Abstract
Purpose
To determine the risk of ipsilateral breast events in patients with ductal carcinoma in situ (DCIS) treated with local excision without irradiation.
Patients and Methods
Patients with either low- or intermediate-grade DCIS measuring 2.5 cm or smaller, or high-grade DCIS measuring 1 cm or smaller who had microscopic margin widths of 3 mm or wider and no residual calcifications on postoperative mammograms were eligible for a prospective trial conducted from 1997 to 2002 by the Eastern Cooperative Oncology Group and North Central Cancer Treatment Group. Patients entered in 2000 and later could take tamoxifen if they wished. Median age at last surgery for the entire population was 60 years (range, 28 to 88 years), and median tumor sizes in the two strata were 6 mm and 5 mm, respectively.
Results
With a median follow-up of 6.2 years, the 5-year rate of ipsilateral breast events in the 565 eligible patients in the low/intermediate grade stratum was 6.1% (95% CI, 4.1% to 8.2%). With a median follow-up of 6.7 years, this incidence for the 105 eligible patients in the high-grade stratum was 15.3% (95% CI, 8.2% to 22.5%).
Conclusion
Rigorously evaluated and selected patients with low- to intermediate-grade DCIS with margins 3 mm or wider had an acceptably low rate of ipsilateral breast events at 5 years after excision without irradiation. Patients with high-grade lesions had a much higher rate, suggesting that excision alone is inadequate treatment. Further follow-up is necessary to document long-term results.
INTRODUCTION
Breast-conserving therapy has become the most common treatment for patients with ductal carcinoma in situ (DCIS) in the United States.1 Four randomized trials have shown that irradiation substantially reduces the risk of recurrence after local excision.2–8 Although mastectomy reduces the risk of local failure to 1% or less at 5 years, the lesser morbidity of breast-conserving therapy has been considered to justify a local failure rate of approximately 5% to 10% at 5 years with irradiation or 8% in patients with receptor-positive disease given tamoxifen in addition to breast irradiation. Retrospective studies have suggested that comparable rates may be achieved for patients with small lesions that have been widely excised, especially those with low- or intermediate-grade histologies, without the addition of breast irradiation.9,10 Many physicians are interested in omitting radiation treatment for some patients with DCIS, as long as the results are acceptable.
We felt that treatment with local excision alone should be studied prospectively in a large, multi-institutional, national study. We hypothesized that a combination of size of lesion, grade, and surgical margin width might define a subset of patients at low risk for local failure without irradiation.
PATIENTS AND METHODS
Objectives
This prospective trial was conducted by the Eastern Cooperative Oncology Group (ECOG) and the North Central Cancer Treatment Group on behalf of the Breast Cancer Intergroup of North America. The protocol was approved by the institutional review boards of the participating centers. All participants gave written informed consent. Its objectives were as follows: (1) to evaluate 5- and 10-year actuarial ipsilateral breast event (IBE) rates after local excision alone for selected patients with DCIS; (2) to evaluate concordance between institutional pathologists and central review pathologists with respect to diagnosis and grading of DCIS; (3) to identify parameters that indicate increased or decreased risk of recurrence in the absence of irradiation; (4) to assess how patients with IBEs were treated at relapse, particularly the use of further breast-conserving therapy; and (5) to evaluate 5- and 10-year actuarial relapse-free, overall, and cause-specific survival. This article will report 5-year results for IBE, relapse-free, and overall survival and the influence of clinical, pathologic, and treatment factors on the risk of IBE.
An IBE was defined as the occurrence of invasive cancer of any histology or DCIS (but not lobular carcinoma in situ) in the treated breast. Sufficient information was not always available to determine the exact location of the second lesion relative to the index lesion. Hence we prefer to use the term IBE rather than local recurrence to avoid the implication that such events necessarily represent a relapse of the initial DCIS resulting from residual disease after surgery, rather than the development of a second primary cancer.
