Abstract
The severity and profile of cognitive dysfunction in first episode schizophrenia and psychotic affective disorders were compared before and after antipsychotic treatment. Parallel recruitment of consecutively admitted study-eligible first-episode psychotic patients (30 schizophrenia, 22 bipolar with psychosis, and 21 psychotic depression) reduced confounds of acute and chronic disease/medication effects as well as differential treatment and course. Patient groups completed a neuropsychological battery and were demographically similar to healthy controls (n=41) studied in parallel. Prior to treatment, schizophrenia patients displayed significant deficits in all cognitive domains. The two psychotic affective groups were also impaired overall, generally performing intermediate between the schizophrenia and healthy comparison groups. No profile differences in neuropsychological deficits were observed across patient groups. Following 6-weeks of treatment, no patient group improved more than practice effects seen in healthy individuals, and level of performance improvement was similar for affective psychosis and schizophrenia groups. Although less severe in psychotic affective disorders, similar profiles of generalized neuropsychological deficits were observed across patient groups. Recovery of cognitive function after clinical stabilization was similar in mood disorders and schizophrenia. To the extent that these findings are generalizable, neuropsychological deficits in psychotic affective disorders, like schizophrenia, may be trait-like deficits with persistent functional implications.
Keywords: Schizophrenia, Bipolar Disorder, Psychotic Depression, Psychosis, Neuropsychology
A growing body of literature describes shared aspects of psychopathology, genetics, neurobiology and treatment efficacy among schizophrenia and psychotic affective disorders (Berrettini, 2000) (Ivleva et al., 2008) (Murray et al., 2004). Cognitive similarities across these disorders have also been identified and may result from overlapping alterations to functional brain systems and related genetic risk factors (Berrettini, 2000) (Bramon and Sham, 2001) (Pearlson et al., 1997). However, cognitive dysfunction in psychotic affective disorders has been reported predominantly in chronic patients during acute episodes (Quraishi and Frangou, 2002) and it remains unclear whether neuropsychological impairments are present at illness onset, vary with clinical symptomatology, and respond differentially to pharmacological treatment.
In the schizophrenia literature, there is considerable interest in neuropsychological deficits as a major cause of functional disability (Green, 2006), a treatment target (Harvey and Cornblatt, 2008), and a phenotype marking familial risk (Berrettini, 2000) (Cannon and Keller, 2006). Overlapping neuropsychological deficits have been reported in schizophrenia and affective psychoses (Daban et al., 2006) (Hill et al., 2004a) (Krabbendam et al., 2005) (Reichenberg et al., 2008) (Schretlen et al., 2007) and in their unaffected relatives (McIntosh et al., 2005) (Pirkola et al., 2005). These overlapping cognitive deficits as well as persistent cognitive and functional impairments observed in psychotic bipolar disorder (Dickerson et al., 2004) (Martinez-Aran et al., 2004) (Tabares-Seisdedos et al., 2008) raise questions about the classic Kraepelinian model by suggesting more similarities regarding persistent functional deficits across the disorders.
A related line of work has compared neurocognitive function in psychotic and nonpsychotic patients with affective disorders. Psychosis in affective disorders has been associated with more severe neuropsychological dysfunction compared to nonpsychotic variants. For example, more severe impairments in executive function (Glahn et al., 2007) and spatial working memory (Glahn et al., 2006) have been observed in bipolar disorder with than without a history of psychosis. More severe cognitive dysfunction has also been reported in psychotic vs. nonpsychotic unipolar depression (Grant et al., 2001) (Hill et al., 2004a) (Jeste et al., 1996).
Few investigations have directly compared affective psychoses and schizophrenia in studies with parallel recruitment of all eligible patients and a broadly focused neuropsychological battery. Such strategies are needed to assess for neuropsychological profile differences, which may indicate differentially affected functional brain systems. Some studies directly comparing schizophrenia and psychotic affective disorders have reported select areas of more severe neuropsychological impairments in schizophrenia (Badcock et al., 2005) (Mojtabai et al., 2000) (Reichenberg et al., 2008). However, the preponderance of available evidence suggests either comparable neurocognitive deficits (Albus et al., 1996) (Hill et al., 2004a) (Jeste et al., 1996) (Rossi et al., 2000) or somewhat greater global dysfunction in schizophrenia compared to psychotic affective disorders (Goldberg et al., 1993) (McClellan et al., 2004) (Mojtabai et al., 2000) (Schretlen et al., 2007).
Given the numerous confounds potentially associated with different course of illness and medications used to treat schizophrenia and affective disorders, comparing disorder-associated neuropsychological function in the early course of illness may provide important insight regarding neuropsychological similarities and differences across these disorders. In addition, studies comparing cognitive abilities during acute episodes of illness to performance after treatment initiation and clinical stabilization may shed light on the impact of acute illness on cognition and deficit persistence across disorders. To our knowledge, no previous investigation has longitudinally compared neuropsychological performance in unipolar depression with psychosis, bipolar disorder with psychosis, and schizophrenia in a parallel recruitment study of first-episode patients. A diverse battery of neuropsychological tests was administered at baseline while antipsychotic-naïve or after a brief treatment discontinuation. Patients who completed 6-weeks of treatment (primarily with risperidone monotherapy) were retested to compare cognitive change at follow-up across the disorders.
