Abstract
Purpose
The objective was to compare laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer.
Patients and Methods
Patients with clinical stage I to IIA uterine cancer were randomly assigned to laparoscopy (n = 1,696) or open laparotomy (n = 920), including hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. The main study end points were 6-week morbidity and mortality, hospital length of stay, conversion from laparoscopy to laparotomy, recurrence-free survival, site of recurrence, and patient-reported quality-of-life outcomes.
Results
Laparoscopy was initiated in 1,682 patients and completed without conversion in 1,248 patients (74.2%). Conversion from laparoscopy to laparotomy was secondary to poor visibility in 246 patients (14.6%), metastatic cancer in 69 patients (4.1%), bleeding in 49 patients (2.9%), and other cause in 70 patients (4.2%). Laparoscopy had fewer moderate to severe postoperative adverse events than laparotomy (14% v 21%, respectively; P < .0001) but similar rates of intraoperative complications, despite having a significantly longer operative time (median, 204 v 130 minutes, respectively; P < .001). Hospitalization of more than 2 days was significantly lower in laparoscopy versus laparotomy patients (52% v 94%, respectively; P < .0001). Pelvic and para-aortic nodes were not removed in 8% of laparoscopy patients and 4% of laparotomy patients (P < .0001). No difference in overall detection of advanced stage (stage IIIA, IIIC, or IVB) was seen (17% of laparoscopy patients v 17% of laparotomy patients; P = .841).
Conclusion
Laparoscopic surgical staging for uterine cancer is feasible and safe in terms of short-term outcomes and results in fewer complications and shorter hospital stay. Follow-up of these patients will determine whether surgical technique impacts pattern of recurrence or disease-free survival.
INTRODUCTION
Uterine cancer is common, with 40,100 cases and 7,470 deaths in the United States in 2008.1 Sites of metastasis include pelvic and para-aortic lymph nodes, adnexa, and peritoneal surfaces.2,3 Surgical treatment and staging are performed according to the International Federation of Gynecology and Obstetrics (FIGO) staging system4 and followed by the American Joint Committee on Cancer.5 Postoperative treatment recommendations, including radiation and/or chemotherapy, are tailored to histologic cell type, nuclear and cytologic grade, depth of myometrial and cervical invasion, lymphovascular space invasion, peritoneal cytology, and stage of disease, with an effort to avoid the toxicity of overtreatment.6–10
Historically, comprehensive surgical staging in endometrial cancer has been accomplished via open laparotomy, including hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic and para-aortic lymphadenectomy, and peritoneal cytology.11 Complete cytoreduction of all sites of metastatic tumor may improve the outcome with adjuvant therapy.12–15
The decade of the 1990s brought the use of minimally invasive surgery to replicate the traditional goals of comprehensive surgical staging of endometrial cancer. Dargent16 and Querleu et al17 in France and Childers et al18 and Spirtos et al19 in the United States demonstrated the adequacy and safety in small single-institution studies. The Gynecologic Oncology Group (GOG) determined that a prospective randomized trial was indicated to compare laparotomy with laparoscopy.
PATIENTS AND METHODS
Study Design
The study was designed to compare laparoscopy with laparotomy for the purpose of complete comprehensive surgical staging of uterine cancer. The primary outcome of the study was recurrence-free survival. Other end points included perioperative adverse events, laparoscopy conversion to laparotomy, length of hospital stay after surgery, operative time, quality of life, sites of recurrence, and survival.
Eligibility requirements were clinical stage I to IIA uterine cancer, adequate bone marrow and renal and hepatic function (defined as WBC ≥ 3,000 cells/μL, platelets ≥ 100,000/μL, creatinine ≤ 2.0 mg/100 mL, bilirubin ≤ 1.5× normal, and AST ≤ 3× normal), and GOG performance status of less than 4. Patients with a prior malignancy were eligible if they had no evidence of cancer. All patients provided a written informed consent that was approved by the institutional review board of the enrolling institution.
Surgical stage was determined according to the rules of FIGO in 1988 and then confirmed by central GOG Pathology Review. Conversion from laparoscopy to laparotomy was a decision of the operating surgeon according to the best interest of the participant. Reasons for conversion were recorded prospectively. Surgeons varied as to whether they completed the lymph node dissection once a positive lymph node was documented.