Eligibility
The trial included two cohorts of patients with nonpalpable DCIS 3 mm or larger considered suitable for breast-conserving therapy who had undergone excision with microscopic margin widths of 3 mm or greater. The first cohort included patients with low or intermediate histologic grade DCIS 2.5 cm or smaller; the second consisted of patients with high histologic grade DCIS 1 cm or smaller. The upper size limit of 2.5 cm for patients with low- and intermediate-grade DCIS was based on the work of Lagios et al.11 The minimum margin width for all patients and the 1 cm upper size limit for patients with high-grade DCIS was based on a consensus of what our surgical and radiation oncology committee members felt they could accept to consider a patient for observation without irradiation after excision. Low/intermediate grade was defined by the presence of nuclear grade 1 or 2 with limited or no foci of necrosis. High grade was defined by the presence of nuclear grade 3 atypia and comedo-type necrosis that was zonal (ie, present in contiguous ductal spaces).12 Lesion size was defined as the largest histologic dimension, including any discontinuous areas. Mammographic estimate of size was allowed where pathologic measurement was impossible (ie, in cases where no residual DCIS was found on excision after initial core biopsy). Postoperative mammograms were required for patients who presented with suspicious microcalcifications; patients with residual suspicious calcifications were ineligible. Patients with clinical evidence of Paget's disease were excluded. Patients could be enrolled up to 6 months after the last definitive breast surgery. Other eligibility criteria included female sex, age 18 years or older, no known HIV infection, and no prior history of invasive or noninvasive breast cancer. Patients with a history of nonmelanoma skin cancer, carcinoma in situ of the cervix, or other invasive malignancies (if they had been cancer-free for at least 10 years) were eligible.
The surgical specimens were to be sequentially sectioned and completely embedded at the treating institution to accurately determine size, grade, and margins. Receptor status of DCIS was not measured routinely during this period. Patients were determined to be eligible and stratified based on the treating institution's pathologic assessment of histologic grade. A complete set of slides documenting these parameters was required to be sent for central pathology review at Vanderbilt University by the pathology study chair (D.P.). Biopsy material obtained from patients who experienced local failure was also to be sent for central review.
Central pathology review was performed for 97% of patients (690 of 711) enrolled in the study. Among the 671 patients found eligible after administrative review (Results), pathology on 654 cases (97.5%) has been centrally reviewed. Of these, 71 cases (10.9%) were considered unacceptable. Analysis of the population characteristics and outcome showed that excluding these 71 patients did not substantially change the results. Hence the results given in the remainder of this article are for the 671 patients eligible before central pathology review, which may present a more realistic picture of this treatment approach in ordinary clinical practice.
Treatment and Follow-Up
All patients were treated with local excision, with a minimum tumor-free margin width of 3 mm achieved by the last (definitive) surgery or the absence of tumor on excision after diagnostic core biopsy or on re-excision. Radiation therapy was not allowed. In 2000 the study was amended to allow adjuvant tamoxifen as a result of the publication of results of the National Surgical Adjuvant Breast and Bowel Project trial B-24.13 Thereafter, the intention to use tamoxifen or not was recorded at patient registration.
Patients were seen every 6 months for an interval history and physical examination, with annual mammograms. In the event of IBE or disease progression elsewhere, treatment was given at the discretion of the treating physicians.
Statistical Methods
Data collection and analysis were performed by the ECOG Coordinating Center. The primary end point was IBE, as defined above. An overall 5-year IBE rate of 10% and an invasive 5-year failure rate of 5% in each stratum were considered acceptable, but higher failure rates were not. The 95% CI on an observed 5-year IBE rate of 10% for 500 eligible and assessable patients per stratum who were all observed for at least 5 years was projected to be 7.5% to 13%. Allowing for up to 15% of the patients to be cancelled, ineligible, or to have problems on central pathology review, the overall accrual goal was therefore set at 588 patients per stratum (1,176 total). After the protocol was amended in 2000, it was assumed that approximately 50% of patients would receive tamoxifen and that tamoxifen would reduce the hazard rates for IBE (both invasive and in situ) by approximately 40%. Thus assuming an IBE rate of 8%, the 95% CI was projected to be 5.8% to 10.7%. The study design also included an early stopping rule based on assuming a constant hazard rate during the early follow-up period to be used to estimate whether the likely 5-year failure rate would exceed that considered to be acceptable. The stopping rule boundaries were not reached at the planned evaluation.
