicted) (all p for trend <0.0001). Likewise, there was a significant, graded, and inverse relationship between lung function and anti-diabetic medication uses (Table 2; p for trend < 0.001). A similar pattern of results but in smaller magnitude was observed for FEV1, leading in all cases, to an inversion of the ratio of FEV1 to FVC.
Subsidiary Analyses
To determine whether diabetes-related differences in inflammatory markers might help explain the relationship of diabetes to lung function, we performed additional analyses after introducing white blood cell count and plasma fibrinogen concentration into fully-adjusted multivariable models. Additional adjustment for these markers attenuated the observed relationships only slightly (data not shown).
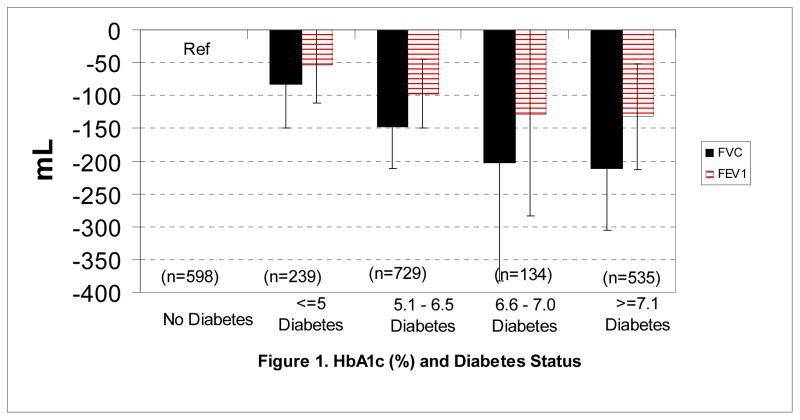
Finally, to confirm the association between glycemia and FVC, we conducted an additional analysis in diabetes cases and a subgroup of individuals without diabetes for which HbA1c levels were available at ARIC Visit 2 (1990 to 1992). As shown in Figure 1, the relationships between poor glycemic control (as indicated by higher %HbA1c) and reduced FVC and FEV1 were significant and graded. In addition, as compared to their non-diabetic counterparts, diabetic adults with % HbA1c less than or equal to 5, 5.1 to 6.5, 6.6 to 7.0, and 7.1+, had FVC (% predicted) that were lower by 2.7% [95%CI: 0.9 to 4.5%], 3.7% [2.0 – 5.4%], 5.5% [0.5 – 10.4%], and 5.8% [3.2 – 8.4%], respectively (p for trend < 0.0001). The corresponding decreases in FEV1 (% predicted) were 2.3% [0.3 – 4.2%], 3.0% [1.2 – 4.8%], 4.5% [0.8 – 9.8%], and 4.7% [1.9 – 7.5%] (p for trend <0.0001). Again, differences in the degree of impairment with increasing glycemia led to an increase in the ratio of FEV1 to FVC with worsening glycemia (p=0.06).
Figure 1.
Adjusted weighted mean differences in baseline FVC and FEV1 by diabetes status and HbA1c level in a subset of 1,637 diabetes cases (incident and prevalent) and 598 non-cases (stratified random sample) at ARIC visit 2 (1990 – 1992) in whom HbA1c was measured. All differences are simultaneously adjusted for age, gender, race, BMI, waist circumference, height, pack-years of smoking, sport activity index, educational level, and ARIC field center. Both p values <0.001 for linear trends. Error bars are 95% CIs.
Prospective Analysis from Baseline to Visit 2
To reduce potential confounding effect from weight gain, we further excluded individuals on the top 1% of increase in BMI or waist circumference from baseline to 3-year follow-up visit (n = 164). During 3 years of follow-up, FVC and FVC (% predicted) declined more rapidly in diabetic adults than in those without diabetes (Table 3). The differences were small (about 6 mL per year), but consistent and significant. Use of anti-diabetes medications showed faster declines in absolute FVC and FVC (%predicted) with graded relationships (p for trend < 0.01). In contrast, lesser decline in FEV1 to FVC ratio was noted in diabetes.