Study Procedures
The technique for surgically staging uterine cancer was defined in accordance to the GOG Surgical Procedures Manual. Cytology was to be obtained on entry into the peritoneal cavity. Pelvic lymph nodes were to be removed from the distal one half of the common iliac artery down to the circumflex iliac vein, and nodal tissue was to be removed anterior to the obturator nerve and surrounding the iliac arteries and vein. The para-aortic nodes included those overlying the vena cava (defined as right), between the vena cava and aorta, and to the left of the aorta (designated left para-aortic). The cephalad boundary of the para-aortic specimen was generally, but not limited to, the inferior mesenteric artery, and the distal boundary was the midpoint of the common iliac artery. The Guidelines for Laparoscopic Pelvic and Aortic Lymph Node Sampling recommend elevation of the inferior mesenteric artery to identify the left ureter and resection of the nodes to the left of the aorta and down the lateral aspect of the left common iliac artery to the midpoint, and the remainder of the lymphadenectomy follows the same boundaries as described earlier.19a Extrafascial hysterectomy and bilateral salpingo-oophorectomy are recommended and outlined in the surgical procedures manual. The technique for laparoscopic hysterectomy was not specified and included laparoscopic-assisted techniques, total laparoscopic approaches, and rarely robotics.
Prospectively completed forms documented reasons for conversion of laparoscopy to laparotomy, operative time, blood loss, transfusions, intraoperative and postoperative complications, use of antibiotics, dates of surgery and discharge, readmissions, reoperations, and subsequent cancer therapy (radiation, chemotherapy, or hormonal therapy) recommended and completed. Intraoperative injuries were coded as yes or no and categorized as involving the bowel, veins, arteries, ureter, bladder, or other site. Postoperative adverse events were recorded on a 6-week follow-up form and classified using the National Cancer Institute Common Toxicity Criteria (version 2.0).20
Standardized pathology evaluation forms were to be completed prospectively by the local GOG pathologist, documenting the number of nodes removed and the number of positive nodes at each of four regions (right pelvic, left pelvic, right para-aortic, and left para-aortic). FIGO staging and prognostic criteria (depth of myometrial invasion, cervical involvement, lymphovascular invasion, metastatic sites, and peritoneal cytology results) were also collected prospectively, along with copies of pathology and cytology reports. Pathology slides were reviewed for central quality control by the assigned GOG gynecologic pathologist (G.S.). A GOG pathology referee (William Rodgers) resolved conflicts that occurred between the local site submitting pathologist and the central pathology (G.S.) review.
Quality-of-life assessments with patient-reported measures were performed in the first 800 patients before random assignment and at 1, 3, and 6 weeks and 6 months after surgery. Postprocedure follow-up forms for recurrence, treatment, survival, cause of death, and new primary cancer were required every 3 months for the first 2 years, then every 6 months for the next 3 years, and then annually thereafter.
Statistical Methods
Random assignment was conducted by a permuted block design such that approximately twice as many registered patients underwent laparoscopy compared with laparotomy. The study was originally designed to accrue 800 patients over a 3-year period to evaluate surgical complications, adverse events, length of hospital stay, and improving quality of life. In 2001, the protocol was amended, and the sample size increased to 2,550 patients to assess whether laparoscopy could be considered not inferior to open laparotomy with regard to recurrence-free survival. Postsurgical complications were graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0)20 and classified as either less than grade 2 or ≥ grade 2. Patients were to be classified as having one or more adverse events versus no adverse events. Assuming that approximately 10% of laparoscopy patients would experience at least one grade 2 or higher postoperative adverse event, 2,550 patients provided 94% power to detect a 5% difference between groups using a two-tailed χ2 test with significance defined as P < .05. If a statistically significant difference was observed in the overall test, comparisons of individual adverse events were to be conducted with adjusted significance levels of P < .005. It was also estimated that 50% of laparotomy patients require extended (> 2 days) hospital stays; the sample size of 2,550 patients provided 91% power for detecting a 7% improvement with laparoscopy using a two-tailed χ2 test with statistical significance defined as P < .05. Continuous data are presented as median and interquartile range (IQR), defined as the 25th to 75th percentile. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. All patients who received surgery are included in the comparisons of short-term clinical outcomes. Logistic regression models were used to assess the influence of patient age, BMI, racial designation/ethnicity (Asian, black, white, Hispanic, and other), and performance status at enrollment (0, 1, or ≥ 2), along with treatment group, on postoperative adverse events or prolonged hospitalization. Logistic regression was also used to assess the influence of patient age, BMI, metastatic disease, and number of patients enrolled per institution on the risk of conversion. Pathology outcomes were assessed using χ2 tests, with statistical significance defined as P ≤ .01.