Method
Participants
Consecutive admissions who presented with a recent onset psychotic disorder were recruited at the University of Illinois Medical Center. Because diagnosis is often unclear during acute episodes, particularly in first episode psychosis patients, patients were followed clinically for several months before a consensus diagnosis could be reached. Diagnosis was determined during multi-disciplinary consensus conferences using all available clinical data including the Structured Clinical Interview for DSM-IV (SCID). Schizoaffective (all depressed type, n=5) and schizophrenia patients were pooled for statistical analysis. Healthy individuals with no history of psychiatric treatment and no Axis I disorder, based on SCID interview, were recruited from the community. As presented in Table 1, there were no group differences in age, sex, race, education, parental socioeconomic status (SES), or estimated premorbid intelligence based on the Reading subtest of the Wide Range Achievement Test (WRAT-III). Although patient groups were also similar on estimates of current intellectual abilities [F=(2,70)2.21, p=.12] based on the Wechsler Abbreviated Scale of Intelligence, we selected groups for similarity on premorbid intelligence estimates to minimize group differences in intellectual potential. No participant had a known history of neurologic disorder, head injury with loss of consciousness (> 10 min.), systemic medical disorder affecting brain function, or significant substance abuse within the preceding 6 months. The study was approved by the University of Illinois Institutional Review Board. All participants provided written consent or, when appropriate, verbal and written assent with written parental consent.
Table 1.
Demographic and Clinical Data for Each Group
| Healthy Comparison (HC) |
Schizophrenia (SZ) |
Bipolar w/ Psychosis (BP) |
Psychotic Depression (PsyDep) |
F/ χ2 (df) | Significant Post-hoc Comparisons |
|
|---|---|---|---|---|---|---|
| Demographics | n=41 | n=30 | n=22 | n=21 | ||
| Age (years) | 24.90(8.79) | 23.03(7.32) | 22.68(6.35) | 24.38(7.70) | 0.56 (3,110)ns | |
| Range | 12-41 | 13-50 | 12-45 | 14-43 | ||
| Sex | χ2=5.24 (3) ns | |||||
| Male | 58.5% | 80.0% | 59.1% | 52.4% | ||
| Female | 41.5% | 20.0% | 40.9% | 47.6% | ||
| Race | χ2=3.82 (6) ns | |||||
| Caucasian | 32.6% | 26.7% | 22.7% | 25.0% | ||
| African-American | 51.2% | 46.6% | 63.6% | 45.0% | ||
| Asian/Latino/Othr | 16.3% | 26.7% | 13.6% | 30.0% | ||
| Annett Handedness scale | 8.66(6.35) | 8.20(5.04) | 8.23(5.87) | 5.24(9.24) | 1.36 (3,110) ns | |
| Education | 12.59(2.78) | 12.13(2.53) | 13.24(3.25) | 12.24(2.28) | 0.77 (3,109) ns | |
| Parental SES | 2.48(0.85) | 2.93(1.02) | 2.70(1.08) | 3.06(1.00) | 2.01 (3,102) ns | |
| WRAT-III Reading | 99.35(7.51) | 93.00(11.69) | 94.77(14.36) | 96.71(10.80) | 2.14 (3,109) ns | |
| Clinical Variables | ||||||
| Median DUP (mos) | 5.5 | 3.0 | 6.0 | |||
| PANSS Total | 81.18(13.33) | 73.14(8.83) | 72.45(17.45) | 3.18 (2,66)* | SZ > BP, PsyDep | |
| PANSS Pos | 23.61(3.88) | 23.48(4.96) | 17.60(4.28) | 13.32 (2,66)§ | PsyDep < SZ, BP | |
| PANSS Neg | 18.93(4.65) | 12.62(5.41) | 16.60(6.08) | 8.46 (2,66)§ | BP < SZ, PsyDep | |
| HDRS | 25.86(9.52) | 29.14(8.62) | 35.25(8.78) | 6.33 (2,66)† | PsyDep > SZ | |
| Simpson-Angus | 0.25(0.65) | 0.10(0.30) | 0.85(2.72) | 1.42 (2,66) ns | ||
| GAF | 37.31(7.05) | 44.05(4.90) | 43.94(7.94) | 8.22 (2,64)§ | SZ < BP, PsyDep | |
p < .05
p ≤ .01
p ≤ .001
Clinical and Neuropsychological Evaluation
Clinical assessment of each patient included the Positive and Negative Syndrome Scale (PANSS), Hamilton Depression Rating Scale (HDRS), Simpson-Angus scale for extrapyramidal symptoms, and the global assessment of function (GAF) scale. The neuropsychological battery included tests of reasoning and flexibility, attention, verbal memory, face memory, working memory, and processing speed. The tests used to evaluate these neuropsychological domains, determined on an a priori basis, are listed in Table 2. Risperidone monotherapy was the preferred treatment for the protocol, but final prescription and all dosage decisions were made by treating psychiatrists based on clinical considerations.
Table 2.
Neuropsychological domains and individual tests comprising each domain with baseline performance by group.
