“Mind the gap” is a warning to subway passengers in London of the sometimes significant space between the train door and station platform. We utilize this phrase to caution researchers to be mindful of the significant gap in our knowledge of age-specific interactions in translational breast cancer research. The relevance of this topic is supported by data showing that many epidemiologic and clinical features of breast cancer demonstrate qualitative age interactions. Here, the term age interaction refers to situations in which breast cancer risk factors, tumor characteristics, and/or clinical trial results differ across age groups. A qualitative age interaction refers to an inverse or crossover effect for different ages. If a qualitative age interaction is suspected, age-related subgroup heterogeneity is likely, and the study should be powered, stratified, or analyzed age specifically to contrast outcomes between younger and older subjects. Failure to do so may blur important age-specific subgroup effects due to averaging of dissimilar cancer populations.1,2
Peto3 distinguished quantitative (noncrossover) from qualitative (crossover) interactions in randomized clinical trials (RCTs). A quantitative interaction occurs if a treatment outcome varies in magnitude but not in direction for subgroups. A qualitative interaction differs in both magnitude and direction, conferring benefit for one subgroup and harm for another. Although quantitative treatment effects are common, qualitative clinical trial results4–6 are rarely reported (Table 1). Furthermore, qualitative age interactions might be overlooked because age is usually treated as a confounding variable rather than as an effect modifier. As a result, many RCTs adjust or match by age to negate its effect rather than stratify by age to observe its full impact. RCTs also are generally underpowered to analyze age-specific effects. In addition, the age distribution of patients in breast cancer RCTs usually differs from that of patients with comparable tumors in the general community, limiting external validity.
Table 1.
Qualitative Age Interactions
| Parameter | Sample Size | RRbefore crossover age in years |
|---|---|---|
| Epidemiologic risk factor | ||
| Parity v nulliparity7 | 577 cases, 826 controls | RRbefore 30-44 > 1.0 |
| Obese v lean BMI8 | Review | RRbefore 50< 1.0 |
| Black v white incidence rates9 | 440,653 cases | RRbefore 40> 1.0 |
| Tumor characteristic | ||
| Poor v good prognostic factors10 | 242,549 cases | RRbefore 50 > 1.0 |
| Breast cancer outcome38 | 1,398 | RR before 35 > 1.0 |
| Clinical trial results | ||
| Adjuvant Rx for ER+ tumors4 | 7,631 cases | RRbefore 35 > 1.0 |
| Neoadjuvant Rx (NSABP B18)5 | 763 preoperative AC, 760 postoperative AC | RRbefore 50 < 1.0 |
| Chemoprevention (fenretinide)6 | 1,432 cases, 1,435 controls | RRbefore 51< 1.0 |
NOTE. Qualitative age interactions are expressed as RRs where one characteristic is compared with a referent characteristic with an assigned RR of 1.0. RR > 1.0 shows increased breast cancer risk or harmful effect while RR < 1.0 shows reduced breast cancer risk or protection compared with the referent characteristic. The crossover age reflects the approximate age of the crossover where RR switches from > 1.0 to < 1.0 or vice versa. RRs in the Table refer to the RR prior to the age of crossover. For example, RRbefore 30-44 years > 1.0 for parity compared with nulliparity; whereas after age 44 years, RR < 1.0 for parity compared with nulliparity. Parity increases breast cancer risk before ages 30-44 years but is protective thereafter.7,12 Obese BMI (body mass index) is protective for premenopausal women before age 50 years but increases risk for postmenopausal women.8 Age-specific incidence rates are higher for blacks than whites prior to age 40 years but higher for whites after age 40 years.9 Poor prognostic factor characteristics (tumor sizes > 2.0 centimeters, positive axillary lymph nodes, high tumor grade, estrogen and progesterone receptor negative expression) are more common among women before age 50 years, whereas good prognostic factors are more common after age 50 years.10 Breast cancer outcome (overall recurrence rate and greater risk for developing metastatic disease) is worse among younger (before age 35 years) than older patients, and this difference is not fully explained by greater frequency of adverse prognostic factors.38 Adjuvant systemic therapy is associated with higher rates of relapse for ER+ breast cancers among women before age 35 years than older women.4 NSABP B18 showed that neoadjuvant (preoperative) chemotherapy was marginally beneficial among women before age 50 years and the reverse was true for older women.5 Fenretinide chemoprevention demonstrated borderline protective effects (P = .045) for premenopausal women before age 51 years and harmful effects for postmenopausal women.6
Abbreviations: RR, relative risk; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; Rx, treatment; NSABP, National Surgical Adjuvant Breast and Bowel Project; AC, doxorubicin and cytoxan.
