Abstract
Purpose
Colorectal cancer (CRC) screening remains underutilized in the United States. Prior studies reporting the cost effectiveness of randomized interventions to improve CRC screening have not been replicated in the setting of small physician practices. We recently conducted a randomized trial evaluating an academic detailing intervention in 264 small practices in geographically diverse New York City communities. The objective of this secondary analysis is to assess the cost effectiveness of this intervention.
Methods
A total of 264 physician offices were randomly assigned to usual care or to a series of visits from trained physician educators. CRC screening rates were measured at baseline and 12 months. The intervention costs were measured and the incremental cost-effectiveness ratio (ICER) was derived. Sensitivity analyses were based on varying cost and effectiveness estimates.
Results
Academic detailing was associated with a 7% increase in CRC screening with colonoscopy. The total intervention cost was $147,865, and the ICER was $21,124 per percentage point increase in CRC screening rate. Sensitivity analyses that varied the costs of the intervention and the average medical practice size were associated with ICERs ranging from $13,631 to $36,109 per percentage point increase in CRC screening rates.
Conclusion
A comprehensive, multicomponent academic detailing intervention conducted in small practices in metropolitan New York was clinically effective in improving CRC screening rates, but was not cost effective.
INTRODUCTION
Colorectal cancer (CRC) screening rates remain lower than screening rates reported for prostate, cervical, and breast cancer; national CRC screening rates have been reported as less than 45%.1 The low rate of CRC screening may be due to a variety of factors related to patients (eg, fear about screening tests, lack of knowledge, disinterest, poor access to care), clinic infrastructure (poor organization, lack of specialists, long waiting lists, complicated referral process), and health care providers (heavy workloads, forgetfulness, concerns about patient noncompliance).2,3 Interventions to improve CRC screening rates have targeted several of these impediments to appropriate screening.
Four randomized trials reported on both the effectiveness and cost effectiveness of interventions designed to improve CRC screening rates.4–11 Some of these interventions involved patient-directed telephone and mailed reminders to those who were nonadherent with CRC screening tests. Other physician-directed interventions involved affixing a patient self-report on CRC screening status to the front of the medical chart or issuing quarterly report cards to physicians with individual and group CRC screening rates for their clinics. The four interventions were effective, with increases in CRC screening rates ranging from 2% to 28%, and incremental cost-effectiveness ratios (ICERs) of $131, $161, $1,161, and $9,639, respectively, per percentage point increase in CRC screening rates (Table 1). Three trials were conducted at large general medicine practices at a university medical center or a Veterans Affairs medical center. The fourth trial was conducted at eight county medical centers in Tampa, FL.
Table 1.
ICERs for Other Large, Randomized Interventions to Improve CRC Screening
| First Author and Year | Total No. of Participants | Target Population | Target Cancer Screening | Intervention | Cost of Intervention(US$) | Baseline Screen Rate (%) | Change in Screening Rate (%) | ICER: Incremental Cost per Percent Increase in Screening(US$) |
|---|---|---|---|---|---|---|---|---|
| Gorin 2008 (manuscript submitted)* | 1,290 patients; 264 physician practices | Primary care physician offices in 2 geographically distinct New York City communities | FOBT, flexible sigmoidoscopy, colonoscopy | Academic detailing intervention, consisting of face-to-face visits from a trained educator and self-learning packets reinforcing CRC screening guidelines | 147,865 | 9-10 (Bronx, Northern Manhattan) 21-28 (Upper East and West sides, Murray Hill) |
7 across both communities | 21,124 (colonoscopy) FOBT and flexible sigmoidoscopy not clinically effective, so ICERs not calculated |
| Wolf 2005,11 Ferreira 20056* | 1,978 patients; 113 provider groups | Primary care physicians within an urban Veterans Affairs Medical Center | FOBT, flexible sigmoidoscopy, colonoscopy | Provider-directed intervention with regular feedback sessions of patient screening rates | 86,753 | 32 | 9 | 9,639 |
| Lairson 2007,7 Pignone 200212 | 1,546 patients | Patients in a large urban, university-based family medicine practice, age 50-74 years and at average risk for CRC | FOBT, flexible sigmoidoscopy, colonoscopy | Patients randomized to 4 groups: Control group Standard intervention, consisting of mailed informational brochure and invitation letter Tailored intervention, consisting of standard intervention + tailored message based on survey data* Tailored intervention + reminder phone call by a trained health educator* |
Control = 0 Standard intervention = 16,254 Tailored intervention = 57,900 Tailored intervention + phone call = 77,200 |
Control = 32 Standard intervention = 46 |
Standard intervention = 14 Tailored intervention = −2 Tailored intervention + phone call = 2 Note: Control group is the standard intervention group |
Standard intervention = 1,161 Tailored intervention = not clinically effective compared with standard intervention, so ICER not calculated Tailored intervention + phone call = 38,600 |
| Shankaran 2007,10 Denberg 20065 | 781 patients | Patients in university-based internal medicine clinics (majority with commercial or university insurance) who received referrals for screening colonoscopy | Colonoscopy | Customized mailed informational brochure | 1,927 | 59 | 12 | 161 |
| Roetzheim 2004,9 Chirikos 20044 | 1,237 patients | Patients enrolled in a county-funded health insurance plan in Florida (do not qualify for Medicaid or Medicare) | FOBT | “SOS Intervention”: cancer screening checklist performed by patients and color-coded chart reminders for physicians. | 3,662 | 12 | 28 | 131 |
Abbreviations: ICER, incremental cost-effectiveness ratio; CRC, colorectal cancer; FOBT, fecal occult blood test.