RESULTS
Study Population
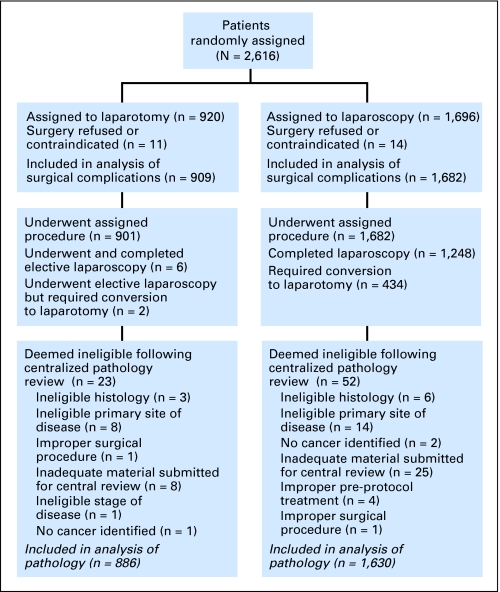
The participants were enrolled between May 1996 and September 2005. A total of 2,616 patients were randomly assigned—920 to laparotomy and 1,696 to laparoscopy (Table 1). Twenty-five patients (14 assigned to laparoscopy and 11 assigned to laparotomy) did not have surgery. Reasons included refusal of randomized treatment, surgery contraindicated as a result of morbidities, insurance issues, and patient moved or decided to have surgery at a non-GOG institution. This resulted in 1,682 laparoscopy patients and 909 laparotomy patients to be included in the analysis of short-term surgical outcomes. After review by the GOG Gynecology and Pathology Committees, 66 laparoscopy and 34 laparotomy patients were found to be ineligible; these patients are excluded from analyses of pathologic results, leaving 1,630 laparoscopy and 886 laparotomy patients for these analyses. A CONSORT diagram of participant flow is shown in Figure 1. The distribution of histologic diagnosis as confirmed by central pathology review was similar in the two treatment arms (Table 2).
Table 1.
Patient Characteristics
| Characteristic | Laparotomy |
Laparoscopy |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 63 | 63 | ||
| Interquartile range | 55-71 | 55-72 | ||
| Weight, kg | ||||
| Median | 74 | 74 | ||
| Interquartile range | 63-89 | 63-88 | ||
| Height, cm | ||||
| Median | 161 | 162 | ||
| Interquartile range | 157-166 | 157-166 | ||
| BMI, kg/m2 | ||||
| Median | 29 | 28 | ||
| Interquartile range | 24-34 | 24-34 | ||
| Race/ethnicity | ||||
| Asian | 34 | 4 | 54 | 3 |
| Black | 37 | 4 | 61 | 4 |
| Hispanic | 45 | 5 | 67 | 4 |
| White | 785 | 86 | 1,495 | 89 |
| Other | 15 | 2 | 10 | 1 |
| Performance status | ||||
| 0 | 821 | 89 | 1,527 | 90 |
| 1 | 89 | 10 | 160 | 9 |
| 2 | 9 | 1 | 5 | < 1 |
| 3 | 1 | < 1 | 2 | < 1 |
Abbreviation: BMI, body mass index.
Fig 1.
CONSORT diagram.
Table 2.