The repeated identification of qualitative age interactions in epidemiologic studies suggests that breast cancer demonstrates age-specific heterogeneity that transcends a simple or single cut point such as menopausal status. For example, parity increases breast cancer risk among women younger than 30 to 44 years but decreases risk among older women (Table 1).7,12 Obesity is protective for women younger than 50 years but increases risk thereafter.8 The Black to White incidence rate crossover shows that age-specific incidence rates are higher for Black than White women before age 40 years, after which the reverse is true.9
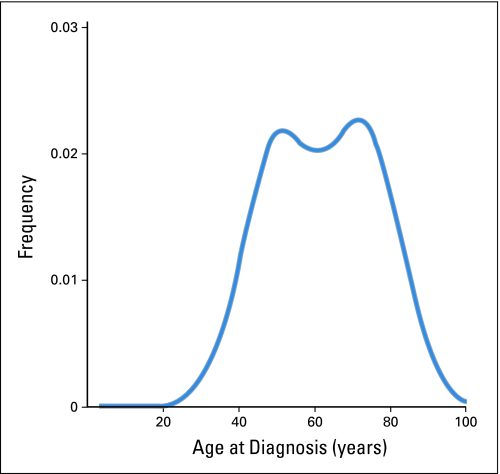
Age at diagnosis may reflect established as well as unknown carcinogenic events and/or exposures that occur during a person's life time. Age may even account for some risk conferred by factors that have not yet been identified. Indeed, traditional breast cancer factors account for less than 50% of breast cancer attributable risk.14 Analyses using novel descriptive and statistical methods also posit that breast cancer may be characterized by early- and late-onset tumor types with modes near ages 50 and 70 years (Figure 1).10,15–18 A bimodal characterization also correlates well with the two main clinical biomarkers (estrogen receptor positive [ER+] and HER2+) and/or molecular breast cancer signatures (luminal and basal-like).19–21 However, with few exceptions,22–24 many clinical studies and most molecular studies do not account for the obvious age-related breast cancer differences.25
Fig 1.
Bimodal age distribution at diagnosis for invasive female breast cancer cases (n = 94,813) in the National Cancer Institute's Surveillance, Epidemiology, and End Results Program during the years 1994 through 1997.16 Kernel density estimation was used to produce smoothed age distributions at diagnosis (or density plots) in single years.17 The kernel smoother estimated the underlying probability density function for breast cancer incidence by age at diagnosis in single years. The area under the curve represented 100% of the patients with breast cancer. Early- and late-onset modes were observed near ages 50 and 70 years, respectively. Although the relative distributions of the early- and late-onset breast cancer populations have fluctuated over time (likely due to complex interactions between age-related biologic factors and screening and/or birth-cohort phenomena), the modal ages of 50 and 70 years have been robust across molecular portraits, demographic and tumor characteristics.10,15,18
The identification of a bimodal rather than normal age distribution at diagnosis for a particular breast cancer characteristic raises concerns that the feature identifies a biologically heterogeneous tumor type. Bimodal breast cancer in the general population also contrasts sharply with the restricted age range of many clinical trials where fewer than 10% of participants may be 65 years or older.26 Furthermore, the modal ages of 50 and 70 years do not sharply divide tumors into distinctive categories, but rather reflect central tendencies for the age distributions of two biologically different cancer populations.27 Tumors that develop at extreme ages are likely to demonstrate predominant early- or late-onset phenotypes, but both tumor types span the entire age range with substantial mixing during midlife. Therefore, chronological age is only a crude proxy for breast tissue age.28 Of note, qualitative age interactions and bimodal cancer populations appear to transcend breast cancer, having been described for Hodgkin's lymphoma,29 hairy cell leukemia,30 nasopharyngeal carcinoma,31 malignant melanoma,32 high- and low-grade serous ovarian carcinomas.33
Age, then, may reflect many fundamental and incompletely understood biologic processes. Carcinogenic mechanisms that may increase with age include methylation of CpG islands in promoter regions of tumor suppressor genes, telomere shortening, and genetic instability. In parallel, many age-related changes occur within the systemic milieu such as declines in immune function. Given these data, one might predict that rates of all types of breast cancer would increase monotonically with age and that tumors among older women would be more aggressive. In fact, the opposite is true, breast cancer incidence rates decelerate with aging near Clemmesen's menopausal hook34 and older women generally have more indolent tumors than younger women (Table 1),11,25,35 at least before age 70 years.37