Physician-directed interventions.
To our knowledge, no prior study has reported on effectiveness and cost effectiveness of efforts to improve CRC screening rates among primary care physicians who work in small urban medical practices. Interventions to improve CRC screening rates in private community practices may be challenging for logistic and financial reasons. Unlike large health maintenance organizations or care networks, small medical settings lack “economies of scale” in contacting larger numbers of patients or reaching out to larger numbers of physicians. Ganz et al13 attempted to reach out to these small practices by conducting a low-intensity quality-improvement program based on a continuing medical education intervention designed to improve CRC screening rates for 36 provider organizations that contracted with a large managed care health plan. Most of the provider organizations included more than 35 primary care physicians. The study found that only 26% of eligible patients received any CRC screening test, with no differences between the intervention or control groups. The current study reports the cost effectiveness of a strategy that attempts to promote CRC screening in small physician practices in a much more intensive way than the approach described by Ganz et al.13
In this article, we review the cost effectiveness of a randomized, multicomponent, provider-directed intervention conducted among small medical practices in two socioeconomically disparate metropolitan New York City communities (Gorin et al, manuscript submitted for publication). This intervention was clinically effective in improving colonoscopy screening across both communities. Our goal in this report is to investigate whether an intensive physician-directed intervention involving evidence-based instruction in established guidelines is an economically feasible strategy to improve CRC screening rates among primary care physicians who work in small group practices.
METHODS
Intervention and Study Design
The academic detailing intervention was conducted in 264 physician offices in two distinct metropolitan communities in New York City. One community consisted of participants in the Upper East and Upper West sides and Murray Hill sections of New York City and is referred to throughout this article as Middle Manhattan. The second community consisted of physician offices in northern Manhattan and South and Central Bronx and is referred to throughout this article as Upper Manhattan/Bronx. A total of 65 physician offices in Middle Manhattan and 71 physician offices in Upper Manhattan/Bronx were randomly assigned to receive the intervention; there were, on average, four practicing physicians per office. All intervention offices received four in-person academic detailing visits from a trained health educator (non–United States licensed, foreign-trained physician) over the course of 12 months. The physicians also received self-learning packets that included professionally designed print materials and scientific articles. Additionally, physicians in the intervention arm received a CD-ROM that included four standardized patient cases addressing barriers to CRC screening that had been identified in physician-completed surveys at the start of the study period. Office-based CRC prevention materials and multilingual patient education materials, evaluated for readability, were shared with the physicians and other staff. A total of 1,290 patient charts from a sample of physician offices (34 offices in the intervention group and 33 offices in the control group) were abstracted and assessed at baseline and 12 months for completion of fecal occult blood test (FOBT), flexible sigmoidoscopy, and colonoscopy. The study used a randomized controlled design. The results of the clinical trial are described in detail in the accompanying article (Gorin et al, manuscript submitted for publication).