Pathology Findings
| Pathology | Laparotomy |
Laparoscopy |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Surgical stage | .841* | ||||
| IA | 310 | 35 | 609 | 37 | |
| IB | 266 | 30 | 451 | 28 | |
| IC | 104 | 12 | 193 | 12 | |
| IIA | 20 | 2 | 37 | 2 | |
| IIB | 32 | 4 | 61 | 4 | |
| IIIA | 42 | 5 | 96 | 6 | |
| IIIC | 84 | 9 | 143 | 9 | |
| IVB | 28 | 3 | 39 | 2 | |
| Unstaged† | 0 | 0 | 1 | < 1 | |
| Tumor type | .415 | ||||
| Endometrioid adenocarcinoma | 727 | 82 | 1,297 | 80 | |
| Anaplastic/other carcinoma | 1 | < 1 | 0 | 0.0 | |
| Clear cell carcinoma | 11 | 1 | 31 | 2 | |
| Mixed epithelial carcinoma | 24 | 3 | 49 | 3 | |
| Serous carcinoma | 94 | 11 | 195 | 12 | |
| Sarcoma | 29 | 3 | 58 | 4 | |
| Peritoneal cytology | 866 | 98 | 1,569 | 96 | .052 |
| Type of nodes | .0009‡ | ||||
| No nodes | 6 | 0.7 | 22 | 1.4 | .124 |
| Para-aortic nodes only | 3 | 0.3 | 6 | 0.4 | .905 |
| Pelvic nodes only | 28 | 3.2 | 109 | 6.8 | .0002§ |
| Both pelvic and para-aortic nodes | 840 | 95.8 | 1,476 | 91.5 | < .0001§ |
| Any pelvic nodes | 868 | 99 | 1,585 | 98 | .183 |
| Median, No. of nodes | 18 | 17 | |||
| IQR, No. of nodes | 12-24 | 12-23 | |||
| Any para-aortic nodes | 843 | 97 | 1,482 | 94 | .002§ |
| Median, No. of nodes | 7 | 7 | |||
| IQR, No. of nodes | 4-11 | 4-11 | |||
Abbreviation: IQR, interquartile range.
Stage I and II v stage III and IV.
Unstaged as a result of surgical complications.
Overall comparison between randomized groups on type of nodes.
Statistically significant at adjusted significance level of P= .01.
Conversion From Laparoscopy to Laparotomy
There were 434 participants (25.8%) randomly assigned to laparoscopy who required conversion to open laparotomy to complete the procedure. Poor exposure was cited in 246 patients (14.6% of patients randomly assigned to laparoscopy arm, or 56.7% of the converted group) as the reason to convert from laparoscopy to laparotomy. Cancer requiring laparotomy for resection was responsible for conversion in 69 patients (4.1% of patients randomly assigned to laparoscopy arm, or 15.9% of patients converted). Excessive bleeding was cited as the reason for conversion in 49 patients (2.9% of the laparoscopy arm, or 11.3% of patients converted), and other reasons for conversion were equipment failure (n = 10) and other cause (n = 70).
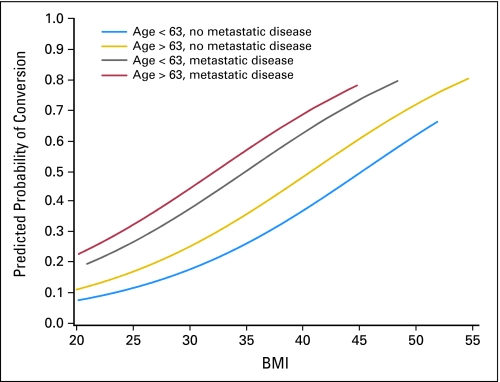
Failure to successfully complete laparoscopy was greater with increasing age (odds ratio [OR] = 1.27; 95% CI, 1.14 to 1.42 for a 10-year increase in age; P < .0001), increasing BMI (OR = 1.11; 95% CI, 1.09 to 1.13 for a one-unit increase in BMI; P < .0001), and metastatic disease (OR = 2.54; 95% CI, 1.90 to 3.41; P < .0001). Figure 2 demonstrates how the estimated risk of conversion increases with increasing BMI for four subgroups; patients with age less than the median age of 63 years and no metastatic disease form the lowest risk group, whereas patients with age greater than the median age and with metastatic disease form the highest risk group; all four subgroups demonstrated increased estimated risk with increasing BMI. After controlling for these important factors, institution size was not a significant determinant of conversion. Table 3 lists the mean and median BMI and observed conversion rates by institution size. Few participants (n = 8) required laparotomy to remove a large uterus, and only two patients were known to have morcelated fibroids to allow the uterus to be removed laparoscopically.
Fig 2.
Predicted probability curve for risk of conversion by body mass index (BMI), age, and metastatic disease.
Table 3.
BMI and Conversion Rates by Institution Enrollment
| No. of Patients Enrolled | BMI (kg/m2) |
Conversion Rate (%) | |
|---|---|---|---|
| Mean | Median | ||
| 1-50 | 29.6 | 28.1 | 27.0 |
| 51-100 | 29.8 | 28.6 | 28.3 |
| 101-150 | 30.5 | 29.7 | 23.5 |
| 151-200 | 29.1 | 27.7 | 14.9 |
| 201-250 | 29.4 | 27.9 | 25.3 |
| 251-300 | 28.7 | 27.2 | 22.5 |
| 300+ | 31.9 | 30.3 | 34.7 |
Abbreviation: BMI, body mass index.