Biologic differences for early- versus late-onset tumors also are reflected in different responses to treatments among younger women (Table 1).36,38 Women younger than 35 years who express ER+ tumors have worse outcomes than older women with ER+ tumors irrespective of treatment.4 This may partly be explained by different patterns of coexpression of key markers at different ages. Neven et al39 reported qualitative age interactions between HER2 and hormone receptor expression. Among HER2 normal tumors, the odds ratio (OR) of being ER+ was 2.6 (95% CI, 1.9 to 3.6) up to age 50 years and age-independent thereafter; the OR of being progesterone receptor positive (PR+) was 2.7 (95% CI, 1.8 to 4.1) up to age 45 and 0.8 (95% CI, 0.8 to 0.9) thereafter. Among HER2+ tumors, the OR of being ER+ and PR+ was 0.8 (95% CI, 0.7 to 1.0) and 0.7 (95% CI, 0.6 to 0.9), respectively. However, an unsupervised analysis of gene expression profiles of ER+ tumors diagnosed in either women 45 years of age or younger or 70 years or older identified six gene clusters, two of which were related to early onset and one to later onset tumors.22 These clusters were unrelated to PR or HER2 status, suggesting biologic complexity related to age at diagnosis that is not completely captured by standard breast cancer markers.
Thus, searching for age-dependent biologic heterogeneity in RCTs would seem to be important. The strength of RCTs derives from their strong internal validity; successful random assignment of subjects coupled with statistical analyses to guarantee comparability among study arms, gives confidence that observed differences in outcomes between groups reflects differences in treatment. However, the external validity or generalizability of RCTs cannot be assumed since RCTs enroll volunteers who meet inclusion criteria.
The source population for a RCT includes all women potentially eligible for the trial; specifically, patients with breast cancer whose tumors have the characteristics under study. RCTs increasingly evaluate drugs that are directed against specific molecular targets, but the population characteristics of patients whose tumors express these targets are often undefined. Early estimates from high risk cohorts indicated that 30% of breast cancers were HER2+, but these estimates may have overstated the true frequency for HER2 expression in the general population.40
Limited access to population-based marker data impairs efforts to assess, the comparability of RCT participants to patients in the community. Generalizability concerns are heightened by data showing that RCT participants are generally healthier, wealthier, younger, and more often white and city dwellers than nonparticipants.26,41 Recognition that defining the population-basis for RCTs is important provides the impetus for conducting studies that include molecular analysis of population-based samples.13,42 A critical aspect of such studies is their ability to characterize the entire population of both cases and controls, permitting unbiased estimation of incidence rates by marker expression. This research is facilitated by technologies such as tissue microarrays that permit the efficient characterization of large sample sets, improved methods for assaying fixed tissues, and new statistical tools for data analysis. In addition, retrospective marker analysis in older studies may provide insights into tumor behavior before adoption of new treatments.42,43 Furthermore, community outcome data may be all that is available to assess new treatments among demographic groups that are under-represented in RCTs.
In conclusion, future breast cancer studies should be designed to permit assessment of age-specific outcomes when possible. The validity of breast cancer RCTs that assess targeted treatments may be strengthened by comparing subject characteristics to those estimated in population-based observational studies. The melding of traditional and novel observational methods can define the population characteristics and suggest hypothesis that would be difficult (if not impossible) to derive in smaller analytic studies and/or RCTs. In addition, differences in the age distributions between RCTs and the general breast cancer population may be masking a fundamentally important biologic variable (aging) that may impact the translation of efficacious treatments to effective patient treatment. This takes on added importance among older patients with breast cancer for whom therapy related complications are as important as cancer cure.44 Therefore, the analysis of age-specific effects in RCTs may be a fundamental way to fill the gap of our incomplete understanding of tumor biology and to optimize treatment until more is known of the obligate determinants for breast cancer.