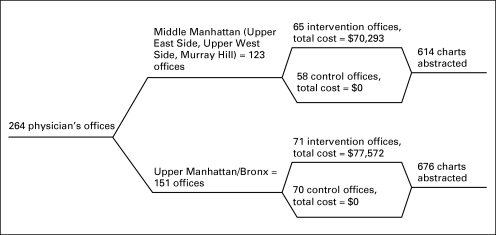
Flow Diagram
Figure 1 represents the sequence of events from initial enrollment to measurement of outcomes. Of note, only patient charts from a random sample of physician offices in both communities were abstracted for review.
Fig 1.
Flow diagram of the academic detailing intervention.
Assumption for the Economic Model
The economic model was based on the health care organization's perspective, as it is most likely to support the costs of cancer screening promotion efforts. The total time spent by each physician on activities related to the academic detailing intervention was less than 4 hours over a 12-month period. Participating physicians were offered a $100 honorarium for their participation; only 74 physicians requested this amount. The remaining physicians involved in this study gave their time voluntarily. Chart review, honoraria, and data abstraction costs were not included, as these were considered study-related costs.
Calculation of Costs
All inputs were included in the cost analysis (Table 2). The cost inputs were divided into two categories: initial development costs (fixed one-time costs including advertising, CD-ROM design, and multilingual translation of materials in the self-learning packets) and intervention delivery costs (cost of printed materials, CD-ROM production, travel, office supplies, and time costs for the detailer). Academic detailers were compensated at an annual health educator salary of $54,780 plus 23.4% benefits rate from the Bureau of Labor Statistics 2007.14 The total fixed costs were divided equally across both communities. The intervention delivery costs were determined for each community based on the number of participating physician offices (Table 2).
Table 2.
Cost of Academic Detailing Intervention
| Cost Input | Total Cost of the Intervention* (US$) | ± 10% Around Cost Estimates (US$) |
|---|---|---|
| Middle Manhattan community (Upper East Side, Upper West Side, Murray Hill) physician offices, n = 123 | ||
| Initial development costs: fixed costs (advertising, CD-ROM design, translation of materials) | 21,484 | 19,336-23,633 |
| Intervention delivery costs | ||
| Printed materials, travel, office supplies | 17,314 | 15,583-19,045 |
| Academic detailer costs† | 31,495 | 28,346-34,644.5 |
| Total cost | 70,293 | 63,263-77,322 |
| Upper Manhattan/Bronx community offices, n = 141 | ||
| Initial development costs: fixed costs (advertising, CD-ROM design, translation of materials) | 21,484 | 19,336-23,633 |
| Intervention delivery costs | ||
| Printed materials, travel, office supplies | 19,847 | 17,862-21,832 |
| Academic detailer costs† | 36,241 | 32,617-39,865 |
| Total cost | 77,572 | 69,815-85,329 |
| Total combined cost (both communities) | 147,865 | 133,078-162,652 |
Two hundred sixty-four physician offices.
Detailing visits conducted by one foreign-trained physician educator over a 12-month period of time. Annual salary based on mean annual health educator salary (bureau of labor statistics 2007) of $54,780 + 23.4% benefits rate.
Calculation of the ICER
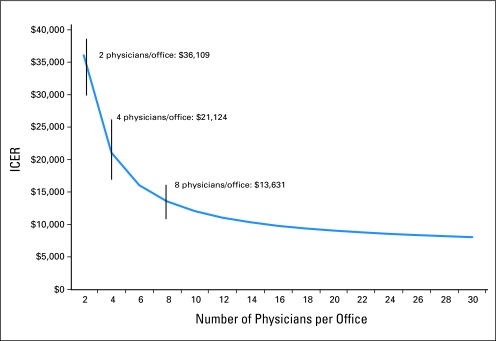
The ICER was calculated on the basis of the total cost of the intervention and the relative change in CRC screening rates between control and intervention groups across both communities. The clinical outcomes used in the calculation of ICER were the completion of colonoscopy, FOBT, and flexible sigmoidoscopy. The ICER is reported as dollars per additional percentage point increase in CRC screening rate. As in prior studies of the cost effectiveness of CRC screening promotion efforts, sensitivity analysis estimates were based on a 10% variation around the point estimates for the various cost inputs.10,11 An additional sensitivity analysis evaluated the ICER for differing numbers of physicians in the practice and, accordingly, varying intervention delivery costs among the practices (Table 3; Fig 2). The average practice setting included four physicians. For this sensitivity analysis, the intervention delivery costs varied, whereas other costs remained constant. Intervention delivery costs were doubled if the practices included an average of two practitioners and were halved for those with eight practitioners.