The analyses presented herein include all eligible patients, using institutional data from the patients' home institutions regarding lesion size, grade, and margin width, unless otherwise stated. For each patient, the failure time data were collected at all recurrences or new primary breast cancers including but not after first distant recurrence. Time to IBE was defined as time from last definitive surgery before study entry to first occurrence of IBE; patients without IBE were censored at date last known to be IBE free. Time to contralateral breast events was defined similarly. Event-time distributions were estimated using Kaplan-Meier analysis,14 with the SE estimated using Greenwood's formula.15 Cox proportional hazards methods were used to estimate hazards ratios and test for significance for event times.16 The proportional hazard assumption was tested using the method of Grambsch and Therneau.17 All P values are two-sided, and CIs are given at the 95% level.
RESULTS
Administrative Data
The study was activated in April 1997 and terminated in October 2002, when the accrual goal was reached for the low/intermediate stratum, with 606 entered patients. At that time, 105 patients with high-grade DCIS had been entered, and the decision was made to stop accrual to the high-grade arm because the accrual of 588 planned patients to that arm could not be completed in a reasonable time frame. Therefore, the final accrual was 711 total patients (575 from ECOG and 136 from North Central Cancer Treatment Group; see Appendix Table A1, online only).
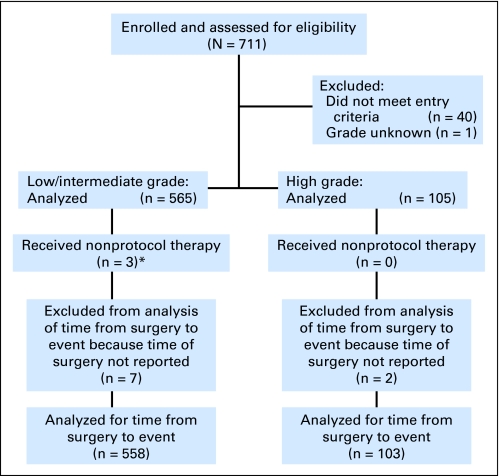
Forty cases (5.6%) were determined to be ineligible after central review of the submitted documentation. Reasons included postexcision mammogram not done or questionable (12 patients), questionable tumor size (10 patients), margins less than 3 mm (eight patients), positive postexcision mammogram (three patients), documents not available for assessment (three patients), microinvasion or invasive cancer (two patients), radiation after excision (one patient), and pathology sampling incomplete (one patient). One additional patient was excluded from analysis secondary to unknown grade. The CONSORT diagram is shown in Figure 1.
Fig 1.
CONSORT diagram for Eastern Cooperative Oncology Group trial 5194. (*) Patients who received nonprotocol therapy or were lost to follow-up were included in the analysis of time from surgery to event.
Patient, Tumor, and Treatment Characteristics
Table 1 lists patient demographics, disease characteristics, and treatment variables. For the low/intermediate patient group, the median patient age was 60 years (range, 28 to 88 years), and the median lesion size was 6 mm (range, 1 to 25 mm), with 76.5% less than 10 mm. Margins were 5 mm or wider in 69.2% of patients and more than 10 mm wide or no tumor found on re-excision in 48.5% of patients. For patients with high-grade disease, the median patient age was 59 years (range, 33 to 87 years), and the median lesion size was 5 mm (range, 2 to 10 mm), with 87.6% less than 10 mm. Margins were 5 mm or wider in 82.9% of patients, and 10 mm or wider or no tumor on re-excision in 53.3% of patients. Fifty-five percent of patients in both the low/intermediate and high-grade patient groups entering after the 2000 addendum allowing adjuvant tamoxifen indicated their intention to take 5 years of tamoxifen, resulting in a total of 31.3% and 28.6%, respectively, of patients with low/intermediate and high-grade DCIS intending to take adjuvant tamoxifen.
Table 1.
