Abstract
Purpose
Children with relapsed neuroblastoma have poor survival. It is crucial to have a reliable method for evaluating functional response to new therapies. In this study, we compared two functional imaging modalities for neuroblastoma: metaiodobenzylguanidine (MIBG) scan for uptake by the norepinephrine transporter and [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) uptake for glucose metabolic activity.
Patients and Methods
Patients enrolled onto a phase I study of sequential infusion of iodine-131 (131I) MIBG (NANT-2000-01) were eligible for inclusion if they had concomitant FDG-PET and MIBG scans. 131I-MIBG therapy was administered on days 0 and 14. For each patient, we compared all lesions identified on concomitant FDG-PET and MIBG scans and gave scans a semiquantitative score.
Results
The overall concordance of positive lesions on concomitant MIBG and FDG-PET scans was 39.6% when examining the 139 unique anatomic lesions. MIBG imaging was significantly more sensitive than FDG-PET overall and for the detection of bone lesions (P < .001). There was a trend for increased sensitivity of FDG-PET for detection of soft tissue lesions. Both modalities showed similar improvement in number of lesions identified from day 0 to day 56 scan and in semiquantitative scores that correlated with overall response. FDG-PET scans became completely negative more often than MIBG scans after treatment.
Conclusion
MIBG scan is significantly more sensitive for individual lesion detection in relapsed neuroblastoma than FDG-PET, though FDG-PET can sometimes play a complementary role, particularly in soft tissue lesions. Complete response by FDG-PET metabolic evaluation did not always correlate with complete response by MIBG uptake.
INTRODUCTION
Neuroblastoma, an embryonal tumor of children that is derived from the peripheral sympathetic nervous system, is frequently metastatic at diagnosis, with long-term survival of less than 40%.1 As new treatment options are tested, it is essential to reliably assess disease response as a measure of therapeutic activity.
Metaiodobenzylguanidine (MIBG) is a guanethidine derivative that is specifically taken up by the norepinephrine transporter (NET) on neuroblastoma cells and provides a highly sensitive and specific agent for imaging when labeled with iodine-123 (123I) and for therapy when labeled with iodine-131 (131I).2 MIBG scintigraphy is well established as an imaging tool for initial diagnosis and staging, as it is concentrated in 90% of neuroblastoma.3,4 However, there is a dearth of data on how and when to use and interpret other complementary imaging studies, such as magnetic resonance imaging (MRI) or positron emission tomography using [18F]fluorodeoxyglucose (FDG-PET).5 FDG-PET is an imaging technique in which an intravenous glucose analog (FDG), labeled with a positron-emitting isotope, fluorine-18, is taken up by cells according to their glucose metabolism. Cells with high metabolism and rapid proliferation can easily be detected by PET because of their high FDG uptake. FDG-PET may visualize neuroblastoma tumors that do not express high levels of the NET and are therefore negative by MIBG.6
Quantization of both MIBG and PET has not been standardized. Semiquantitative scoring systems have been tested for MIBG, which are correlated with response and, in some cases, event-free survival.7,8 To document change on PET scans, a standardized uptake value is measured for each lesion that can be observed over time to determine response to treatment, but no systematic definition of response has been validated for this modality.
We report here the comparison of overall disease response and response by 123I-MIBG and FDG-PET in patients treated on a single study (N2000-01) with rapid-sequence double infusion of 131I-MIBG. We also compared lesions identified on concomitant MIBG and FDG-PET scans for concordance overall, in bone, and in soft tissue.
PATIENTS AND METHODS
Patients and Therapy
Eligible patients had poorly responsive or progressive high-risk neuroblastoma and at least one MIBG-positive lesion. Patients were 1 to 30 years of age at the time of enrollment and had normal organ function. Patients were treated in this dose-escalation study (New Approaches to Neuroblastoma Therapy [NANT] –2000-01) with 131I-MIBG on days 0 and 14 and received autologous peripheral-blood stem-cell infusion on day 28. Disease evaluation was performed on day 56. 123I-MIBG scans were done prior to therapy and on day 56; FDG-PET scans were required pretherapy and were repeated on days 13 and 56 if initially positive. The pretherapy scans were performed less than 6 weeks before study entry and more than 2 weeks after any antitumor therapy. All scans were done according to institutional radiology guidelines. Details of the therapy administration, supportive care, and outcome were previously reported.9 The protocol was sponsored by the NANT consortium and was approved by the United States Food and Drug Administration. The study enrolled 21 patients from 2001 to 2005. Patients with at least one concomitant MIBG and FDG-PET scan available for review in the appropriate format were eligible for this report. Of the 23 FDG-PET scans with concomitant MIBG scans reviewed, 19 scans were FDG-PET and four scans were FDG-PET/computed tomography (CT). The study was approved by NANT institutional review boards, and informed consent was obtained for all patients. Participating NANT investigators and institutions are listed in the Appendix (online only).