| Neuropsychological Domain | HC | SZ | BP | PsyDep |
|---|---|---|---|---|
| Processing Speed | ||||
| Trail Making Test: Part A time1 | 26.12± 8.05 | 33.58± 21.14 | 31.18± 13.32 | 26.47± 7.80 |
| Controlled Oral Word Association2 | ||||
| Letters (mean C,F,L) | 13.49± 3.43 | 10.68± 3.54 | 12.93± 4.69 | 11.19± 3.46 |
| Categories (mean animal, fruit) | 21.09± 4.50 | 15.10± 3.57 | 22.89± 5.53 | 17.22± 4.51 |
| Cogtest3: Finger Tapping (dom. hand) | 196.5± 31.22 | 194.9± 28.86 | 179.7± 29.42 | 187.3± 28.55 |
| Reasoning & Flexibility | ||||
| Trail Making Test: Part B time1 | 64.16± 24.83 | 91.36± 37.57 | 82.71± 43.55 | 81.71± 34.13 |
| Penn Conditional Exclusion Test3 | ||||
| Total Trials | 66.67± 27.70 | 89.55± 31.76 | 77.95± 28.90 | 89.78± 24.07 |
| Category 2 Trials | 18.57± 8.18 | 16.97± 8.25 | 17.73± 15.09 | 26.94± 19.28 |
| Cogtest4 Set Shifting Test | ||||
| Reaction Time increase after shift | 39.09± 42.06 | 84.43± 98.19 | 54.09± 55.50 | 52.29± 69.10 |
| Error increase after shift | 0.37± 3.26 | 4.50± 9.49 | 1.14± 12.24 | 0.50± 7.50 |
| Verbal Memory | ||||
| CVLT-II (raw scores) | ||||
| Total Trials 1-5 | 53.58± 10.59 | 38.48± 12.00 | 48.45± 11.67 | 44.74± 11.50 |
| Short Delay Free Recall | 10.95± 2.83 | 7.31± 3.59 | 10.23± 2.71 | 8.74± 2.56 |
| Long Delay Free Recall | 11.29± 2.87 | 7.38± 3.64 | 9.91± 3.41 | 9.26± 3.02 |
| Recognition Discriminability | 3.24± 0.71 | 2.45± 0.91 | 3.10± 0.81 | 2.83± 0.78 |
| Face Memory | ||||
| Penn Face Memory Test3: Imm. Recog | 31.74± 3.63 | 27.77± 4.96 | 31.27± 3.97 | 28.94± 5.75 |
| Penn Face Memory Test3: Delay Recog | 33.98± 3.56 | 30.44± 5.41 | 31.91± 4.25 | 32.44± 5.45 |
| Working Memory | ||||
| Cogtest4: Spatial Working Memory | ||||
| Direct touch distance (mm) | 4.11± 1.57 | 4.46± 2.74 | 5.59± 2.99 | 4.39± 2.48 |
| 2 sec Delay Error (mm) | 15.03± 4.78 | 19.56± 5.39 | 15.79± 5.07 | 16.68± 5.84 |
| 12 sec Delay Error (mm) | 23.28± 8.44 | 30.44± 11.75 | 26.66± 10.61 | 28.28± 9.38 |
| WMS-III: Digit Span backward (raw) | 7.30± 2.37 | 5.31± 2.29 | 5.55± 1.79 | 7.00± 2.85 |
| WMS-III: Spatial Span backward (raw) | 7.47± 2.15 | 4.93± 1.98 | 6.36± 2.40 | 6.26± 1.94 |
| Attention | ||||
| Penn CPT3: d-prime | 3.13± 1.21 | 2.12± 1.36 | 2.18± 1.46 | 2.18± 1.34 |
| WMS-III: Digit Span forward (raw) | 10.77± 2.51 | 9.38± 2.34 | 10.27± 2.75 | 10.16± 2.63 |
| WMS-III: Spatial Span forward (raw) | 8.33± 2.11 | 6.72± 2.03 | 8.09± 2.31 | 7.53± 2.17 |
Halstead-Reitan Neuropsychological Battery (Reitan and Wolfson, 1993);
Multilingual Aphasia Examination (MAE) (Benton AL and Hamsher K, 1976);
Penn Computerized Neuropsychological Battery (Gur et al., 2001);
Cogtest (Barua P et al., 2002); CVLT-II: California Verbal Learning Test – Second Edition (Delis DC et al., 2000); WMS-III: Wechsler Memory Scale – Third Edition (Psychological Corporation, 1997).
Medication
Multiple studies have documented the beneficial and adverse neurobehavioral effects of antipsychotic treatments (Harvey et al., 2000) (Purdon et al., 2000) (Reilly et al., 2007) (Reilly et al., 2006). Over half (52.1%) of patients in this study were naïve to antipsychotics at enrollment (13 SZ, 13 BP, 12 PsyDep). In addition to antipsychotic naïve patients, we recruited patients with either brief recent treatment (acute treatment in an ER, less than one week of active treatment immediately prior to recruitment) or limited lifetime exposure to antipsychotic treatment. One patient reported 16-weeks of lifetime antipsychotic treatment, and the remainder of the previously treated sample had been prescribed antipsychotic medications over their lifetime for less than three weeks (mean=2.8±2.1 weeks), often with limited treatment adherence. With the exception of one patient who received a single lifetime dose of risperidone (1.0 mg) in the emergency room over 48 hours before neuropsychological testing, all patients were studied after a minimum antipsychotic free period of at least 72 hours. This brief washout was utilized to reduce acute drug effects at baseline testing, such as sedation, and to permit diagnostic evaluation, medical work-up and a detailed history before treatment initiation. There were no significant differences between antipsychotic naïve and previously treated patients in terms of demographic, clinical, or neuropsychology domain measures.
Five patients were treated with antidepressants (SSRIs) at baseline testing (SZ=1, BP=2, PsyDep=2). Six patients with an affective disorder had previously been treated with anticonvulsants, but those had been tapered and discontinued at least 72 hours before baseline testing. Four patients were prescribed low dose lorazepam (1-2 mg) as a sleep aid 19-48 hours prior to baseline testing. Although the half-life for lorazepam is 10-20 hours, the duration of action is typically 6-8 hours for hypnotic and cognitive effects of single doses (Banen and Resnick, 1973) (Wyeth, 2002). One patient’s anxiety was sufficiently severe that he was given 1 mg lorazepam prior to leaving the inpatient unit for neuropsychological testing. Given the low doses, short duration of action, and the likelihood that the negative effects of sleep deprivation, fatigue, and/or agitation associated with untreated anxiety would more severely compromise test performance than these rescue medications, these patients were retained for analysis to maintain sample representativeness.
Follow-up Studies
It is unclear whether cognitive deficits are more persistent after recovery in schizophrenia than in mood disorders. Longitudinal analyses were undertaken to assess the possibility of differential response to treatment and clinical stabilization. With the exception of the alternate form of the CVLT-II, the same neuropsychological tests were administered at follow-up. The retention rate from baseline to the 6-week follow-up testing was similar for the patient groups (59.7%) and healthy participants (55.8%), however the affective groups (BP=14, PsyDep=9) had a marginally lower retention rate (χ2 =2.94, p=.09) that those in the schizophrenia group (22 of 30). Treatment nonadherence is a major challenge facing clinicians treating first-episode psychotic disorders (Coldham et al., 2002) and the primary reason for drop-outs in this study. Within each diagnostic group, no significant differences in age, parental SES, estimated premorbid intelligence, and baseline global neuropsychological performance were found between participants who completed baseline and follow-up neuropsychological assessments.