Patient Demographics and Disease Characteristics
| Characteristic | Low/Intermediate (n = 565) |
High (n = 105) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age at last surgery, years* | ||||
| Median | 60 | 59 | ||
| Range | 28-88 | 33-87 | ||
| 28-49 | 113 | 20.0 | 25 | 23.8 |
| 50-64 | 249 | 44.1 | 42 | 40.0 |
| 65-88 | 203 | 35.9 | 38 | 36.2 |
| Race/ethnicity | ||||
| White | 523 | 92.6 | 95 | 90.5 |
| Hispanic | 8 | 1.4 | 1 | 1.0 |
| Black | 16 | 2.8 | 5 | 4.8 |
| Other | 14 | 2.5 | 0 | |
| Unknown | 4 | 0.7 | 4 | 3.8 |
| Menopausal status† | ||||
| Premenopausal | 135 | 23.9 | 28 | 26.7 |
| Postmenopausal | 430 | 76.1 | 77 | 73.3 |
| Margin width, mm (institutional measure) | ||||
| < 5 | 174 | 30.8 | 18 | 17.1 |
| 5-10 | 117 | 20.7 | 31 | 29.5 |
| ≥ 10 | 274 | 48.5 | 56 | 53.3 |
| Lesion size, mm (institutional measure) | ||||
| Median | 6 | 5 | ||
| Range | 1-25 | 2-10 | ||
| < 5 | 161 | 28.5 | 34 | 32.4 |
| 5-< 10 | 271 | 48.0 | 58‡ | 55.2 |
| ≥ 10§ | 133 | 23.5 | 13 | 12.4 |
| Method of detection | ||||
| Microcalcifications | 401 | 71.0 | 89 | 84.8 |
| Density or mass | 93 | 16.5 | 3 | 2.9 |
| Both | 40 | 7.1 | 8 | 7.6 |
| Incidental finding | 20 | 3.5 | 5 | 4.8 |
| Other | 9 | 1.6 | 0 | |
| Unknown | 2 | 0.4 | 0 | |
| Bloody nipple discharge | ||||
| Yes | 13 | 2.3 | 1 | 1.0 |
| No | 544 | 96.3 | 103 | 98.1 |
| Unknown | 8 | 1.4 | 1 | 1.0 |
| Tamoxifen use before study entry | ||||
| Yes | 68 | 12.0 | 11 | 10.5 |
| No | 494 | 87.4 | 94 | 89.5 |
| Unknown | 3 | 0.5 | 0 | |
| Hormone replacement therapy before study entry | ||||
| Yes | 240 | 42.5 | 47 | 44.8 |
| No | 318 | 56.3 | 58 | 55.2 |
| Unknown | 7 | 1.2 | 0 | |
Age at study entry used for nine cases whose date of last surgery was unavailable.
Patients younger than 50 years were assumed to be premenopausal when menopausal status was not recorded.
Includes one patient with tumor smaller than 10 mm, not otherwise described.
Tumor measured as exactly 10 mm for high-grade stratum.
IBEs
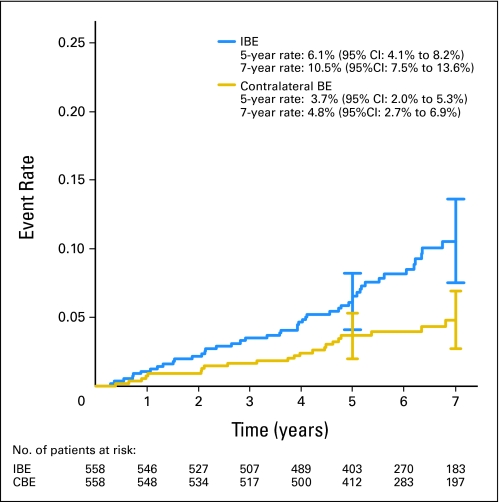
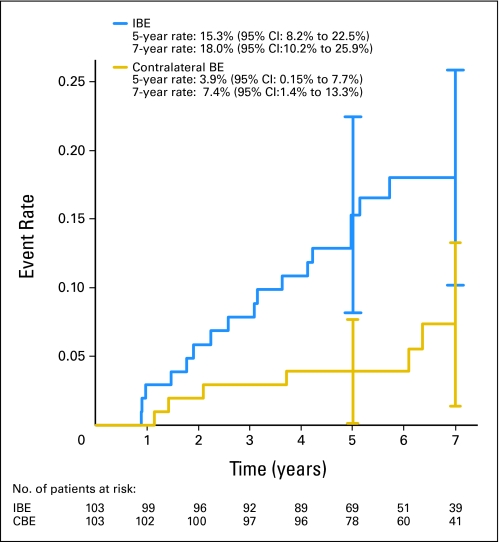
The median follow-up time was 6.3 years for all patients (6.2 and 6.7 years for the low/intermediate-grade and high-grade strata, respectively). In the low/intermediate-grade stratum, there were 49 IBEs. Fifty-three percent were invasive (with or without DCIS), and 46.9% were DCIS only. The 5-year IBE rate was 6.1% (95% CI, 4.1% to 8.2%), and the 7-year rate was 10.5% (95% CI, 7.5% to 13.6%; Fig 2). There were 23 new primary contralateral breast cancers, 65.2% of which were invasive and 34.8% DCIS only, for a 5-year rate of 3.7% (95% CI, 2.0% to 5.3%) and a 7-year rate of 4.8% (95% CI, 2.7% to 6.9%). In the high-grade stratum, there were 17 IBEs, 35.3% of which were invasive and 64.7% DCIS only. The 5-year IBE rate was 15.3% (95% CI, 8.2% to 22.5%), and the 7-year rate was 18.0% (95% CI, 10.2% to 25.9%; Fig 3). There were six contralateral breast events (all invasive), for a 5-year rate of 3.9% (95% CI, 0.15% to 7.7%) and a 7-year rate of 7.4% (95% CI, 1.4% to 13.3%).