Scan Review and Evaluation
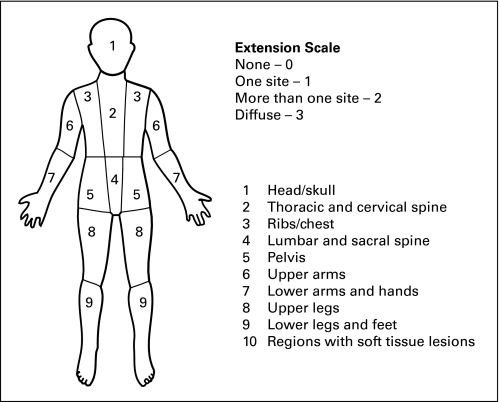
FDG-PET and MIBG scans were retrospectively reviewed by at least two nuclear medicine physicians. Data regarding anatomic location and type of lesion (osteomedullary or soft tissue) were recorded. FDG-PET– and MIBG-positive lesions were each mapped onto anterior-view body images (Appendix Fig A1, online only). Time-matched FDG-PET and MIBG images were viewed simultaneously. Initial radiology reports were reviewed when clarification was needed. Because these studies were completed at multiple large children's cancer centers over a 4-year time period, there was variability in the body views available. All that were scored had sufficient data.
We analyzed concordance and nonconcordance of lesions, before and after MIBG therapy, by the two modalities for all anatomically distinct locations, as well as separately for osteomedullary and soft tissue lesions. The definition of concordance was a single anatomic lesion identified by both imaging modalities (MIBG and FDG-PET) at one time point, whereas a nonconcordant lesion was identified by one of the two modalities, but not the other at a single time point. The total number of lesions in a patient was defined as the sum of all anatomically different lesions seen by either modality both prior to and after therapy.
Overall disease response was determined by central review using NANT response criteria, a modification of the International Neuroblastoma Response Criteria. This included Response Evaluation Criteria in Solid Tumors scores for CT scans with solid lesions, semiquantitative MIBG scores using the Curie method,7,10 and bone marrow, which had to become tumor free to qualify as response.9
Statistics
The lesions identified on FDG-PET and MIBG scans at study enrollment and at day 56 were compared. The concordance rate was calculated as the number of lesions seen by both modalities divided by the number of lesions seen by at least one of the imaging methods. McNemar's test, based on the nonconcordant lesions (ie, FDG-PET positive and MIBG negative v FDG-PET negative and MIBG positive) was used to formally compare the two modalities at study enrollment, at day 56, and overall. All reported P values are based on McNemar's test unless otherwise described and are two-sided.
RESULTS
Twenty-one patients were enrolled onto the NANT 2000-01 trial, of whom 14 patients had both FDG-PET and MIBG scans and were eligible for review (Appendix Table A1, online only). This analysis yielded 23 paired studies, with 13 pairs at study enrollment and 10 pairs at day 56. Eight patients also had the day 13 FDG-PET scan. Nine patients had concomitant MIBG and FDG-PET scans completed and available for review both at study enrollment and on day 56 and thus were included in our analysis of lesion changes by modality after therapeutic MIBG treatment.
All Lesions
We identified a total of 139 distinct lesions by adding all lesions on pre- and post-therapy scans. There was a 39.6% concordance rate for all lesions combined, including both bone and soft tissue lesions (Table 1). Nonconcordant lesions that were MIBG positive/FDG-PET negative (71 [51.1%] of 139 lesions) were more frequent than nonconcordant lesions that were MIBG negative/FDG-PET positive (13 [9.4%] of 139 lesions), with P < .001 (Table 2; Fig 1). In the pre–MIBG therapy images, 88 lesions were identified on the two modalities, with an overall concordance rate of 43.2%, as compared with post–MIBG therapy, with 51 total lesions and a concordance rate of 33.3% (P = .284). Pretherapy, nonconcordant MIBG-positive/FDG-PET–negative lesions comprised 39 (44.3%) of 88 lesions, whereas MIBG-negative/FDG-PET–positive lesions comprised 11 (12.5%) of 88 lesions (P < .001). Post-therapy, nonconcordant MIBG-positive/FDG-PET–negative lesions were more frequent (32 [62.7%] of 51 lesions), and nonconcordant MIBG-negative/FDG-PET–positive lesions were quite rare (two [3.9%] of 51 lesions; P < .001). Therefore, for all lesions combined, MIBG imaging was significantly more sensitive in detecting disease than FDG-PET imaging for both pre- and post-therapy scans. Because most patients on this trial had a preponderance of bone lesions, we sought to compare whether the greater MIBG sensitivity reflected its performance in bone lesions or whether it was equally superior in soft tissue.
Table 1.