Treatment with antipsychotic medications was initiated or resumed shortly after neuropsychological testing. Risperidone was the first line treatment (mean dose=2.65± 1.72 mg). Only three patients (SZ=2, BP=1) who completed both pre and post-treatment assessments were prescribed a different antipsychotic prior to follow-up testing, with deviations from first line treatment based on the clinical judgment of the treating physician. Seven patients (SZ=5, BP=1, PsyDep=1) were prescribed benzotropine (mean=1.5± 0.87 mg) for extra-pyramidal symptoms (and did not differ cognitively from the remainder of the patient sample); and three patients (BP=2, PsyDep=1) were also prescribed a mood stabilizer. Six patients (SZ=1, BP=1, PsyDep=4) received SSRI treatment during the 6-week treatment period, including the four patients already treated with SSRIs at baseline who continued treatment throughout the study period.
Data Analysis
To provide a standard metric for comparison across neuropsychological tests and domains, scores were standardized (z-score) to the baseline performance of the healthy comparison group. To meet the assumptions for parametric analysis, baseline healthy comparison neuropsychological variables were assessed for normality of distribution by calculating two-tailed tests of skewness and kurtosis. Non-normal distributions were normalized using natural log, square, or cubic transformations. Examination of specific test scores revealed some extreme scores and, consistent with previous reports (Saykin et al., 1994) (Hill et al., 2004b), several subjects (SZ=9, BP=7, HC=2, PsyDep=4) had scores truncated to z=-4.0 on at least one test variable. Following normalization and truncation, scores for each of the six neuropsychological domains were computed as the mean of test scores comprising each function (Hill et al., 2004a). A composite of global neuropsychological function was computed as the mean of the six z-transformed domain scores and compared across groups using one-way ANOVA. Potential profile differences were assessed with two-way repeated measures MANOVA in which neuropsychological domain (processing speed, reasoning and flexibility, attention, verbal memory, face memory, working memory, attention) was the within subjects factor and diagnosis (HC, SZ, BP, PsyDep) was the between subjects factor. Due to unequal cell size, Pillai’s test was used for both univariate and multivariate ANOVA. Demographic variables were not used as covariates due to the lack of group differences on these measures.
Results
Clinical Ratings
Age of first contact with the mental health system was similar across patient groups [F(2,37)=1.06, p=.36], as was time from psychosis onset to study enrollment [F(2,67)=2.16, p=.12]. Patients with bipolar disorder had lower negative symptom ratings and the psychotic depression patients endorsed significantly more depressive symptoms and fewer positive symptoms of psychosis (see Table 1). There were no significant patient group differences regarding length of previous employment [F(2,67)=0.37, p=.69] or highest occupation level [χ2(2)=2.67, p=.26]. Following 6-weeks of treatment patients showed clinically and statistically significant reductions in PANSS positive [F(1,42)=48.18, p<.001], negative [F(1,42)=8.38, p<.01], and overall psychiatric symptoms [F(1,42)=51.88, p<.001] as well as fewer depressive symptoms [F(1,42)=59.98, p<.001].
Neuropsychological Studies
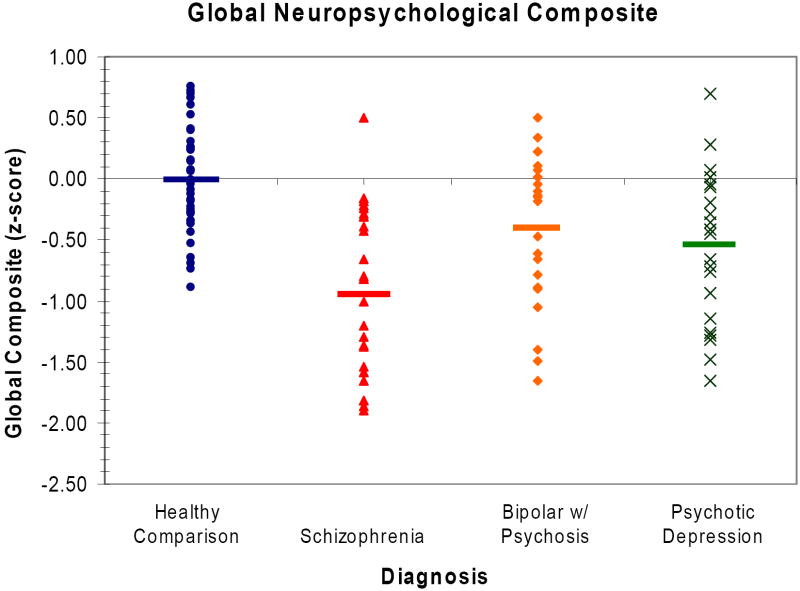
Global neuropsychological performance differed significantly across diagnostic groups [F(3,110)=15.03, p<.001]. Simple contrasts among groups, using a Hochberg correction (Hochberg, 1988) for multiple comparisons, revealed that all patient groups were impaired relative to healthy individuals. Furthermore, the schizophrenia group displayed more severe impairment than the psychotic bipolar group on the generalized neuropsychological composite score (Figure 1).
Figure 1.
Mean z-scores across all neuropsychological domains, reflecting a composite index of neurocognitive deficit, in groups with schizophrenia, psychotic bipolar disorder and psychotic unipolar depression at baseline standardized to the matched healthy comparison group.
Profile Comparisons
A repeated measures MANOVA was used to test for differences in neuropsychological profiles across disorders. Results indicated a significant main effect of diagnosis [F(3,97) =14.44, p<.001], a marginal but nonsignificant difference in performance deficits across neuropsychological domains [F(5,93) =2.23, p=.06], and a nonsignificant diagnosis by neuropsychological domain interaction indicating no difference in profiles of neuropsychological deficits across participant groups [F(15,285) =0.67, p=.81] (Figure 2). Given the effect of diagnosis and the marginal significance of deficits across neuropsychological domains, univariate ANOVA were conducted separately on each neuropsychological function in an exploratory manner to identify any domains where patient group differences might be suggested (Table 3). After controlling for multiple comparisons using the Hochberg approach (Hochberg, 1988), the only patient group differences revealed by simple contrasts were less severe deficits for verbal memory and reasoning and flexibility in psychotic bipolar disorder compared to schizophrenia.