Operative Results
The median operative time for the open laparotomy arm was 130 minutes (IQR, 102 to 167 minutes), and for the laparoscopy arm, it was 204 minutes (IQR, 160 to 252 minutes; P < .001).
Intraoperative Complications
Intraoperative complications were not statistically significantly different between the two treatment groups (8% for laparotomy v 10% for laparoscopy, P = .106; Table 4). The percentage of patients with arterial bleeding was slightly higher in the laparoscopy group than in the laparotomy group (1.8% v 0.7%, respectively). Of the 30 laparoscopy patients who reported arterial bleeding, 11 were controlled without conversion to laparotomy.
Table 4.
Complications and Adverse Events
| Complications and Adverse Events | Laparotomy |
Laparoscopy |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Intraoperative complications | |||||
| Any | 69 | 8 | 160 | 10 | .106 |
| Bowel | 16 | 2 | 37 | 2 | |
| Vein | 23 | 3 | 45 | 3 | |
| Artery | 6 | 1 | 30 | 2 | |
| Bladder | 7 | 1 | 21 | 1 | |
| Ureter | 6 | 1 | 14 | 1 | |
| Other | 13 | 1 | 26 | 2 | |
| Postoperative adverse events (grade ≥ 2) | |||||
| Any | 191 | 21 | 240 | 14 | < .001 |
| Urinary tract infection | 27 | 3 | 35 | 2 | |
| Fever | 33 | 4 | 55 | 3 | |
| Pelvic cellulitis | 8 | 1 | 14 | 1 | |
| Abscess | 6 | 1 | 17 | 1 | |
| Venous thrombophlebitis | 12 | 1 | 14 | 1 | |
| Pulmonary embolus | 12 | 1 | 20 | 1 | |
| Bowel obstruction | 12 | 1 | 14 | 1 | |
| Ileus* | 68 | 8 | 66 | 4 | |
| Pneumonia | 19 | 2 | 15 | 1 | |
| Wound infection | 33 | 4 | 53 | 3 | |
| Urinary fistula | 1 | < 1 | 6 | < 1 | |
| Bowel fistula | 2 | < 1 | 6 | < 1 | |
| Congestive heart failure | 11 | 1 | 12 | 1 | |
| Arrhythmia* | 22 | 2 | 15 | 1 | |
| Perioperative and postoperative period | |||||
| Blood transfusion | 66 | 7 | 143 | 9 | .280 |
| Antibiotics | 211 | 23 | 274 | 16 | < .001 |
| Readmission | 59 | 7 | 96 | 6 | .413 |
| Reoperation | 22 | 2 | 48 | 3 | .523 |
| Treatment-related deaths | 8 | 1 | 10 | < 1 | .404 |
| Hospital stay > 2 days | 845 | 94 | 867 | 52 | < .001 |
Significantly different at adjusted significance level of P = .005.
Postoperative Period
Complications ≥ grade 2 were more common in laparotomy patients than laparoscopy patients (21% v 14%, respectively; P < .001), even after controlling for patient age, race/ethnicity, BMI, and performance status. Ileus occurred significantly more often in laparotomy patients than laparoscopy patients (7% v 4%, respectively; P < .004), as did cardiac arrhythmia (2% v 1%, respectively). Other complications were not significantly different at the adjusted significance level of P = .005.
Postoperative intravenous antibiotic use was documented in 13% of laparotomy patients and in 8% of laparoscopy patients (P < .001). Oral antibiotic use was reported in 16% and 12% of patients assigned to laparotomy and laparoscopy, respectively (P = .003).
Readmission (7% for laparotomy v 6% for laparoscopy) and reoperation (2% for laparotomy v 3% for laparoscopy) rates were not significantly different between the study arms. There were 18 deaths in the 30-day perioperative or postoperative period in which surgery may have been a contributing factor. Perioperative deaths occurred in 10 laparoscopy patients and eight laparotomy patients (0.59% v 0.88%, respectively; P = .404) and were mainly secondary to thromboembolic events (pulmonary embolus, n = 10; complications requiring reoperation, n = 3; hemorrhage, n = 1; progressive stage IVB cancer and chemotherapy, n = 2; and infection/sepsis, n = 2).