Concordance of 139 Individual Total Lesions on MIBG and FDG-PET
| Site of Lesion | Patient ID | MIBG Pretherapy | PET Pretherapy | Con Pretherapy | MIBG Post-Therapy | PET Post-Therapy | Con Post-Therapy | MIBG Total | PET Total | Con Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Bone | 5 | 5 | 3 | 0.60 | 3 | 0 | 0 | 8 | 3 | 0.38 |
| 6 | 2 | 0 | 0 | — | — | — | 2 | 0 | 0 | |
| 7 | 7 | 6 | 0.86 | 4 | 3 | 0.75 | 11 | 9 | 0.82 | |
| 8 | 12 | 9 | 0.75 | 9 | 7 | 0.45 | 21 | 16 | 0.61 | |
| 9 | 4 | 1 | 0.25 | 1 | 0 | 0 | 5 | 1 | 0.20 | |
| 10 | 4 | 8 | 0.50 | — | — | — | 4 | 8 | 0.50 | |
| 11 | 1 | 1 | 1 | 2 | 1 | 0.50 | 3 | 2 | 0.67 | |
| 12 | 4 | 0 | 0 | 0 | 0 | — | 4 | 0 | 0 | |
| 13 | 10 | 8 | 0.80 | 8 | 4 | 0.50 | 18 | 12 | 0.67 | |
| 14 | — | — | — | 8 | 0 | 0 | 8 | 0 | 0 | |
| 15 | 10 | 0 | 0 | — | — | — | 10 | 0 | 0 | |
| 16 | 4 | 1 | 0 | 9 | 0 | 0 | 13 | 1 | 0 | |
| 19 | 8 | 1 | 0.13 | 0 | 0 | — | 8 | 1 | 0.13 | |
| Total lesions | 71 | 38 | 33/76 | 44 | 15 | 13/46 | 115 | 53 | 46/122 | |
| 43.4% | 28.3% | 37.7% | ||||||||
| Soft tissue | 4 | 2 | 1 | 0.50 | — | — | — | 2 | 1 | 0.50 |
| 8 | 1 | 3 | 0.33 | 2 | 1 | 0.50 | 3 | 4 | 0.40 | |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | |
| 10 | 1 | 3 | 0.33 | — | 0 | — | 1 | 3 | 0.33 | |
| 11 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0.50 | |
| 16 | 1 | 2 | 0.50 | 1 | 1 | 1 | 2 | 3 | 0.67 | |
| Total lesions | 6 | 11 | 5/12 | 5 | 4 | 4/5 | 11 | 15 | 9/17 | |
| 41.7% | 80% | 52.9% | ||||||||
| All lesions | 4 | 2 | 1 | 0.50 | — | — | — | 2 | 1 | 0.50 |
| 5 | 5 | 3 | 0.60 | 3 | 0 | 0 | 8 | 3 | 0.38 | |
| 6 | 2 | 0 | 0 | — | — | — | 2 | 0 | 0 | |
| 7 | 7 | 6 | 0.86 | 4 | 3 | 0.75 | 11 | 9 | 0.82 | |
| 8 | 13 | 12 | 0.67 | 11 | 8 | 0.46 | 24 | 20 | 0.57 | |
| 9 | 5 | 2 | 0.40 | 2 | 1 | 0.50 | 7 | 3 | 0.43 | |
| 10 | 5 | 11 | 0.45 | — | — | — | 5 | 11 | 0.45 | |
| 11 | 1 | 2 | 0.50 | 3 | 2 | 0.67 | 4 | 4 | 0.60 | |
| 12 | 4 | 0 | 0 | 0 | 0 | — | 4 | 0 | 0 | |
| 13 | 10 | 8 | 0.80 | 8 | 4 | 0.50 | 18 | 12 | 0.67 | |
| 14 | — | — | — | 8 | 0 | 0 | 8 | 0 | 0 | |
| 15 | 10 | 0 | 0 | — | — | — | 10 | 0 | 0 | |
| 16 | 5 | 3 | 0.14 | 10 | 1 | 0.10 | 15 | 4 | 0.12 | |
| 19 | 8 | 1 | 0.13 | 0 | 0 | — | 8 | 1 | 0.13 | |
| Total lesions | 77 | 49 | 38/88 | 49 | 19 | 17/51 | 126 | 68 | 55/139 | |
| 43.2% | 33.3% | 39.6% |
NOTE. Only patients with bone or soft tissue lesions are included in that respective section of the table. Total lesions indicates the number of distinct anatomical lesions seen by a single modality or by the combined modalities.
Abbreviations: MIBG, metaiodobenzylguanidine; FDG-PET, [18F]fluorodeoxyglucose positron emission tomography; Con, concordance, which indicates the fraction of anatomically distinct lesions identified on both types of scan.
Table 2.
Nonconcordant Lesions on MIBG and FDG-PET Imaging
| Site of Lesion | Pretherapy |
Post-Therapy |
Overall |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIBG | PET | MIBG Positive/PET Negative | MIBG Negative/PET Positive | P | MIBG | PET | MIBG Positive/PET Negative | MIBG Negative/PET Positive | P | MIBG | PET | MIBG Positive/PET Negative | MIBG Negative/PET Positive | P | |
| Bone | < .001 | < .001 | < .001 | ||||||||||||
| No. | 71 | 38 | 38 | 5 | 44 | 15 | 31 | 2 | 115 | 53 | 69 | 7 | |||
| % | 50 | 6.6 | 67.4 | 4.3 | 56.6 | 5.8 | |||||||||
| Soft tissue | .059 | 0 | .16 | ||||||||||||
| No. | 6 | 11 | 1 | 6 | 5 | 4 | 1 | — | 11 | 15 | 2 | 6 | |||
| % | 8.3 | 50 | 20 | 11.8 | 35.3 | ||||||||||
| All lesions | < .001 | < .001 | < .001 | ||||||||||||
| No. | 77 | 49 | 39 | 11 | 49 | 19 | 32 | 2 | 126 | 68 | 71 | 13 | |||
| % | 44.3 | 12.5 | 62.7 | 3.9 | 51.1 | 9.4 | |||||||||
NOTE. P value compares the proportion of nonconcordant MIBG-positive/FDG-PET–negative lesions with the proportion of nonconcordant MIBG-negative/FDG-PET–positive lesions.