Figure 2.
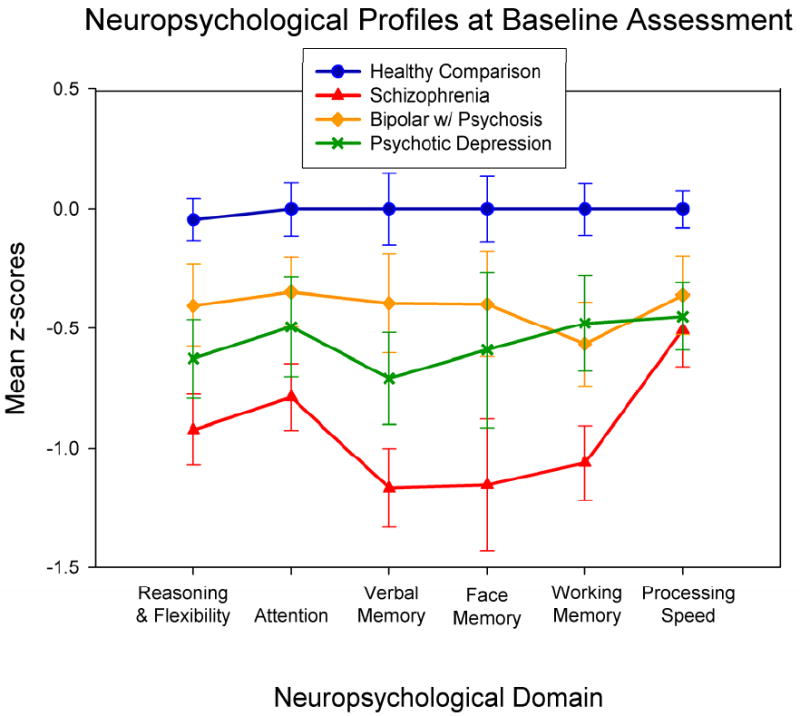
Neuropsychological performance profiles (anchored to normalized baseline performance of the healthy comparison group) show similar profiles of dysfunction across neuropsychological domain in the patient groups, albeit differing in severity.
Table 3.
Results of the baseline univariate ANOVA for each neuropsychological domain to clarify the multivariate effect of diagnosis.
| Neuropsychological Function | F | df | p | Significant Pair-wise Comparisons |
|---|---|---|---|---|
| Reasoning & Flexibility | 9.12 | 3,110 | <.001 | HC > SZ, PsyDep; BP > SZ |
| Attention | 6.21 | 3,110 | .001 | HC > SZ |
| Verbal Memory | 9.27 | 3,103 | <.001 | HC > SZ, PsyDep; BP > SZ |
| Face Memory | 5.38 | 3,104 | .002 | HC > SZ |
| Working Memory | 10.31 | 3,109 | <.001 | HC > SZ, BP |
| Processing Speed | 3.86 | 3,109 | .011 | HC > SZ |
HC = healthy comparison, SZ= schizophrenia, BP = bipolar disorder, PsyDep = psychotic depression
Follow-up – Treatment effects
All patient groups showed significant clinical improvements at follow-up on PANSS Total, PANSS Positive, and GAF scores (see Table 1). While all patient groups reported fewer depressive symptoms at follow-up [main effect of time F(1,39)=124.35, p<.001], the psychotic depression group showed greater reductions [time by diagnosis interaction F(2,39)=9.62, p<.001]. The schizophrenia group showed significant reductions in negative symptoms at follow-up [F(1,18)=8.91, p=.008], but there was no significant change in negative symptom ratings for affective disorder groups over time.
Contrasting neuropsychological performance before and after acute treatment using repeated measures MANOVA indicated significant main effects of time [F(1,56)=16.12, p<.001], diagnosis [F(3,56)=15.54, p<.001], and neuropsychological domain [F(5,52)=2.47, p=.04], but no significant interaction terms. The significant main effect of time, combined with a nonsignificant time by diagnosis interaction [F(3,56)=0.17, p=.92], indicate that the combination of practice effects, drug treatment and clinical stabilization in the patient groups were no greater than practice effects alone in the healthy comparison group (Figure 3). Similarly, the nonsignificant domain by group by time interaction provides no evidence for differential improvement over time or across neuropsychological domains in the patient groups.
Figure 3.
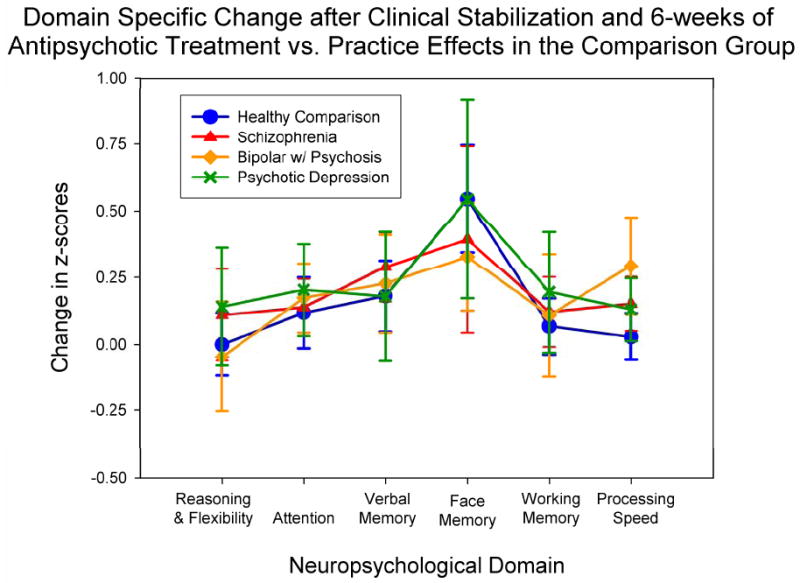
Neuropsychological change at the 6-week follow-up, relative to the initial assessment, indicates similar modest improvement in test scores at follow-up in patient groups and in the healthy comparison group.