Hospital Length of Stay
The proportion of patients requiring more than 2 days of hospitalization after surgery was significantly smaller in patients randomly assigned to laparoscopy compared with laparatomy (52% v 94%, respectively; P < .0001), even after controlling for age, race/ethnicity, BMI, and performance status. The median length of stay for laparotomy patients was 4 days (IQR, 3 to 5 days), and the median length of stay for the intent-to-treat laparoscopy arm patients was 3 days (IQR, 2 to 4 days). Patients completing laparoscopy had a median length of stay of 2 days, and patients who converted to laparotomy had a median length of stay of 4 days.
Surgical Staging
Lymph nodes were histologically documented from the pelvis in 99% of laparotomy patients and 98% of laparoscopy patients (P = .183; Table 2). In patients who underwent laparotomy and laparoscopy, para-aortic lymph nodes were documented in 97% and 94%, respectively (P = .002), and both para-aortic and pelvic lymph nodes were identified in 96% and 92%, respectively (P < .001). Peritoneal fluid or washings were examined cytologically in 98.0% of laparotomy patients and 96% of laparoscopy patients (P = .052). The proportion of participants randomly assigned to the laparotomy and laparoscopy arms found to have advanced surgical stage (FIGO stage IIIA, IIIC, or IV) was not significantly different between groups (17% v 17%, respectively; P = .851).
DISCUSSION
This prospective, multi-institutional, randomized trial has documented the feasibility and improved safety profile of laparoscopic comprehensive surgical staging for uterine cancer when compared with the same procedures undertaken via laparotomy. The patterns of recurrence and survival results will be reported when the data are mature. Laparotomy remains an option, especially in the morbidly obese, those with a large uterus, and those with metastatic disease.
Pelvic and para-aortic nodes were not removed in 8% of laparoscopy patients and 4% of laparotomy patients (P < .0001), and peritoneal cytology was not documented in a small percentage of both the laparoscopy and laparotomy groups (3.7% v 2.2%, respectively). Advanced surgical stage (stage IIIA, IIIC, or IVB) was detected in 17% of laparoscopy patients and 17% of laparotomy patients, suggesting that neither treatment arm demonstrated improved ability to detect metastatic disease (P = .841). Lymph node metastases were found in 9% of participants and were similar in both groups.
Differences in location and frequency of lymph nodes removed could be secondary to intraoperative decisions based on perceived risk of metastatic disease versus the perceived risk of operative morbidity, or other individual surgeon bias. Some surgeons could have perceived a benefit of laparoscopy, even when the full staging could not be completed.
With a seemingly high conversion rate of 25.8% (95% CI, 23.7% to 28.0%), this study supports attempting the procedure laparoscopically and converting to laparotomy when necessary. This study documents that the failure of completion of laparoscopic staging is associated with increasing BMI, metastatic disease, and increasing patient age, and in that order of importance. Conversion from laparoscopy to laparotomy occurred in 17.5% of patients with BMI of 25 kg/m2 and in 26.5% of patients with BMI of 34 to 35 kg/m2, whereas 57.1% of patients with BMI greater than 40 kg/m2 required conversion. Analysis of institutional variability was attempted by examining whether conversion rates were lower in institutions contributing large numbers of participants. However, other factors such as physician preference, conventions of individual institutions, and surgeon experience level are also likely important factors that are more difficult to quantify. The length of stay was the same for laparotomy patients and those converted to laparotomy, revealing that the risk of initiating the procedure laparoscopically was acceptable.
Assessing the comorbid conditions of patients and the risks and benefits of comprehensive surgical staging will continue to influence which operative approach the surgeon may choose. Vaginal hysterectomy alone may be appropriate in some patients.21,22 Laparoscopically assisted vaginal hysterectomy and bilateral salpingo-oophorectomy with peritoneal cytology, followed by frozen section on the uterus to determine risk factors for metastatic disease, before decision making about whether to proceed with staging, may be in the best interest of selected patients.
The data presented here can provide guidance for surgeons to better select patients most likely to require laparotomy. Early reports of laparoscopic surgical staging limited patient eligibility to a BMI of 30 kg/m2 or 180 kg.16–19 The current study also initially considered women with a BMI of greater than 35 kg/m2 to be ineligible but did not obtain information on larger patients.