Abbreviations: MIBG, metaiodobenzylguanidine; FDG-PET, [18F]fluorodeoxyglucose positron emission tomography.
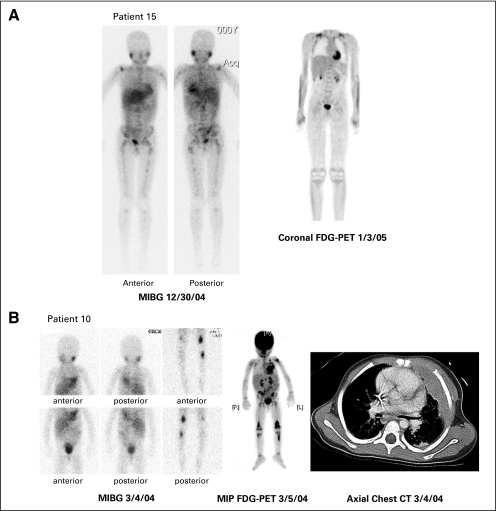
Fig 1.
Comparison of concomitant iodine-123 (123I) metaiodobenzylguanidine (MIBG) and [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) scans. (A) Patient with extensive MIBG uptake but no FDG-PET uptake. Ten lesions were identified on pretherapy MIBG scan: thoracic and lumbar spine, right and left proximal humerus, right shoulder, right and left pelvis, right and left proximal femur, and right and left tibia. Physiologic uptake is seen in the myocardium, liver, bladder, and salivary glands. No lesions were identified on pretherapy FDG-PET. Physiologic uptake was seen in the myocardium, brown fat in supraclavicular regions bilaterally, kidneys, bladder, and bone marrow within the spine. (B) Patient with partially concordant 123I-MIBG and FDG-PET scans. Five of 11 lesions were concordant on pretherapy maximum intensity projection (MIP) FDG-PET and MIBG: left chest soft tissue, left chest wall bone, left proximal tibia, and right and left distal femur. Six of 11 lesions were pretherapy FDG-PET positive only: left humerus, left supraclavicular soft tissue, right supraclavicular soft tissue, lower thoracic, left sacroiliac, and right proximal tibia. On the MIBG scan, normal uptake is visualized in the myocardium, salivary glands, liver, and bladder. The intense uptake in the left chest wall lesions impedes visualization of the normal myocardial uptake. The FDG-PET scan shows normal uptake in the myocardium, brain, kidneys, and bladder. The right proximal tibia shows the least FDG uptake on FDG-PET and is not visualized by MIBG. Soft tissue versus bone was difficult to discern so additional information from computed tomography (CT) was used. The chest CT shows the left chest soft tissue and bone lesions.
Bone
A total of 122 bone lesions were identified with a concordance rate of 37.7% (Table 1). Similar to the total lesion analysis, the rate of lesions that were nonconcordant MIBG positive/FDG-PET negative (69 [56.6%] of 122 lesions) exceeded that of lesions that were nonconcordant MIBG negative/FDG-PET positive (seven [5.8%] of 122; P < .001). This remained true when cranial lesions were excluded: MIBG positive/FDG-PET negative, 58 of 108 lesions, and MIBG negative/FDG-PET positive, seven of 108 lesions (P < .001). The pre–MIBG therapy scan review identified 76 unique bone lesions with a concordance rate of 43.4%. Nonconcordant MIBG-positive/FDG-PET–negative lesions comprised 38 (50%) of 76 lesions, whereas MIBG-negative/FDG-PET–positive lesions comprised five (6.6%) of 76 lesions (P < .001; Table 2). The post-therapy scan review identified 46 bone lesions with a concordance rate of 28.3%. Again, the nonconcordant MIBG-positive/FDG-PET–negative lesions were far more frequent than the nonconcordant MIBG-negative/FDG-PET –positive lesions (31 [67.4%] of 46 lesions v two [4.3%] of 46 lesions; P < .001). Of the 122 distinct lesions identified, 14 (11%) were in cranial bones. Of these 14 cranial lesions, three lesions were also viewed on FDG-PET. In sum, for bone lesions alone, MIBG imaging was significantly more sensitive for the detection of disease than FDG-PET imaging, either in total or with exclusion of cranial lesions.
Soft Tissue
A total of 17 soft tissue lesions were documented with a concordance rate of 52.9% (Table 1). Unlike the overall lesion and bone lesion analysis, the soft tissue lesion analysis found that the nonconcordant MIBG-positive/FDG-PET–negative lesions (two [11.8%] of 17 lesions) were less frequent than the nonconcordant MIBG-negative/FDG-PET–positive lesions (six [35.3%] of 17 lesions), though the difference was not significant (P = .16; Table 2). The six MIBG-negative/FDG-PET –positive lesions were lymph nodes not clearly identified as enlarged on CT imaging. Analysis of the limited number of soft tissue lesions on pretherapy scans revealed 12 lesions with a concordance rate of 41.7%. The nonconcordant MIBG-positive/FDG-PET–negative lesions (one [8.3%] of 12 lesions) were less frequent than the nonconcordant MIBG-negative/FDG-PET–positive lesions (six [50%] of 12 lesions; P = .059). There were only five soft tissue lesions detected on the post-therapy imaging studies with 80% concordance. The one lesion that was not concordant was MIBG positive/FDG-PET negative. For soft tissue lesions, MIBG only detected two (25%) of the eight discordant lesions, whereas for bone lesions, MIBG detected 69 (91%) of the 76 discordant lesions (P < .001 using Fisher's exact test). These data suggest that FDG-PET may be more sensitive than MIBG for the detection of soft tissue lesions.