Discussion
This study is the first to compare demographically similar groups of first episode schizophrenia patients with first episode psychosis unipolar and bipolar affective disorder patients across a wide range of neuropsychological tests. Importantly, the groups were assessed with minimal acute, chronic and differential drug treatments, evaluated using the same battery of tests, studied in parallel with a healthy comparison group, and there were no group differences in age, sex, education, premorbid intellectual abilities, and parental SES. Furthermore, our recruitment strategy identified all suitable patients regardless of disorder, rather than comparing independently ascertained and recruited samples. Finally, a uniform treatment approach emphasizing monotherapy with the second generation antipsychotic risperidone was utilized to minimize confounds related to differential treatments across affective and nonaffective disorders.
Modest and nonsignificant improvement in neuropsychological status was observed after treatment across patient groups despite dramatic changes in mood state from acutely ill to clinically stable. That is, analysis of follow-up data after 6-weeks of primarily risperidone monotherapy failed to reveal greater improvement in the affective disorder groups relative to the schizophrenia group and no patient group showed greater improvement than practice effects observed in the healthy comparison group. To the extent that these patient samples represent their respective disorders, these findings suggest that, like schizophrenia, neuropsychological deficits in psychotic affective disorders may be persistent and an ongoing cause of disability in affective disorders with psychosis.
Baseline Profile Similarity and Severity Differences
Consistent with prior neuropsychological studies (Bilder et al., 2000) (Gur et al., 2001) (Hill et al., 2004b) (Reichenberg et al., 2008), the schizophrenia group displayed a profile of generalized dysfunction relative to demographically similar controls at baseline. Global neuropsychological deficits relative to controls in the two affective disorder groups were statistically comparable and intermediate between the healthy and schizophrenia groups. There was no evidence for differential profiles of neuropsychological dysfunction between the schizophrenia group and patients with psychotic affective disorders. A similar constellation of neuropsychological abilities, and by inference their relevant brain systems, appear to be disrupted across these psychotic disorders (Albus et al., 1996) (Jeste et al., 1996) (Mojtabai et al., 2000) (Schretlen et al., 2007) , yet the degree to which these systems are impacted may be more severe in schizophrenia.
The pattern of comparable neuropsychological profiles with variable level of deficit observed in our previous comparison of first-episode schizophrenia and unipolar psychotic depression (Hill et al., 2004a), was replicated and extended in this study with an independent sample. In both studies, effects of chronic illness and differential chronic drug treatment across disorders were minimized by studying early course patients. Previous studies, typically with chronic patients, have reported more severe impairments in schizophrenia compared to affective disorders, but qualitatively similar neuropsychological profiles (Albus et al., 1996) (Reichenberg et al., 2008) (Schretlen et al., 2007), particularly for psychotic affective disorders (Jeste et al., 1996) (Mojtabai et al., 2000). In the present study, the similarity of profiles was further supported by post hoc analyses in which the only significant patient group differences observed were less severe verbal memory and reasoning and flexibility impairments in the bipolar disorder group than in schizophrenia.
Stability of Neuropsychological Abilities across Psychotic Disorders
The classic Kraepelinian model of dementia praecox and manic-depression conceptualized the cognitive deficits in schizophrenia as being more severe and trait-like than in mood disorders. The present findings are consistent with that model. However, the Kraepelinian model also proposed considerably more differentiation of the disorders in terms of the persistence of these deficits than is evident in the present data. Early support for the Kraepelinian model came from studies comparing schizophrenia to mood disorders without separating psychotic from nonpsychotic affective disorder patients. Persistent cognitive impairments in patients with affective disorders and no history of psychosis appear to be modest (Grant et al., 2001).
Studies investigating the stability of cognitive dysfunction in affective disorders during and after psychotic episodes are rare and available data with adults with chronic bipolar disorder (Balanza-Martinez et al., 2005) and pediatric bipolar disorder (Pavuluri et al., 2008) suggest persistent deficits. The present study longitudinally compared adults with first-episode psychotic bipolar disorder onward against two additional first episode psychosis groups to find that performance improvements over time (reflecting the combined effects of practice, medication and clinical stabilization) did not exceed practice effects alone in the healthy comparison sample or differ from improvement in the other disorders. The observation of modest change in the schizophrenia sample is consistent with previous studies reporting that cognitive change in first-episode schizophrenia patients, following pharmacological treatment and clinical stabilization, was both modest (Keefe et al., 2007) and on par with practice effects observed in demographically similar healthy comparison groups (Goldberg et al., 2007) (Hill et al., 2004b). With appropriate regard for inferential limitations imposed by the small sample sizes, significant attrition in the follow-up study, and the lack of longer follow-up, the present findings point to persistent cognitive deficits in psychotic affective disorders that are not significantly altered by acute therapy and clinical stabilization. The stability of these deficits and the presence of significant neuropsychological deficits in pediatric bipolar disorder (Pavuluri et al., 2008), raises the possibility, as proposed in schizophrenia, that cognitive deficits in affective psychotic disorders may be neurodevelopmental in origin (Murray et al., 2004) and result from a cascade of atypical maturational events that disrupt a wide range of neurobehavioral brain systems in a relatively static manner into adult life.
To the extent that the present findings generalize to larger samples, the observed similarity of neuropsychological profiles across psychotic disorders may indicate a final common pathway of neuropsychological deficits resulting from disorder-related alterations of integrated functional brain systems. It is possible that the neurobiological features underlying these deficits have either similar etiological mechanisms or that they represent common behavioral deficits with different etiologies. Recent reports of similar genetic findings across schizophrenia and psychotic mood disorders (Bramon & Sham, 2001; Badner & Gershon, 2002), involving cognition related genes, are consistent with at least a partially common etiology model, but the degree to which parallel behavioral deficits have similar and differing etiologies remains a question for future research.