Fig 2.
Ipsilateral breast events (IBEs) and contralateral breast events (CBEs) in patients with low-or intermediate-grade ductal carcinoma in situ. Vertical bars represent 95% CIs. Numbers at risk are given beneath the x-axis.
Fig 3.
Ipsilateral breast events (IBEs) and contralateral breast events (CBEs) in patients with high-grade ductal carcinoma in situ. Vertical bars represent 95% CIs. Numbers at risk are given beneath the x-axis.
The 5-year rates of IBE in relation to individual patient, tumor, and treatment characteristics are listed in Table 2. In a Cox proportional hazards model with grade as the only covariate, grade (low/intermediate v high) was significantly related to the risk of IBE (P = .024), with a hazard ratio of 0.53 (95% CI, 0.31 to 0.92) in favor of low/intermediate grade. Separate models were therefore created to examine the impact of other factors on the risk of IBE for each stratum. For each stratum, the variables included were institutional lesion size (< 10 mm v ≥ 10 mm), institutional margin (< 10 mm v ≥ 10 mm), age at last surgery as a continuous variable, intent to use tamoxifen or not, and tamoxifen use or not before study entry. The only significant variable in either model was age in the high-grade stratum, with a hazard ratio of 0.95 (95% CI, 0.91 to 0.99; P = .016), which reflects the effect of a 1-year increase in age.
Table 2.
Five-Year Ipsilateral Breast Event Rates for Eligible Patients
| Variable | Low/Intermediate Grade |
High Grade |
||||
|---|---|---|---|---|---|---|
| No. | 5-Year Rate (%) | 95% CI | No. | 5-Year Rate (%) | 95% CI | |
| Age, years | ||||||
| < 45 | 49 | 8.5 | 0.5 to 16.5 | 11 | 54.4 | 25.1 to 84.0 |
| ≥ 45 | 509 | 5.9 | 3.8 to 8.0 | 92 | 10.3 | 3.9 to 16.7 |
| Margin size, mm | ||||||
| < 10 | 284 | 5.6 | 2.8 to 8.3 | 48 | 14.8 | 4.7 to 24.9 |
| ≥ 10 | 274 | 6.7 | 3.6 to 9.7 | 55 | 15.9 | 5.7 to 26.1 |
| Lesion size, mm | ||||||
| < 10 | 426 | 5.5 | 3.3 to 7.8 | 90 | 12.7 | 5.7 to 20.0 |
| ≥ 10* | 132 | 8.1 | 3.3 to 12.9 | 13 | 32.9 | 6.4 to 59.3 |
| Intent to use tamoxifen | ||||||
| Yes | 175 | 7.5 | 3.4 to 11.6 | 29 | 16.6 | 1.1 to 32.0 |
| No† | 383 | 5.5 | 3.1 to 7.8 | 74 | 15.3 | 7.0 to 23.7 |
| Prior tamoxifen use | ||||||
| Yes | 67 | 1.5 | 0 to 4.4 | 11 | ‡ | |
| No | 488 | 6.8 | 4.5 to 9.1 | 92 | 17.1 | 9.2 to 25.0 |
Tumor measured as exactly 10-mm for high grade stratum.