Response
Nine patients with concomitant FDG-PET and MIBG scans both pre– and post–MIBG therapy were evaluated for response by each modality (Table 3). Four patients were alive with disease at the time of last follow-up, with a median time to follow-up of 19.7 months. Of the nine patients, two had resolution of all lesions on FDG-PET and a decrease in number of lesions on MIBG. Three patients showed a decrease in number of lesions by both FDG-PET and MIBG. One patient had an increase in number of lesions on MIBG but no change on FDG-PET, one patient had a decrease in number of lesions on FDG-PET and no change on MIBG, and one patient had an increase in number of lesions on MIBG and decrease in lesions on FDG-PET. One patient was not assessable because pretherapy FDG-PET was negative. Overall, seven of eight patients with FDG-PET positivity prior to therapy showed a decrease in the number of FDG-PET–positive lesions after therapy, although response to treatment as seen by FDG-PET imaging was not a variable in determining overall disease response on this study. Five of these patients with improvement on FDG-PET imaging also had evidence of overall objective disease response (one partial response, four mixed responses [MRs]) using International Neuroblastoma Response Criteria. Two patients with improvement by FDG-PET did not demonstrate overall objective disease response (one patient with stable disease, one patient with progressive disease). Six of nine patients showed a decreased semiquantitative MIBG extension score after therapy. Of these, five had an overall disease response of partial response or MR. As seen in Table 3, patients were classified as achieving MR or stable disease with improvement of disease as assessed by MIBG imaging because of persistent bone marrow disease (n = 3) or lack of CT response by Response Evaluation Criteria in Solid Tumors (n = 2). One additional patient had negative FDG-PET scans both before and after therapy, but improved by MIBG. Overall, five of the eight patients evaluated for FDG-PET had a concordant improvement in both FDG-PET and MIBG scans after therapy.
Table 3.
Disease Response by MIBG Semiquantitative Score and Number of FDG-PET Lesions
| Patient ID | MIBG Extension Score Pretherapy | MIBG Extension Score Post-Therapy | Relative Score* | No. of FDG-PET Lesions Detected Pretherapy | No. of FDG-PET Lesions Detected Post-Therapy | FDG-PET Ratio† | BM Response | CT Response | Overall Response | Survival |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Outcome | ||||||||||
| 5 | 7 | 3 | 0.4 | 3 | 0 | 0 | — | — | PR | 437 | Dead |
| 7 | 12 | 12 | 1 | 6 | 3 | 0.5 | CR | — | MR | 662 | Dead |
| 8 | 23 | 17 | 0.7 | 12 | 8 | 0.7 | CR‡ | PR | MR | 166 | Dead |
| 9 | 12 | 4 | 0.3 | 2 | 1 | 0.5 | SD | SD | MR | 559 | Alive |
| 11 | 2 | 6 | 3 | 2 | 2 | 1 | — | — | PD | 552 | Dead |
| 12 | 4 | 0 | 0 | 0 | 0 | — | SD | SD | MR | 902 | Alive |
| 13 | 13 | 10 | 0.8 | 8 | 4 | 0.5 | SD | — | SD | 195 | Alive |
| 16 | 8 | 16 | 2 | 3 | 1 | 0.3 | SD | PR | PD | 91 | Dead |
| 19 | 16 | 5 | 0.3 | 1 | 0 | 0 | SD | — | MR | 640 | Alive |
NOTE. FDG-PET was not used in the determination of overall response.
Abbreviations: MIBG, metaiodobenzylguanidine; FDG-PET, [18F]fluorodeoxyglucose positron emission tomography; BM, bone marrow; CT, computed tomography; PR, partial response; CR, complete response; MR, mixed response; SD, stable disease; PD, progressive disease.
MIBG post-therapy extension score/MIBG pretherapy extension score.
Number of FDG-PET lesions post-therapy/number of FDG-PET lesions pretherapy.
BM went from neuroblastoma to all ganglioneuroma.
In comparing the day 13 FDG-PET and on-study FDG-PET, three of seven patients showed improvement by FDG-PET after the first MIBG treatment. An additional two of five patients showed further improvement from day 13 to day 56 (Table 4).
Table 4.
Number of Lesions Identified by Sequential FDG-PET Analysis
| Patient | Day 0 | Day 13 | Day 56 |
|---|---|---|---|
| 4 | 1 | 1 | — |
| 5 | 3 | 0 | 0 |
| 8 | 12 | 13 | 8 |
| 9 | 2 | 1 | 1 |
| 10 | 11 | 11 | — |
| 11 | 2 | 2 | 1 |
| 19 | 1 | 0 | 0 |
Abbreviation: FDG-PET, [18F]fluorodeoxyglucose positron emission tomography.