Implications for Treatment
The observation of persistent neuropsychological disturbances in psychotic affective disorders may indicate a need to broaden interest in neuropsychological deficits both as a cause of morbidity and as a potential treatment target in psychotic mood disorders to parallel the developing interest in these areas in schizophrenia. The notion that neuropsychological dysfunction plays a key role in poor functional outcomes has become widely accepted in the schizophrenia literature (Green, 2006). While there is an emerging literature linking neurocognitive deficits in bipolar disorder to reduced functional capacity (Dickerson et al., 2004) (Martinez-Aran et al., 2004) (Pavuluri et al., 2008) (Tabares-Seisdedos et al., 2008), very few studies have investigated cognition and functional abilities in unipolar psychotic disorders of early adult life.
Cognitive deficits have been suggested as treatment targets in schizophrenia based on the stability of neuropsychological deficits over time, the limited beneficial impact of antipsychotic treatment on cognitive impairments, and the strong relationship between cognitive deficits and functional disability. Research along these lines in affective disorders is less established, but there has been some relevant work with elderly patients (Raskin et al., 2007). The present findings suggest that efforts to develop cognition enhancing treatments may need to be considered for psychotic affective disorders as well as schizophrenia. For such efforts to succeed, more work is needed to establish neurocognitive similarities and differences across psychotic disorders, to learn about the cause of neurocognitive deficits, and ascertain the stability of these deficits over lengthier periods and their functional implications.
Limitations
The present study was designed to assess the early course of cognitive dysfunction beginning with the first episode of psychosis, thus study duration was relatively short and may not detect differential deterioration that occurs over more extended periods of years or decades. Second, a high attrition rate is a problem for early course of illness patients as treatment nonadherence or clinical preference for additional or different treatments (particularly for affective patients) reduce the number and potentially, the representativeness of patients available for our follow-up study. As evidenced here, small sample sizes are also an obstacle for this type of research, particularly the longitudinal component. While it is difficult to make firm conclusion with small samples, we believe that comparing groups before and after treatment is an excellent approach to comparing disease and treatment effects on cognition across psychotic disorders and for addressing questions about the state-dependence of cognitive impairments across psychotic disorders. In light of the small samples and despite the lack of differences between drop-outs and completers, additional studies are needed to support the present findings in larger samples with longer follow-up durations. Furthermore, the generalizability of these findings may be restricted to psychotic patients who are willing and able to complete neuropsychological testing prior to treatment initiation and adhere to their prescribed medication regimen. Finally, although nonparametric comparisons indicated no group differences in sex distribution, it should be noted that the male to female ratio in the schizophrenia group was more than double the ratio in other groups. Given the sex difference on tests of verbal memory and motor speed (Rubin et al., 2008) (Fiszdon et al., 2003) (Sota and Heinrichs, 2003) (Gur et al., 2001) this ratio may partially account for the discrepancy between verbal memory and processing speed in the schizophrenia group and, perhaps, the absence of group differences on face memory. Larger studies are needed to investigate the impact of gender differences on cognitive deficits across psychotic disorders.
Concluding Remarks
The present data indicate that neuropsychological dysfunction is present early in the course of both depression and bipolar disorder with psychosis, and that these deficits appear to be similar in form, albeit less severe, to those seen in first-episode schizophrenia. Improvement in neuropsychological performance after acute pharmacologic treatment (typically risperidone monotherapy) and clinical stabilization was not more robust in patients with affective psychoses than schizophrenia. For all patient groups, improvement was no greater than the practice effects seen in the healthy comparison group at retesting. Thus, although further empirical support is needed, the present findings suggest that all three of the major psychotic disorders of early adult life are associated with diffuse and persistent neurocognitive dysfunction. Considering the impact of psychosis on cognition and functional status in general, these findings may have important clinical implications for prognosis and treatment development for psychotic affective disorders.
The present findings point to a need for longer term neuropsychological follow-up of early course patients with psychotic affective disorders to evaluate whether differential persistence or progressive changes exist over the long-term course of illness compared to schizophrenia. Second, the findings point to a need for neuroimaging and other approaches to learn more about potentially unique causes of neuropsychological deficits associated with different psychotic disorders. Finally, our findings highlight the need for more studies investigating the relationship between what appear to be persistent neuropsychological deficits and functional disability in mood disorders with psychotic features to determine whether, like schizophrenia, cognitive impairment should come into focus as a treatment target for psychotic affective disorders.
Acknowledgments
This project was supported by the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) and the National Institutes of Health (NIMH: MH077862, MH062134, MH080066, and MH072767).