Thromboembolic events are common in gynecologic cancer patients as a result of the combination of long duration of pelvic surgery, hypercoagulability from cancer, and immobilization (Virchow's triad: stasis, injury, and hypercoagulable state). Prevention of this complication continues to be an avenue for improvement in the surgical treatment of women with uterine cancer.
Experience may improve patient selection for laparoscopy and allow for early recognition of the need to perform a laparotomy. Peritoneal cytology should be obtained on access to the peritoneal cavity followed by the dissection of the para-aortic lymph nodes first to demonstrate the feasibility of completion of the laparoscopic staging. The hysterectomy and bilateral salpingo-oophorectomy can be performed last, except when the size of the uterus may be the limiting factor. Immediate laparotomy, when the completion of staging via laparoscopy is recognized as not feasible, may decrease complications, operative time, and cost.
Patients treated by laparoscopy had a superior quality of life through 6 weeks after surgery compared with patients treated by laparotomy; quality-of-life results will be reported separately.23 Successful laparoscopy, being a less invasive procedure, results in less pain, faster recovery, and a significantly reduced length of hospital stay. Endometrial cancer is an ideal cancer for minimally invasive surgery to be translated into short-term quality-of-life improvements. Robotics was not studied in this clinical trial and may decrease conversion rates in large BMI patients.
This study indicates that comprehensive surgical staging of endometrial cancer can be performed using laparoscopy without increased intraoperative injuries, with fewer postoperative complications, and with shorter hospital stay. This makes attempting laparoscopy, when assumed to be feasible, worth the extra operative time and surgeon training. Survival results will be reported when the data are mature. There remains controversy on the therapeutic value of surgical staging in uterine cancer. Gynecologic oncologists in the United States perceive a benefit from surgical resection of all metastatic cancer and tailoring postoperative treatment, with chemotherapy or radiation, to the pathologic findings.24–30
Appendix
The following Gynecologic Oncology Group (GOG) institutions participated in the GOG 2222 (LAP2): Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, University of Pennsylvania Cancer Center, University of California at San Diego, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, The University of Texas M. D. Anderson Cancer Center, University of Massachusetts Medical School, Women's Cancer Center, University of Oklahoma, Tacoma General Hospital, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Fletcher Allen Health Care, University of Wisconsin Hospital, Women and Infants Hospital, and Community Clinical Oncology Program (CCOP).
Footnotes
Supported by National Cancer Institute Grant No. CA 27469 to the Gynecologic Oncology Group (GOG) Administrative Office and Grant No. CA 37517 to the GOG Statistical and Data Center.
Presented in part at the 11th Biennial Meeting of the International Gynecologic Cancer Society, October 14-18, 2006, Santa Monica, CA; the 37th Annual Meeting of the Society of Gynecologic Oncologists, March 22-26, 2006, Palm Springs, CA; and the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joan L. Walker, Nick M. Spirtos
Administrative support: Joan L. Walker
Provision of study materials or patients: Joan L. Walker, Nick M. Spirtos, Richard Barakat, Michael L. Pearl, Sudarshan K. Sharma
Collection and assembly of data: Joan L. Walker, Nick M. Spirtos, Richard Barakat, Michael L. Pearl
Data analysis and interpretation: Joan L. Walker, Marion R. Piedmonte, Nick M. Spirtos, Scott M. Eisenkop, John B. Schlaerth, Robert S. Mannel, Gregory Spiegel, Michael L. Pearl
Manuscript writing: Joan L. Walker, Marion R. Piedmonte, Nick M. Spirtos, Scott M. Eisenkop, Robert S. Mannel, Gregory Spiegel, Michael L. Pearl
Final approval of manuscript: Joan L. Walker, Marion R. Piedmonte, Scott M. Eisenkop, John B. Schlaerth, Robert S. Mannel, Gregory Spiegel, Richard Barakat, Michael L. Pearl, Sudarshan K. Sharma
REFERENCES
- 1.American Cancer Society. Cancer facts and figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Morrow CP, Bundy BN, Kurman FJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 3.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. Int J Gynaecol Obstet. 2003;83(suppl 1):79–118. doi: 10.1016/s0020-7292(03)90116-0. [DOI] [PubMed] [Google Scholar]