Acknowledgment
Supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. We thank the reviewers for their helpful comments.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: William F. Anderson, Ismail Jatoi, Mark E. Sherman
Administrative support: William F. Anderson
Provision of study materials or patients: William F. Anderson
Collection and assembly of data: William F. Anderson
Data analysis and interpretation: William F. Anderson, Ismail Jatoi, Mark E. Sherman
Manuscript writing: William F. Anderson, Ismail Jatoi, Mark E. Sherman
Final approval of manuscript: William F. Anderson, Ismail Jatoi, Mark E. Sherman
REFERENCES
- 1.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 2.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine: Reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 3.Peto R. Statistical Aspects of Cancer Trials. London, United Kingdom: Chapman and Hall; 1982. [Google Scholar]
- 4.Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: Need for tailored treatments. J Natl Cancer Inst Monogr. 2001:44–51. doi: 10.1093/oxfordjournals.jncimonographs.a003459. [DOI] [PubMed] [Google Scholar]
- 5.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 7.Lubin JH, Burns PE, Blot WJ, et al. Risk factors for breast cancer in women in northern Alberta, Canada, as related to age at diagnosis. J Natl Cancer Inst. 1982;68:211–217. [PubMed] [Google Scholar]
- 8.Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997;216:28–43. doi: 10.3181/00379727-216-44153b. [DOI] [PubMed] [Google Scholar]
- 9.Anderson WF, Rosenberg PS, Menashe I, et al. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100:1804–1814. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson WF, Jatoi I, Devesa SS. Distinct breast cancer incidence and prognostic patterns in the NCI's SEER program: Suggesting a possible link between etiology and outcome. Breast Cancer Res Treat. 2005;90:127–137. doi: 10.1007/s10549-004-3777-3. [DOI] [PubMed] [Google Scholar]
- 11.Remvikos Y, Magdelenat H, Dutrillaux B. Genetic evolution of breast cancers. III: Age-dependent variations in the correlations between biological indicators of prognosis. Breast Cancer Res Treat. 1995;34:25–33. doi: 10.1007/BF00666488. [DOI] [PubMed] [Google Scholar]
- 12.Janerich DT, Hoff MB. Evidence for a crossover in breast cancer risk factors. Am J Epidemiol. 1982;116:737–742. doi: 10.1093/oxfordjournals.aje.a113462. [DOI] [PubMed] [Google Scholar]
- 13.Sherman ME, Rimm DL, Yang XR, et al. Variation in breast cancer hormone receptor and HER2 levels by etiologic factors: A population-based study. Int J Cancer. 2007;121:1079–1085. doi: 10.1002/ijc.22812. [DOI] [PubMed] [Google Scholar]
- 14.Madigan MP, Ziegler RG, Benichou J, et al. Proportion of breast cancer cases in the United States explained by well-established risk factors. J Natl Cancer Inst. 1995;87:1681–1685. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 15.Anderson WF, Pfeiffer RM, Dores GM, et al. Comparison of age frequency distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1899–1905. doi: 10.1158/1055-9965.EPI-06-0191. [DOI] [PubMed] [Google Scholar]
- 16.SEER-13. Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2007. Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence-SEER 13 Regs Limited-Use, Nov 2006 sub (1992-2004) http://www.seer.cancer.gov. [Google Scholar]
- 17.Anderson WF, Chatterjee N, Ershler WB, et al. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 18.Anderson WF, Reiner AS, Matsuno RK, et al. Shifting breast cancer trends in the United States. J Clin Oncol. 2007;25:3923–3929. doi: 10.1200/JCO.2007.11.6079. [DOI] [PubMed] [Google Scholar]