Funding for this study was provided by NARSAD and NIMH Grants MH077862, MH062134, MH080066, and MH072767; neither NARSAD nor the NIMH had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Drs. Hill, Reilly, and Sweeney designed the study. Dr. Harris collected and managed data. Drs. DeLeon, Marvin, and Rosen provided recruitment referrals and patient clinical care. Dr. Hill undertook the statistical analysis and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albus M, Hubmann W, Wahlheim C, Sobizack N, Franz U, Mohr F. Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand. 1996;94:87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- Badcock JC, Michiel PT, Rock D. Spatial working memory and planning ability: contrasts between schizophrenia and bipolar I disorder. Cortex. 2005;41:753–763. doi: 10.1016/s0010-9452(08)70294-6. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Tabares-Seisdedos R, Selva-Vera G, Martinez-Aran A, Torrent C, Salazar-Fraile J, Leal-Cercos C, Vieta E, Gomez-Beneyto M. Persistent cognitive dysfunctions in bipolar I disorder and schizophrenic patients: a 3-year follow-up study. Psychother Psychosom. 2005;74:113–119. doi: 10.1159/000083170. [DOI] [PubMed] [Google Scholar]
- Banen DM, Resnick O. Lorazepam versus glutethimide as a sleep-inducing agent for geriatric patient. Journal of the American Geriatric Society. 1973;21:507. doi: 10.1111/j.1532-5415.1973.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Barua P, Bilder R, Small A, Sharma T. Standardization and cross-validation study of cogtest: An automated neurocognitive battery for use in clinical trials of schizophrenia. Schizophr Bull. 2002;31:318. [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination Manual University of Iowa. Department of Neurology; Iowa City: 1976. [Google Scholar]
- Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep. 2001;3:332–337. doi: 10.1007/s11920-001-0030-1. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr Scand. 2002;106:286–290. doi: 10.1034/j.1600-0447.2002.02437.x. [DOI] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Balanza-Martinez V, Salazar-Fraile J, Selva-Vera G, Vieta E. Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychother Psychosom. 2006;75:72–84. doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – Second Edition (CVLT-II) manual. The Psychological Corporation; New York, NY: 2000. [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings CR, Origoni AE, Cole S, Yolken RH. Association between cognitive functioning and employment status of persons with bipolar disorder. Psychiatr Serv. 2004;55:54–58. doi: 10.1176/appi.ps.55.1.54. [DOI] [PubMed] [Google Scholar]
- Fiszdon JM, Silverstein SM, Buchwald J, Hull JW, Smith TE. Verbal memory in schizophrenia: sex differences over repeated assessments. Schizophr Res. 2003;61:235–243. doi: 10.1016/s0920-9964(02)00285-2. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap ME, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disord. 2006;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Gold JM, Greenberg R, Griffin S, Schulz SC, Pickar D, Kleinman JE, Weinberger DR. Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry. 1993;150:1355–1362. doi: 10.1176/ajp.150.9.1355. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25:777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Cornblatt BA. Pharmacological treatment of cognition in schizophrenia: an idea whose method has come. Am J Psychiatry. 2008;165:163–165. doi: 10.1176/appi.ajp.2007.07111810. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Moriarty PJ, Serper MR, Schnur E, Lieber D. Practice-related improvement in information processing with novel antipsychotic treatment. Schizophr Res. 2000;46:139–148. doi: 10.1016/s0920-9964(00)00033-5. [DOI] [PubMed] [Google Scholar]
- Hill SK, Keshavan MS, Thase ME, Sweeney JA. Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatry. 2004a;161:996–1003. doi: 10.1176/appi.ajp.161.6.996. [DOI] [PubMed] [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res. 2004b;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of signicance. Biometrika. 1988;75:800–802. [Google Scholar]
- Ivleva E, Thaker G, Tanaka S. Comparing genes and phenomenology in the major psychoses: Schizophrenia and bipolar 1 disorder. Schizophr Bull. 2008;34:734–742. doi: 10.1093/schbul/sbn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry. 1996;153:490–496. doi: 10.1176/ajp.153.4.490. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, Lieberman JA. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugue E, Daban C, Salamero M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- McClellan J, Prezbindowski A, Breiger D, McCurry C. Neuropsychological functioning in early onset psychotic disorders. Schizophr Res. 2004;68:21–26. doi: 10.1016/S0920-9964(03)00058-6. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. Br J Psychiatry. 2005;186:378–385. doi: 10.1192/bjp.186.5.378. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Bromet EJ, Harvey PD, Carlson GA, Craig TJ, Fennig S. Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry. 2000;157:1453–1460. doi: 10.1176/appi.ajp.157.9.1453. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, West A, Jindal K, Hill SK, Sweeney JA. Longitudinal study of neurocognitive function in pediatric bipolar disorder: three-year followup shows patients lagging behind healthy youth. J Am Acad Child Adolesc Psychiatry. 2008 doi: 10.1097/CHI.0b013e318196b907. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppa T, Haukka J, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:930–936. doi: 10.1016/j.biopsych.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Memory Scale-Third Edition (WMS-III) Harcourt Brace & Company; San Antonio, TX: 1997. [Google Scholar]
- Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Gen Psychiatry. 2000;57:249–258. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, Dinkel JJ, Rotz BT, Mohs RC. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164:900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological Function and Dysfunction in Schizophrenia and Psychotic Affective Disorders. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol Psychiatry. 2007;62:818–821. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. Theory and clinical interpretation. 2. Neuropsychology Press; Tucson, AZ: 1993. The Halstead-Reitan Neuropsychological test battery. [Google Scholar]
- Rossi A, Arduini L, Daneluzzo E, Bustini M, Prosperini P, Stratta P. Cognitive function in euthymic bipolar patients, stabilized schizophrenic patients, and healthy controls. J Psychiatr Res. 2000;34:333–339. doi: 10.1016/s0022-3956(00)00025-x. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Haas GL, Keshavan MS, Sweeney JA, Maki PM. Sex difference in cognitive response to antipsychotic treatment in first episode schizophrenia. Neuropsychopharmacology. 2008;33:290–297. doi: 10.1038/sj.npp.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pulver AE, Rivkin P, Rao VA, Diaz-Asper CM, Dickerson FB, Yolken RH, Pearlson GD. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sota TL, Heinrichs RW. Sex differences in verbal memory in schizophrenia patients treated with “typical” neuroleptics. Schizophr Res. 2003;62:175–182. doi: 10.1016/s0920-9964(02)00373-0. [DOI] [PubMed] [Google Scholar]
- Tabares-Seisdedos R, Balanza-Martinez V, Sanchez-Moreno J, Martinez-Aran A, Salazar-Fraile J, Selva-Vera G, Rubio C, Mata I, Gomez-Beneyto M, Vieta E. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J Affect Disord. 2008 doi: 10.1016/j.jad.2007.12.234. [DOI] [PubMed] [Google Scholar]
- Wyeth. Product information: Ativan (R), lorazepam. Philadelphia, PA: 2002. [Google Scholar]