Table 3.
Adjusted mean changes [95% confidence intervals] † in FVC, FEV1, and FEV1 to FVC ratio over 3 years of follow-up by diabetes status at baseline.
| ΔFVC (ml/yr) | ΔFEV1 (ml/yr) | ΔFVC %pred (%/yr) | ΔFEV1 %pred (%/yr) | ΔFEV1/FVC (%/yr) | |
|---|---|---|---|---|---|
| No Diabetes | ↓ 58 [56 – 59] | ↓ 47 [45 – 48] | ↓ 0.96 [0.9 – 1.0] | ↓ 0.7 [0.7 – 0.8] | ↓ 0.1 [0.07 − 0.1] |
| Diabetes | ↓ 64 [59 – 69] | ↓ 49 [46 – 53] | ↓ 1.1 [1.0 – 1.3] | ↓ 0.9 [0.7 – 1.0] | ↓ 0.05 [−0.02 − 0.1] |
| P value | 0.01 | 0.13 | 0.009 | 0.08 | 0.22 |
| Diabetes by fasting glucose (mg/dL) | |||||
| < 140 | ↓ 63 [53 – 72] | ↓ 49 [42 – 56] | ↓ 1.1 [0.8 – 1.3] | ↓ 0.8 [0.6 – 1.0] | ↓ 0.1 [−0.03 – 0.2] |
| 140 – 199 | ↓ 61 [52 – 69] | ↓ 47 [40 – 53] | ↓ 1.1 [0.9 – 1.3] | ↓ 0.8 [0.6 – 1.0] | ↓ 0.1 [−0.1 – 0.2] |
| 200+ | ↓ 56 [46 – 65] | ↓ 47 [40 – 54] | ↓ 0.9 [0.7 – 1.2] | ↓ 0.8 [0.5 –1.0] | ↓ 0.2 [0.01 – 0.3] |
| P for trend‡ | 0.59 | 0.89 | 0.65 | 0.88 | 0.83 |
| Diabetes by duration (years) | |||||
| <=5 | ↓ 57 [48 – 67] | ↓ 45 [38 – 52] | ↓ 1.0 [0.7 – 1.3] | ↓ 0.7 [0.5 – 1.0] | ↓ 0.1 [−0.1 – 0.1] |
| 6 – 9 | ↓ 63 [47 – 79] | ↓ 52 [40 – 63] | ↓ 1.1 [0.7 – 1.5] | ↓ 0.9 [0.5 – 1.3] | ↓ 0.1 [−0.3 – 0.1] |
| >=10 | ↓ 68 [57 – 79] | ↓ 50 [41 – 58] | ↓ 1.3 [1.0 – 1.6] | ↓ 0.9 [0.6 – 1.2] | ↑ 0.03 [−0.1 – 0.2] |
| Unknown | ↓ 65 [59 – 72] | ↓ 51 [46 – 56] | ↓ 1.2 [1.0 – 1.3] | ↓ 0.9 [0.7 – 1.1] | ↓ 0.1 [−0.2 – 0.0] |
| P for trend in known duration‡ | 0.07 | 0.42 | 0.05 | 0.31 | 0.11 |
| Diabetes by medications | |||||
| No medication | ↓ 63 [57 – 69] | ↓ 48 [44 – 53] | ↓ 1.1 [0.9 – 1.3] | ↓ 0.8 [0.7 – 1.0] | ↓ 0.1 [−0.02 – 0.1] |
| Oral agents | ↓ 57 [48 – 66] | ↓ 50 [43 – 57] | ↓ 1.0 [0.7 – 1.2] | ↓ 0.9 [0.6 – 1.1] | ↓ 0.2 [0.1 – 0.3] |
| Insulin (alone or with oral) | ↓ 79 [68 – 90] | ↓ 52 [44 – 61] | ↓ 1.5 [1.2 – 1.9] | ↓ 0.9 [0.6 – 1.2] | ↑0.2 [−0.4 −0.1] |
| P for trend‡ | 0.001 | 0.40 | 0.001 | 0.33 | 0.0003 |
Adjusted for baseline age, gender, race, height, BMI, waist circumference, pack-years of smoking, sport activity index, education level, baseline lung function, BMI change from baseline to year 3, waist change from baseline to year 3, incident asthma or chronic lung disease or use of medications for the conditions at 3-year follow-up, and ARIC center.