- 5.American Joint Committee on Cancer. Cancer Staging Manual. ed 6. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 6.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- 8.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 9.Straughn JM, Huh WK, Orr JW, Jr, et al. Stage IC adenocarcinoma of the endometrium: Survival comparisons of surgically staged patients with and without adjuvant radiation therapy. Gynecol Oncol. 2003;89:295–300. doi: 10.1016/s0090-8258(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 10.Fanning J, Hoffman ML, Andrews SJ, et al. Cost-effectiveness analysis of the treatment for intermediate risk endometrial cancer: Postoperative brachytherapy vs. observation. Gynecol Oncol. 2004;93:632–636. doi: 10.1016/j.ygyno.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 12.Bristow RE, Zerbe MJ, Rosenshein NB, et al. Stage IVB endometrial carcinoma: The role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85–91. doi: 10.1006/gyno.2000.5843. [DOI] [PubMed] [Google Scholar]
- 13.Awtrey CS, Cadungog MG, Leitao MM, et al. Surgical resection of recurrent endometrial carcinoma. Gynecol Oncol. 2006;102:480–488. doi: 10.1016/j.ygyno.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Lambrou NC, Gomez-Marin O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with stage III and stage IV endometrial carcinoma: A study of morbidity and survival. Gynecol Oncol. 2004;93:653–658. doi: 10.1016/j.ygyno.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Thomas MB, Mariani A, Cliby WA, et al. Role of cytoreduction in stage III and IV uterine papillary serous carcinoma. Gynecol Oncol. 2007;107:190–193. doi: 10.1016/j.ygyno.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Dargent D. Laparoscopic surgery and gynecologic cancer. Curr Opin Obstet Gynecol. 1993;5:294–300. [PubMed] [Google Scholar]
- 17.Querleu D, Leblanc E, Castelain B. Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol. 1991;164:579–581. doi: 10.1016/s0002-9378(11)80025-6. [DOI] [PubMed] [Google Scholar]
- 18.Childers JM, Spirtos NM, Brainard P, et al. Laparoscopic staging of the patient with incompletely staged early adenocarcinoma of the endometrium. Obstet Gynecol. 1994;83:597–600. doi: 10.1097/00006250-199404000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Spirtos NM, Schlaerth JB, Spirtos TW, et al. Laparoscopic bilateral pelvic and paraaortic lymph node sampling: An evolving technique. Am J Obstet Gynecol. 1995;173:105–111. doi: 10.1016/0002-9378(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 19a.Gynecologic Oncology Group. Guidelines for Laparoscopic Pelvic and Aortic Lymph Node Sampling. https://gogmember.gog.org/manuals/pdf/surgman.pdf.
- 20.National Cancer Institute. Common Toxicity Criteria version 2.0. http://ctep.cancer.gov/protocol Development/electronic_applications/docs/ctcv20_4-30-992.pdf. [PubMed]
- 21.Chan JK, Lin YG, Monk BJ, et al. Vaginal hysterectomy as primary treatment of endometrial cancer in medically compromised women. Obstet Gynecol. 2001;97:707–711. [PubMed] [Google Scholar]
- 22.Bloss JD, Berman ML, Bloss LP, et al. Use of vaginal hysterectomy for the management of stage I endometrial cancer in the medically compromised patient. Gynecol Oncol. 1991;40:74–77. doi: 10.1016/0090-8258(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 23.Kornblith AB, Huang HQ, Walker JL, et al. Quality of life of patients with endometrial cancer undergoing laparoscopic staging compared to laparotomy: A Gynecologic Oncology Group study. J Clin Oncol. doi: 10.1200/JCO.2009.22.3529. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creutzberg CL. Lymphadenectomy in apparent early-stage endometrial carcinoma: Do numbers count? J Clin Oncol. 2005;23:3653–3655. doi: 10.1200/JCO.2005.11.947. [DOI] [PubMed] [Google Scholar]
- 25.Partridge EE, Shingleton HM, Menck HR. The National Cancer Data Base report on endometrial cancer. J Surg Oncol. 1996;61:111–123. doi: 10.1002/(SICI)1096-9098(199602)61:2<111::AID-JSO5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Maggino T, Romagnolo C, Landoni F, et al. An analysis of approaches to the management of endometrial cancer in North America: A CTF study. Gynecol Oncol. 1998;68:274–279. doi: 10.1006/gyno.1998.4951. [DOI] [PubMed] [Google Scholar]
- 27.Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomized study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 29.Kilgore LC, Partridge EE, Alvarez RD, et al. Adenocarcinoma of the endometrium: Survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 30.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]