- 19.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 20.Sorlie T, Wang Y, Xiao C, et al. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: Gene expression analyses across three different platforms. BMC Genomics. 2006;7:127. doi: 10.1186/1471-2164-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 22.Yau C, Fedele V, Roydasgupta R, et al. Aging impacts transcriptomes but not genomes of hormone-dependent breast cancers. Breast Cancer Res. 2007;9:R59. doi: 10.1186/bcr1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders CK, Acharya CR, Hsu DS, et al. Age-specific differences in oncogenic pathway deregulation seen in human breast tumors. PLoS ONE. 2008;3:e1373. doi: 10.1371/journal.pone.0001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 25.Thomas GA, Leonard RC. How age affects the biology of breast cancer. Clin Oncol (R Coll Radiol) 2009;21:81–85. doi: 10.1016/j.clon.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 27.Anderson WF, Matsuno RK. Breast cancer heterogeneity: A mixture of at least two main types? J Natl Cancer Inst. 2006;98:948–951. doi: 10.1093/jnci/djj295. [DOI] [PubMed] [Google Scholar]
- 28.Pike MC, Krailo MD, Henderson BE, et al. ‘Hormonal’ risk factors: ‘Breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 29.MacMahon B. Epidemiology of Hodgkin's disease. Cancer Res. 1966;26:1189–1201. [PubMed] [Google Scholar]
- 30.Dores GM, Matsuno RK, Weisenburger DD, et al. Hairy cell leukemia: A heterogeneous disease? Br J Haematol. 2008;142:45–51. doi: 10.1111/j.1365-2141.2008.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haugen M, Bray F, Grotmol T, et al. Frailty modeling of bimodal age-incidence curves of nasopharyngeal carcinoma in low-risk populations. Biostatistics. 2009;10:501–514. doi: 10.1093/biostatistics/kxp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson WF, Pfeiffer RM, Tucker MA, et al. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer. 2009;115:4176–4185. doi: 10.1002/cncr.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimley PM, Matsuno RK, Rosenberg PS, et al. Qualitative age interactions between low and high grade serous ovarian carcinomas. Cancer Epidemiol Biomarkers Prev. 2009;18:2256–2261. doi: 10.1158/1055-9965.EPI-09-0240. [DOI] [PubMed] [Google Scholar]
- 34.Clemmesen J. Carcinoma of the breast. Br J Radiol. 1948;21:583–590. doi: 10.1259/0007-1285-21-252-583. [DOI] [PubMed] [Google Scholar]
- 35.Clark GM. The biology of breast cancer in older women. J Gerontol. 1992;47:19–23. [PubMed] [Google Scholar]
- 36.Fowble BL, Schultz DJ, Overmoyer B, et al. The influence of young age on outcome in early stage breast cancer. Int J Radiat Oncol Biol Phys. 1994;30:23–33. doi: 10.1016/0360-3016(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 37.Wildiers H, Van Calster B, van de Poll-Franse LV, et al. Relationship between age and axillary lymph node involvement in women with breast cancer. J Clin Oncol. 2009;27:2931–2937. doi: 10.1200/JCO.2008.16.7619. [DOI] [PubMed] [Google Scholar]
- 38.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 39.Neven P, Van Calster B, Van den Bempt I, et al. Age interacts with the expression of steroid and HER-2 receptors in operable invasive breast cancer. Breast Cancer Res Treat. 2008;110:153–159. doi: 10.1007/s10549-007-9687-4. [DOI] [PubMed] [Google Scholar]
- 40.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 41.Britton A, McKee M, Black N, et al. Threats to applicability of randomised trials: Exclusions and selective participation. J Health Serv Res Policy. 1999;4:112–121. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 42.Anderson WF, Luo S, Chatterjee N, et al. Human epidermal growth factor receptor-2 and estrogen receptor expression: A demonstration project using the residual tissue respository of the Surveillance, Epidemiology, and End Results (SEER) program. Breast Cancer Res Treat. 2009;113:189–196. doi: 10.1007/s10549-008-9918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 44.Giordano SH, Hortobagyi GN. Time to remove the subspecialty blinders: Breast cancer does not exist in isolation. J Natl Cancer Inst. 2008;100:230–231. doi: 10.1093/jnci/djn015. [DOI] [PubMed] [Google Scholar]