P values correspond to tests for linear trend across categories.
To address the observation that lung function loss in the non-diabetes comparisons was greater than the previous report (26), we limited the analysis to non-diabetic adults with baseline age less than 55 years, BMI less than 25, never smoked, without lung diseases nor asthma, and in professional, technical, or service occupations (n=681). In this sub-group analysis, the adjusted annual FVC and FEV1 decreases were still 50 ml [95% CI: 45 – 54 ml] and 45 ml [41–48 ml], respectively.
Additional cross-sectional and prospective analyses were conducted among lifetime non-smokers only. All results and patterns observed in never smokers were similar and comparable to the total study population.
CONCLUSIONS
In cross-sectional analyses, middle-aged adults with type 2 diabetes had significantly lower FVC, FEV1, FVC (% predicted), and FEV (% predicted) compared to their non-diabetic counterparts. These relationships were graded (by fasting glucose, HbA1c, diabetes duration, intensity of anti-diabetic treatment) and were independent of traditional risk factors. In prospective analyses, FVC declined faster in diabetic adults than in their non-diabetic counterparts. Again, these associations were independent of known risk factors (i.e. age, smoking, central obesity) for lung function decline and showed graded associations with indicators of diabetes severity.
In this study, the non-diabetic group had annual FVC decrease by 58 ml per year, which was higher than 25 to 35 ml per year from previous literature (26). Further research is needed to explain the possible differences. On the other hand, an absolute difference of 6 ml more decline per year due to diabetes (64 vs. 58 ml/year) deserves clinical attention.
Our results are generally consistent with prior cross-sectional studies, which demonstrated lower FVC and FEV1 in adults with prevalent diabetes as compared to their non-diabetic counterparts (1–5, 7), especially when diabetes was of longer duration (2,3), required insulin treatment (1), and when diabetic individuals had existing complications of the disease (3, 7). Furthermore, in non-diabetic adults, lower FVC and FEV1 were associated with higher fasting glucose (1, 2) and with hyperinsulemia and estimated insulin resistance (4, 5, 16).
Three previous studies offer prospective data on diabetes and subsequent lung function (6, 8, 27). Lange et al followed 17,506 Danish adults in the Copenhagen City Heart Study for 15 years (6). At baseline, FVC and FEV1 were consistently lower in diabetic individuals with ~8% difference in FVC between diabetes and non-diabetes (similar to what we found in ARIC: 103% vs. 96%). However, longitudinal analyses showed no influence of diabetes on subsequent declines. FVC declined approximately 24 ml per year in women, and 39 ml per year in men in diabetic individuals. Davis et al (8) followed 125 Australian patients with type 2 diabetes for a mean of 7 years. FVC and FEV1 continued to decline at an annual rate of 68, and 71 mL per year, respectively. Declines in FVC and FEV1 were more rapid in patients with higher baseline HbA1c. Nevertheless, no non-diabetic control group was assembled for comparison. Litonjua et al conducted a nested case-control analysis in 352 men who developed diabetes and 352 non-diabetic men in the Normative Aging Study (27). The study found that, although cases had lower FEV1 and FVC at all time points, cases had only 5.4 mL/year greater declines compared with controls after diabetes diagnosis (p=0.2; median follow-up : 11.9 years). Like other case-control studies, it was possible that only healthy subjects who were at risk for diabetes completed the lung function tests.
Although the underlying mechanism relating diabetes to reduced lung function remains unclear, previous studies suggest several possible explanations including glycosylation of chest wall and bronchial tree proteins (9), thickening of basal lamina (17), and perhaps related to increased susceptibility to respiratory infections (18). Additionally, hyperglycemia, inflammation and diabetes-related oxidative stress have been shown to induce muscle dysfunction (19).