Includes those cases entered before the 2000 addendum allowing tamoxifen use.
No events observed by 5 years.
Treatment of Recurrence and Other End Points
Of the 49 patients in the low/intermediate-grade stratum with IBE, 21 patients were treated with salvage breast-conserving therapy and 24 patients were treated with mastectomy; four patients were treated with systemic therapy only after biopsy. Seven of the 49 patients in the low/intermediate-grade stratum received chemotherapy. Of the 17 patients in the high-grade stratum with IBE, four patients were treated with salvage breast-conserving therapy and 13 patients were treated with mastectomy. Five of the 17 patients in the high-grade group received chemotherapy.
In the low/intermediate group, there were three patients with ipsilateral nodal failure and one patient with simultaneous nodal failure and distant metastases. In the high-grade group, one patient developed an ipsilateral nodal failure and one developed distant metastases. The respective 5-year disease-free survival rates in the two strata were 85.6% (95% CI, 82.6% to 88.6%) and 77.7% (95% CI, 69.4% to 85.9%).
Forty-one of 565 patients in the low/intermediate-grade group and five of 105 patients in the high-grade group have died. The respective 5-year survival rates were 95.7% (95% CI, 94.0% to 97.4%) and 97.0% (95% CI, 93.6% to 100%). So far, no death has been due to breast cancer.
DISCUSSION
Four randomized trials have demonstrated that irradiation reduces the risk of IBEs after excision of DCIS from approximately 15% to 20% at 5 years and 25% to 30% at 10 years to approximately 5% to 9% and 15%, respectively, for relatively unselected patients. However, none of these trials have shown a difference in the rates of distant metastases, breast cancer–specific survival, or overall survival between irradiated and unirradiated patients.2–8 This is in contrast to the situation for invasive cancer, where the use of radiotherapy significantly improves these outcomes.18 Hence the potential side effects of radiation therapy, although small,19 must be weighed more carefully in making treatment decisions for patients with DCIS.
Several investigators have reported rates of local recurrence of 5% to 15% at 5 to 10 years in retrospective studies of selected patients treated with local excision alone with very wide margins.9,10,20–22 However, our study is the first large, multi-institutional prospective trial showing that rigorous mammographic and pathologic evaluation and selection criteria can yield comparable results. It should be noted that the patients entered onto this trial had a more favorable median lesion size and width of margins than the entry criteria required.
We believe the IBE rate of 6% at 5 years for the low/intermediate-grade stratum would be acceptable to many patients and physicians, whereas the rate of 15% at 5 years for the high-grade group would not be, especially because we expect higher rates at 10 years. These findings are similar to those of a prospective trial of local excision alone at the Harvard-affiliated medical institutions.23 With a median follow-up of 43 months in that study, the crude local failure rate was 6% (nine of 146 patients) for those with highest nuclear grade 1 or 2 present, compared with 40% (four of 10) for those with any nuclear grade 3 component.
The increase in IBE rates beyond 5 years (Figs 2 and 3) warrants caution regarding the clinical implications of our results. This increase and the shapes of the curves are similar to the findings in a study of 268 patients treated with lumpectomy and radiation.24 Patients with high-risk histology (presence of nuclear grade 3 plus comedo-type necrosis) in that study had a 5-year failure rate of 12%, compared with 3% for patients with lower-risk DCIS; however, at 10 years the respective rates were 18% and 15% Thus, substantially longer observation is warranted to determine whether omission of radiation treatment is appropriate for some patients with DCIS.
Radiation Therapy Oncology Group trial 98-04 randomly allocated patients with DCIS to receive radiation therapy or not after excision, using the same selection criteria as in our protocol. The trial closed in 2006, accruing 636 patients. The results will be important in showing how much irradiation will reduce the local failure rate for such highly selected patients and the long-term clinical significance of such therapy.