DISCUSSION
This analysis investigated the role of FDG-PET metabolic imaging and MIBG imaging in staging and response assessment in neuroblastoma. The nonconcordance found with both MIBG-positive/FDG-PET–negative lesions and MIBG-negative/FDG-PET–positive lesions demonstrates that MIBG is more sensitive overall and for the detection of bone disease, but both imaging modalities have the potential to identify lesions not visualized on the other scan, contributing unique information about disease sites.
Current imaging recommendations to stage neuroblastoma are based on the International Neuroblastoma Staging System first developed in 1988 and revised in 1993.10 The International Neuroblastoma Risk Group is in the process of proposing new modifications to this system.11 The most recent published imaging recommendations for assessment of disease extent are CT and/or MRI scan of the primary tumor site and to assess for spread to the neck, thorax, abdomen, or pelvis. MIBG scan is the preferred method for evaluation of metastatic disease, but if unavailable or negative, a technetium-99m bone scan should be completed.12 Imaging to assess disease response usually includes CT or MRI and MIBG if lesions were previously MIBG positive. Several studies have shown the important role that MIBG imaging plays in assessing disease response.7,13,14 FDG-PET imaging may be completed at the discretion of the treating physician but is not a formally recommended imaging modality in neuroblastoma.
False-negative MIBG imaging can occur in tumors with low expression of the NET, in CNS metastases, or in small tumors in bone marrow.15,16 False-positive results of MIBG imaging can occur due to physiologic uptake within remaining adrenal tissue, bowel, brown fat, salivary glands, myocardium, intestines, and thyroid.17,18 Fully mature neuroblastoma (ganglioneuroma) will also concentrate MIBG in approximately 20% of cases.19 Several pilot studies have demonstrated that FDG uptake in neuroblastoma can be used for detection of primary and metastatic disease.6,20 Additionally, FDG-PET can be useful in staging and monitoring treatment response in patients with MIBG-negative tumors.21
One advantage of FDG-PET is the detailed information about both the anatomic location of the tumor through high-quality three-dimensional images and the metabolic activity of the tumor. A limiting factor is the normal high physiologic uptake of FDG in the brain, making FDG-PET less effective for imaging cranial vault lesions. Bone marrow may also demonstrate increased uptake due to bone marrow hyperplasia after myelosuppressive chemotherapy. Normal uptake is also seen in the tonsils, salivary glands, liver, spleen, myocardium, adenoids, brown fat, epiphyseal cartilage in children, ovaries, and in areas of active muscle contractility, including the vocal cords.22
In a retrospective analysis, Kushner et al20 compared 92 concomitant images in 51 patients with high-risk neuroblastoma in varying phases of treatment and with heterogeneous disease status, finding an overall concordance for presence of disease of 50%. Our study analyzed individual lesion concordance in a homogeneous relapse population and is therefore not directly comparable, but we found a lower concordance rate for individual lesions of 39.6%. Kushner et al also found that FDG-PET imaging showed more sites of soft tissue disease than MIBG in 16 of 36 studies, supporting the results of our study. For bone lesions, FDG-PET more effectively detected disease in extracranial sites, unlike our results, which demonstrate MIBG to be superior to FDG-PET for the detection of all osteomedullary disease. The Kushner et al report suggests that PET, along with bone marrow testing, is sufficient for extracranial disease detection, whereas our data suggest that MIBG is usually more sensitive for disease detection, except in MIBG-negative patients or some soft tissue lesions. The greater sensitivity of MIBG seen in our study may be attributable to the differences in the patient population. The current study included only patients who demonstrated positive MIBG uptake and was comprised of patients who experienced relapse, in whom soft tissue involvement would be expected to be lower and bone and bone marrow disease more prevalent.23
MIBG was more sensitive than FDG-PET overall and for bone lesions for both pre- and post-therapy imaging. FDG-PET seemed to have greater sensitivity for soft tissue lesions, but the relative rarity of soft tissue lesions in this population precluded valid comparison. The study was limited by the inability to confirm active neuroblastoma by biopsy in nonconcordant lesions. Additionally, because the images reviewed were collected from a multicenter study, there was variation in the type of image available for review. Some MIBG scans only had snapshot imaging available for review, whereas others had full-body images and single-photon emission CT. Future prospective studies should use prescribed imaging protocols and electronic transmission of data to minimize scan variability.
The day 13 FDG-PET images show a similar percentage of patients had improvement between study enrollment and day 13 (three of seven patients) and between days 13 and 56 (two of five patients). One patient had a single additional lesion identified on day 13 from study enrollment. This patient then had resolution of five lesions between days 13 and 56. These data suggest that the metabolically active lesions vary in the timing of their response to 131I-MIBG.
Future directions for investigation include determination of the prognostic implications of residual MIBG positivity. In cases where FDG-PET has become negative but MIBG is positive, biopsy might confirm whether the tumor had matured or was only temporarily “stunned” for metabolic activity. Neither FDG-PET nor MIBG scans are sufficiently sensitive to detect small amounts of bone marrow disease, as shown in this study and previously.20,24 Therefore, bilateral bone marrow biopsies continue to be an essential component of disease evaluation. Larger prospective studies of FDG-PET at diagnosis, during, and after therapy correlated with survival would help to determine the relative significance of these modalities.