Other studies of lung function in the prediabetic individual complicate causal inferences. In particular, several recent prospective studies, including the ARIC Study, have demonstrated that reduced lung function is an independent predictor of incident type 2 diabetes (4, 20, 21). In the present study, the associations between diabetes status and lung function were more significant cross-sectionally than prospectively. These results suggested the notion that abnormalities in lung function precede diabetes, then continue after diabetes onset.
Furthermore, it is possible that our findings in ARIC fit into the broadening picture of mild organ dysfunction associated with altered gene expression found in the common soil underlying diabetes. The effects could be mediated by pro-inflammatory master regulator molecules such as the mediators of the nuclear factor kappa B (NFκB) and activator protein (AP)-1 pathways, which themselves might be subject to further inflammation by hyperglycemia (22, 23). In this study, however, addition of inflammation markers to the models, though slightly reducing the size of the decline associated with diabetes, did not provide clear evidence of a role for inflammatory.
Attention to the lung as a possible target organ of diabetes-related injury has been highlighted recently by the approval of delivering insulin by inhalation (24). A recent meta-analysis of randomized controlled trials of at least 12 weeks’ duration (25) reported a greater decrease in FEV1 from baseline among those taking inhaled insulin than did those in the comparison group (weighted mean difference, − 0.31 L (CI: − 0.043 to − 0.020 L).
Strengths of the present study included a community-based population, standardized spirometric techniques, extensive data on potential confounders, and the large, biracial, community-based sample, which increased precision of our estimates and permitted multivariable statistical adjustments. Nonetheless, several limitations should be kept in mind. First, although the availability of standardized follow-up data on lung function was a definite strength, the interval was short--only 3 years. Second, HbA1c was measured only at year 3 of follow-up, limiting its usefulness in longitudinal analyses. Finally, given the strong relation between type 2 diabetes and central adiposity, even the most meticulous adjustment for BMI and waist circumference leaves some concern about the possibility of residual confounding.
In summary, this study supports the notion that lower lung function, particularly decreased vital capacity, not only precedes the onset of diabetes but continues, at an accelerated pace, with the onset of the disease. Additional research is required to identify pathophysiologic mechanisms and to determine clinical significance of this association. In the meantime, clinicians should pay heightened attention to pulmonary function in their patients with type 2 diabetes.
Acknowledgments
The Atherosclerosis Risk in Communities Study is supported by contracts from the National Heart, Lung, and Blood Institute (N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022), and by National Institute of Diabetes, Digestive, and Kidney Diseases Grant (5R01-DK56918), Bethesda, MD. Dr. Punjabi was supported by National Institutes of Health Grants (HL04065 and HL75078). Dr. Wang was supported by a grant from the National Institutes of Health, NCRR, Bethesda, MD (M01-RR02719). Dr. Duncan received support from a Centers of Excellence Grant of CNPq (the Brazilian National Research Council). Dr. Selvin was supported by NIH/NIDDK grant K01 DK076595. Dr. Brancati was supported by a grant from the National Institutes of Health, NIDDK, Bethesda, MD (K24 DK62222). Analysis of this manuscript was partially supported by Pfizer. The authors thank the staff and participants in the ARIC study for their important contributions.