Overall survival has been excellent to date in our trial, with no patient dying of breast cancer so far, but further follow-up is required to validate the long-term safety of this approach. Future goals include analyzing the results of central pathology review and assessing IBE risk in relation to biologic markers using patients' banked tissue blocks.
In conclusion, rigorously evaluated and selected patients with low- to intermediate-grade DCIS with margins 3 mm or wider had an acceptably low rate of ipsilateral breast events at 5 years after excision without irradiation. Patients with high-grade lesions had a much higher rate of ipsilateral breast events, suggesting that excision alone is inadequate treatment. Further follow-up is necessary to document long-term results and optimize patient selection for this approach.
Acknowledgment
We thank Gloria Robinson at the Eastern Cooperative Oncology Group Coordinating Center for data management.
Appendix
Table A1.
Accrual by Group and Institution
| Institution | No. of Patients | Accrual (%) |
|---|---|---|
| Thomas Jefferson University | 3 | 0.4 |
| Johns Hopkins University | 55 | 7.7 |
| Mayo Clinic Rochester | 35 | 4.9 |
| Rochester, University of | 20 | 2.8 |
| Tufts-New England Medical Center | 73 | 10.3 |
| Albert Einstein College of Medicine | 5 | 0.7 |
| Case Western-MetroHealth Medical Center | 29 | 4.1 |
| Fox Chase Cancer Center | 42 | 5.9 |
| Pennsylvania, University of | 19 | 2.7 |
| New York University Medical Center | 17 | 2.4 |
| Pittsburgh, University of | 9 | 1.3 |
| Northwestern University | 53 | 7.4 |
| St Francis Hospital of Oklahoma CCOP | 14 | 2.0 |
| Wisconsin, University of | 54 | 7.6 |
| Wisconsin, Medical College of | 13 | 1.8 |
| Northern New Jersey CCOP, Hackensack Medical Center | 6 | 0.8 |
| Indiana University Cancer Center | 16 | 2.2 |
| Vanderbilt University | 33 | 4.6 |
| Emory University | 7 | 1.0 |
| Marshfield Clinic | 5 | 0.7 |
| Stanford University | 15 | 2.1 |
| Abbott Northwestern Hospital | 7 | 1.0 |
| Miami, University of | 1 | 0.1 |
| Moffitt Cancer Center | 2 | 0.3 |
| Lankenau Hospital | 9 | 1.3 |
| Allegheny University-Allegheny General Hospital | 6 | 0.8 |
| Washington Medical Center | 1 | 0.1 |
| West Michigan Cancer Center | 6 | 0.8 |
| Cancer Institute of New Jersey | 11 | 1.5 |
| Beth Israel Deaconess Medical Center | 8 | 1.1 |
| Ochsner Clinic | 1 | 0.1 |
| Total accrual, ECOG | 575 | 80.8 |
| Mayo Clinic | 71 | 10.6 |
| Sanford Cancer Center-Oncology Clinic | 3 | 0.4 |
| Duluth Clinic | 8 | 1.2 |
| MeritCare Hospital CCOP | 11 | 1.6 |
| CentraCare Clinic | 2 | 0.3 |
| Rapid City Regional Hospital | 4 | 0.6 |
| Cedar Rapids Oncology Associates | 2 | 0.3 |
| Lincoln General Hospital | 1 | 0.1 |
| Mid Dakota Clinic | 1 | 0.1 |
| Ochsner Clinic | 1 | 0.1 |
| Mercy Hospital Medical Center | 3 | 0.4 |
| Carle Clinic | 5 | 0.7 |
| Siouxland Hematology-Oncology Association | 8 | 1.2 |
| Bismarck Cancer Center | 1 | 0.1 |
| Mercy Medical Center–North Iowa | 7 | 1.0 |
| Poudre Valley Hospital | 3 | 0.4 |
| St Joseph Mercy Hospital–Oakland | 1 | 0.1 |
| Wichita CCOP | 4 | 0.6 |
| Total accrual, NCCTG | 136 | 19.1 |
Abbreviations: CCOP, Community Clinical Oncology Program; ECOG, Eastern Cooperative Oncology Group; NCCTG, North Central Cancer Trial Group.