Our study suggests that for patients with MIBG-positive relapsed neuroblastoma, MIBG is more sensitive than FDG-PET for disease detection and response evaluation. However, given the fact that FDG-PET can identify disease in MIBG-negative lesions, it may serve as a complementary imaging modality in selected patients. Our study demonstrated examples of FDG-PET detection of soft tissue disease not seen on MIBG imaging, even in patients who had other MIBG-positive lesions. FDG-PET indication of response was seen in all assessable patients who showed disease response by MIBG and in two additional patients who had stable and progressive disease by MIBG. The implications of the additional information provided by FDG-PET for disease staging and response evaluation in newly diagnosed patients have not yet been shown. The overall higher sensitivity of MIBG imaging supports continued preferential use of MIBG scans as surveillance imaging for patients not receiving therapy as well as for response evaluation during therapy for relapse, because bone and bone marrow are the most frequent sites of progression.23
Appendix
Fig A1.
Body image used for scoring.
Table A1.
Patient Characteristics
| Patient ID | Bone Sites | BM Sites | Soft Tissue | Age (years) | Prior BMT | Prior RT | Total MIBG (mCi) | Total (mCi/kg) | MIBG Scan, Day 0 or 56 | FDG-PET, Any |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | Yes | 8.8 | Yes | Yes | 516 | 23.5 | Yes | No |
| 2 | Yes | No | Yes | 3.5 | Yes | Yes | 325 | 25 | Yes | No |
| 3 | Yes | No | Yes | 8.3 | Yes | Yes | 521 | 21.7 | Yes | No |
| 4 | Yes | No | Yes | 6.5 | No | Yes | 655 | 31.2 | Yes | Yes |
| 5 | Yes | No | No | 10.6 | Yes | Yes | 953 | 30.7 | Yes | Yes |
| 6 | Yes | Yes | No | 6.5 | No | Unknown | 759 | 34.5 | Yes | Yes |
| 7 | Yes | Yes | No | 13.5 | Yes | Yes | 976 | 31.5 | Yes | Yes |
| 8 | Yes | Yes | Yes | 8.2 | No | Yes | 689 | 31.3 | Yes | Yes |
| 9 | Yes | Yes | Yes | 8.1 | No | No | 1,280 | 49.2 | Yes | Yes |
| 10 | Yes | Yes | Yes | 2.8 | No | Yes | 423 | 35.3 | Yes | Yes |
| 11 | Yes | No | Not measurable | 4.9 | No | No | 685 | 40.3 | Yes | Yes |
| 12 | Yes | Yes | Not measurable | 7.1 | Yes | No | 1,047 | 49.9 | Yes | Yes |
| 13 | Yes | Yes | Not measurable | 5.3 | No | Yes | 581 | 32.3 | Yes | Yes |
| 14 | Yes | No | No | 6.1 | Yes | Yes | 665 | 23.8 | Yes | Yes |
| 15 | Yes | Yes | No | 11.9 | No | No | 1,206 | 35.5 | Yes | Yes |
| 16 | Yes | Yes | Yes | 4.9 | Yes | Yes | 601 | 37.6 | Yes | Yes |
| 17 | Yes | No | Yes | 6.5 | Yes | Yes | 786 | 43.7 | Yes | No |
| 18* | Yes | Yes | Not measurable | 5.8 | Yes | Yes | 664 | 21.4 | No | No |
| 19 | Yes | Yes | No | 8.2 | Yes | Yes | 1,244 | 41.5 | Yes | Yes |
| 20 | Yes | Yes | Yes | 3.8 | No | Yes | 702 | 41.3 | Yes | No |
| 21 | Yes | No | No | 4.6 | Yes | Yes | 857 | 42.9 | Yes | No |
Abbreviations: BM, bone marrow; BMT, prior myeloablative chemotherapy with hematopoietic stem-cell transplantation; RT, radiation therapy; MIBG, metaiodobenzylguanidine; FDG-PET, [18F]fluorodeoxyglucose positron emission tomography.
This patient only received one of the two MIBG infusions.
Table A2.
Participating NANT Investigators and Institutions
| Investigator | Institution |
|---|---|
| Judy Villablanca, MD | Children's Hospital Los Angeles4650 Sunset Blvd Los Angeles, CA 90027 |
| Katherine K. Matthay, MD | University of California, San Francisco School of Medicine505 Parnassus Avenue, M647San Francisco, CA 94143 |
| Clare Twist, MD | Lucille Salter Packer Children's Hospital300 Pasteur Drive Stanford, CA 94305 |
| John M. Maris, MD | Children's Hospital of Philadelphia324 South 34th Street Philadelphia, PA 19104 |
| Susan Cohn, MD | Children's Memorial Hospital2300 Children's Plaza Chicago, IL 60614 |
Abbreviation: NANT, New Approaches to Neuroblastoma Therapy.