References
- 1.Lange P, Groth S, Kastrup J, et al. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur R respir J. 1989;2:14–19. [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Frette C. NIDDM, impaired glucose tolerance, and pulmonary function in older adults. The Rancho Bernardo Study. Diabetes Care. 1996;19:1441–1444. doi: 10.2337/diacare.19.12.1441. [DOI] [PubMed] [Google Scholar]
- 3.Davis TME, Knuiman M, Kendall P, et al. Reduced pulmonary function and its association in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Research and Clinical Practice. 2000;50:153–159. doi: 10.1016/s0168-8227(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J. 1998;12(3):641–5. doi: 10.1183/09031936.98.12030641. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia. 2004;47(2):195–203. doi: 10.1007/s00125-003-1310-6. [DOI] [PubMed] [Google Scholar]
- 6.Lange P, Parner J, Schnohr P, Jensen G. Copenhagen City Heart Study: longitudinal analysis of ventilatory capacity in diabetic and nondiabetic adults. Eur Respir J. 2002;20(6):1406–12. doi: 10.1183/09031936.02.00050502. [DOI] [PubMed] [Google Scholar]
- 7.Marvisi M, Bartolini L, del Borrello P, Brianti M, Marani G, Guariglia A, Cuomo A. Pulmonary function in non-insulin-dependent diabetes mellitus. Respiration. 2001;68:268–72. doi: 10.1159/000050509. [DOI] [PubMed] [Google Scholar]
- 8.Davis WA, Knuiman M, Kendall P, Grange V, Davis TM. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27(3):752–7. doi: 10.2337/diacare.27.3.752. [DOI] [PubMed] [Google Scholar]
- 9.Sandler M. Is the lung a ‘target organ’ in diabetes mellitus? Arch Intern Med. 1990;150(7):1385–8. [PubMed] [Google Scholar]
- 10.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.Ferris BG, Jr, Speizer FE, Bishop Y, et al. Spirometry for an epidemiologic study: Deriving optimum summary statistics for each subject. Bull Europ Physiopath Resp. 1978;14:146–166. [PubMed] [Google Scholar]
- 12.Gardner RM, et al. ATS statement. Snowbird workshop on standarization of spirometry. Am Rev Respir Dis. 1979;119:831. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 13.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Coresh J, Jordahl J, Boland L, Steffes M. Stability of Hemoglobin A1c (HbA1c) Measurements from Frozen Whole Blood Samples Stored for Over a Decade. Diabetic Medicine. 2005;22(12):1726–30. doi: 10.1111/j.1464-5491.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 15.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Amer Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 16.McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;15;161(6):546–56. doi: 10.1093/aje/kwi076. [DOI] [PubMed] [Google Scholar]
- 17.Weynand B, Jonckheere A, Frans A, Rahier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration. 1999;66:14–19. doi: 10.1159/000029331. [DOI] [PubMed] [Google Scholar]
- 18.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- 19.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43(2):289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 20.Engstrom G, Janzon L. Risk of developing diabetes is inversely related to lung function: a population-based cohort study. Diabet Med. 2002;19(2):167–70. doi: 10.1046/j.1464-5491.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- 21.Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Brancati FL. Vital capacity as a predictor of incident type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(6):1472–9. doi: 10.2337/diacare.28.6.1472. [DOI] [PubMed] [Google Scholar]
- 22.Patti ME. Gene expression in the pathophysiology of type 2 diabetes mellitus. Curr Diab Rep. 2004;4(3):176–81. doi: 10.1007/s11892-004-0020-x. [DOI] [PubMed] [Google Scholar]
- 23.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–54. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 24.Cefalu WT. Concept, strategies, and feasibility of noninvasive insulin delivery. Diabetes Care. 2004;27(1):239–46. doi: 10.2337/diacare.27.1.239. [DOI] [PubMed] [Google Scholar]
- 25.Ceglia L, Lau J, Pittas AG. Meta-Analysis: Efficacy and safety of inhaled insulin therapy in adults with diabetes mellitus. Ann Intern Med. 2006;145(9):665–75. doi: 10.7326/0003-4819-145-9-200611070-00009. [DOI] [PubMed] [Google Scholar]
- 26.Tager IB, Segal MR, Speizer FE, Weiss ST. The natural history of forced expiratory volumes. Effect of cigarette smoking and respiratory symptoms. Am Rev Respir Dis. 1988;138(4):837–49. doi: 10.1164/ajrccm/138.4.837. [DOI] [PubMed] [Google Scholar]
- 27.Litonjua AA, Lazarus R, Sparrow D, Demolles D, Weiss ST. Lung function in type 2 diabetes: the Normative Aging Study. Respir Med. 2005;99(12):1583–90. doi: 10.1016/j.rmed.2005.03.023. [DOI] [PubMed] [Google Scholar]