Footnotes
See accompanying editorial on page 5303
Supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA40057, CA07190, CA25224, and CA16116 and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, chair). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Presented in part at the 29th Annual San Antonio Breast Cancer Symposium, December 14-18, 2006, San Antonio, TX.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002934.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lorie L. Hughes, David L. Page, Robert Gray, Lawrence J. Solin, Nancy E. Davidson, William C. Wood
Provision of study materials or patients: Lorie L. Hughes, Lawrence J. Solin, Nancy E. Davidson, Mary Ann Lowen, James N. Ingle, William C. Wood
Collection and assembly of data: Lorie L. Hughes, Molin Wang, David L. Page, Robert Gray
Data analysis and interpretation: Lorie L. Hughes, Molin Wang, David L. Page, Robert Gray, Lawrence J. Solin, Abram Recht, William C. Wood
Manuscript writing: Lorie L. Hughes, Molin Wang, David L. Page, Robert Gray, Lawrence J. Solin, Nancy E. Davidson, James N. Ingle, Abram Recht, William C. Wood
Final approval of manuscript: Lorie L. Hughes, Molin Wang, David L. Page, Robert Gray, Lawrence J. Solin, Nancy E. Davidson, Mary Ann Lowen, James N. Ingle, Abram Recht, William C. Wood
REFERENCES
- 1.Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 2.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 4.Wapnir I, Dignam J, Julian TB, et al. Long-term outcomes after invasive breast tumor recurrence (IBTR) in women with DCIS in NSABP B-17 and B-24. J Clin Oncol. 2007;25:7s. abstr 520. [Google Scholar]
- 5.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: Randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 6.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: Ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—A study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 7.Ringberg A, Nordgren H, Thorstensson S, et al. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast: Results from the Swedish randomised trial. Eur J Cancer. 2007;43:291–298. doi: 10.1016/j.ejca.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ. N Engl J Med. 1999;340:1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald HR, Silverstein MJ, Lee LA, et al. Margin width as the sole determinant of local recurrence after breast conservation in patients with ductal carcinoma in situ of the breast. Am J Surg. 2006;192:420–422. doi: 10.1016/j.amjsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Lagios M, Page DL. Lagios experience. In: Silverstein MJ, Lagios MD, Poller DN, et al., editors. Ductal Carcinoma In Situ of the Breast. Baltimore, MD: William & Wilkins; 1997. pp. 361–365. [Google Scholar]
- 12.Silverstein MJ, Lagios MD, Recht A, et al. Image-detected breast cancer: State of the art diagnosis and treatment. J Am Coll Surg. 2005;201:586–597. doi: 10.1016/j.jamcollsurg.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley and Sons; 1980. [Google Scholar]
- 16.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighed residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 18.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro CL, Recht A. Side effects of adjuvant therapy for breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 20.de Mascarel I, Bonichon F, MacGrogan G, et al. Application of the Van Nuys prognostic index in a retrospective series of 367 ductal carcinomas in situ of the breast examined by serial macroscopic sectioning: Practical considerations. Breast Cancer Res Treat. 2000;61:151–159. doi: 10.1023/a:1006437902770. [DOI] [PubMed] [Google Scholar]
- 21.MacAusland SG, Hepel JT, Chong FK, et al. An attempt to independently verify the utility of the Van Nuys Prognostic Index for ductal carcinoma in situ. Cancer. 2007;110:2648–2653. doi: 10.1002/cncr.23089. [DOI] [PubMed] [Google Scholar]
- 22.Di Saverio S, Catena F, Santini D, et al. 259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: A retrospective review with long term follow up. Breast Cancer Res Treat. 2008;109:405–416. doi: 10.1007/s10549-007-9668-7. [DOI] [PubMed] [Google Scholar]
- 23.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 24.Solin L, Kurtz J, Fourquet A, et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. J Clin Oncol. 1996;14:754–763. doi: 10.1200/JCO.1996.14.3.754. [DOI] [PubMed] [Google Scholar]