Footnotes
Supported in part by National Institutes of Health (NIH) Grants No. NCI T32 CA128583-01, NCI R21 CA97758, NCI PO1 81403, and NCRR UCSF-CTSI UL1 RR024131, and the Dougherty Foundation, Alex Lemonade Foundation, Campini Foundation, V-Foundation, Mildred V. Strouss Chair, and Conner Fund.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Denah R. Taggart, Randall Hawkins, Katherine K. Matthay
Financial support: Katherine K. Matthay
Administrative support: Denah R. Taggart, Alekist Quach, Randall Hawkins
Provision of study materials or patients: Judith G. Villablanca, Hollie A. Jackson, Carina Mari Aparici, Katherine K. Matthay
Collection and assembly of data: Denah R. Taggart, Myo M. Han, Alekist Quach, David Carlson, John Maris, Randall Hawkins
Data analysis and interpretation: Denah R. Taggart, Myo M. Han, Susan Groshen, Wei Ye, Carina Mari Aparici, David Carlson, John Maris, Randall Hawkins, Katherine K. Matthay
Manuscript writing: Denah R. Taggart, Randall Hawkins, Katherine K. Matthay
Final approval of manuscript: Denah R. Taggart, Myo M. Han, Alekist Quach, Susan Groshen, Wei Ye, Judith G. Villablanca, Hollie A. Jackson, Carina Mari Aparici, David Carlson, John Maris, Randall Hawkins, Katherine K. Matthay
REFERENCES
- 1.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Mairs RJ, Zalutsky MR. New MIBG preparation to improve targeted radiotherapy and reduce toxic side-effects in neuroblastoma patients undergoing combination treatment. Br J Cancer. 1995;72:250. doi: 10.1038/bjc.1995.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung A, Shapiro B, Hattner R, et al. Specificity of radioiodinated MIBG for neural crest tumors in childhood. J Nucl Med. 1997;38:1352–1357. [PubMed] [Google Scholar]
- 4.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36:228–247. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Olivier P, Colarinha P, Fettich J, et al. Guidelines for radioiodinated MIBG scintigraphy in children. Eur J Nucl Med Mol Imaging. 2003;30:B45–50. doi: 10.1007/s00259-003-1138-9. [DOI] [PubMed] [Google Scholar]
- 6.Shulkin BL, Hutchinson RJ, Castle VP, et al. Neuroblastoma: Positron emission tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose compared with metaiodobenzylguanidine scintigraphy. Radiology. 1996;199:743–750. doi: 10.1148/radiology.199.3.8637999. [DOI] [PubMed] [Google Scholar]
- 7.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 8.Messina JA, Cheng SC, Franc BL, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer. 2006;47:865–874. doi: 10.1002/pbc.20777. [DOI] [PubMed] [Google Scholar]
- 9.Matthay KK, Quach A, Huberty J, et al. Iodine-131–metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: A new approach to the Neuroblastoma Therapy Phase 1 study. J Clin Oncol. 2009;27:1020–1025. doi: 10.1200/JCO.2007.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 11.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howman-Giles R, Shaw PJ, Uren RF, et al. Neuroblastoma and other neuroendocrine tumors. Semin Nucl Med. 2007;37:286–302. doi: 10.1053/j.semnuclmed.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein HM, Cohn SL, Shore RM, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–3915. doi: 10.1200/JCO.2004.07.144. [DOI] [PubMed] [Google Scholar]
- 14.Perel Y, Conway J, Kletzel M, et al. Clinical impact and prognostic value of metaiodobenzylguanidine imaging in children with metastatic neuroblastoma. J Pediatr Hematol Oncol. 1999;21:13–18. doi: 10.1097/00043426-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kushner BH, Yeh SD, Kramer K, et al. Impact of metaiodobenzylguanidine scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol. 2003;21:1082–1086. doi: 10.1200/JCO.2003.07.142. [DOI] [PubMed] [Google Scholar]
- 16.Matthay KK, Brisse H, Couanet D, et al. Central nervous system metastases in neuroblastoma: Radiologic, clinical, and biologic features in 23 patients. Cancer. 2003;98:155–165. doi: 10.1002/cncr.11448. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand MJ. Meta-iodobenzylguanidine in children. Semin Nucl Med. 1993;23:231–242. doi: 10.1016/s0001-2998(05)80104-7. [DOI] [PubMed] [Google Scholar]
- 18.Pfluger T, Schmied C, Porn U, et al. Integrated imaging using MRI and 123I metaiodobenzylguanidine scintigraphy to improve sensitivity and specificity in the diagnosis of pediatric neuroblastoma. AJR Am J Roentgenol. 2003;181:1115–1124. doi: 10.2214/ajr.181.4.1811115. [DOI] [PubMed] [Google Scholar]
- 19.Lonergan GJ, Schwab CM, Suarez ES, et al. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: Radiologic-pathologic correlation. Radiographics. 2002;22:911–934. doi: 10.1148/radiographics.22.4.g02jl15911. [DOI] [PubMed] [Google Scholar]
- 20.Kushner BH, Yeung HW, Larson SM, et al. Extending positron emission tomography scan utility to high-risk neuroblastoma: Fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol. 2001;19:3397–3405. doi: 10.1200/JCO.2001.19.14.3397. [DOI] [PubMed] [Google Scholar]
- 21.Kushner BH. Neuroblastoma: A disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–1188. [PubMed] [Google Scholar]
- 22.Nanni C, Rubello D, Castellucci P, et al. 18F-FDG PET/CT fusion imaging in paediatric solid extracranial tumours. Biomed Pharmacother. 2006;60:593–606. doi: 10.1016/j.biopha.2006.07.091. [DOI] [PubMed] [Google Scholar]
- 23.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]