Table 3.
ICERs for the Academic Detailing Intervention
| Cost-Effectiveness Measure | FOBT (US$) | Flexible Sigmoidoscopy (US$) | Colonoscopy (US$) |
|---|---|---|---|
| Increase in screening rates, % | Screening rates did not increase | Screening rates did not increase | 7% |
| Total cost of intervention | 147,865 | 147,865 | 147,865 |
| ICER per percentage point increase in screening | * | * | 21,124 |
| Sensitivity estimate, % (±10%) | * | * | 19,011-23,236 |
| Total initial development costs | 42,968 | 42,968 | 42,968 |
| Total intervention delivery costs (4 physicians per office) | 104,897 | 104,897 | 104,897 |
| Sensitivity estimate (intervention delivery costs: 8 physicians per office 2×) | 52,449 | 52,449 | 52,449 |
| Sensitivity estimate (intervention delivery costs: 2 physicians per office 1/2×) | 209,794 | 209,794 | 209,794 |
| ICER sensitivity estimate | * | * | 13,631-36,109 |
Abbreviations: ICERs, incremental cost-effectiveness ratios; FOBT, fecal occult blood test.
ICER cannot be calculated because the intervention is not clinically effective.
Fig 2.
Estimated incremental cost effectiveness ratio (ICER) per percentage point increase in screening based on the number of physicians per office.
RESULTS
At baseline, more patients in Upper Manhattan/Bronx had Medicaid insurance (36%) compared with Middle Manhattan (6.5%). In Middle Manhattan, baseline screening rates for colonoscopy, FOBT, and flexible sigmoidoscopy were 28.0%, 11.5%, and 6.4%, respectively, in the control offices and 21.1%, 8.4%, and 3.2%, respectively, in the intervention offices. In Upper Manhattan/Bronx, baseline screening rates for colonoscopy, FOBT, and flexible sigmoidoscopy were 10.3%, 9.9%, and 1.9%, respectively, in the control offices and 9.1%, 11.0%, and 8.3%, respectively, in the intervention offices.
At a follow-up of 12 months, there was no statistically significant difference in uptake of FOBT or flexible sigmoidoscopy between control and intervention groups (P = .47 for FOBT and P = .81 for flexible sigmoidoscopy). In Upper Manhattan/Bronx, there was a 7.1% increase in colonoscopy screening in the intervention compared with the control group; in Middle Manhattan, there was a 5.7% increase in colonoscopy screening in the intervention compared with the control group. For the composite of both communities, there was a 7% greater increase in screening by colonoscopy in the intervention group compared with the control group (odds ratio [OR] = 1.93; 95% CI, 1.11 to 3.37; P = .02). A subgroup analysis also revealed that patients who were uninsured or had Medicaid insurance were less likely than the rest of the sample to undergo colonoscopic screening at follow-up, though this difference was small (OR = 0.96; 95% CI, 0.93 to 0.99; P = .01; v OR = 0.98; 95% CI, 0.97 to 0.99; P = .01, respectively). Stratifying by community, the postintervention increase in CRC screening was 40% higher in Middle Manhattan than in Upper Manhattan/Bronx; baseline screening rates were approximately twice as high in Middle Manhattan.
The total cost of the intervention across both communities was $147,865. The ICER of this physician-directed intervention is $21,124 per percentage point increase in colonoscopy screening rate (Table 3). Sensitivity estimates based on a 10% variation around the cost inputs indicated that the estimated cost per additional percentage increase in colonoscopy screening ranged from $19,011 to $23,236. Sensitivity estimates based on varying the intervention delivery costs by size of the physician offices estimated the cost per additional percentage increase in colonoscopy screening ranged from $13,631 (for medical practices with eight practitioners) to $36,109 (for medical practices with two practitioners).
The academic detailing intervention was not associated with a statistically significant increased rate of CRC screening by either FOBT or flexible sigmoidoscopy. As such, the ICER cannot be calculated for these two other screening modalities.
DISCUSSION
To our knowledge, this study is the first to assess the cost-effectiveness of an intensive provider-directed intervention designed to improve CRC screening rates among practitioners who work in small group practices. The current study was conducted among 264 physician offices in New York City and consisted of multicomponent academic detailing, including face-to-face visits. The clinical trial reported a 7% increase in colonoscopy screening in the practices that were included in the intervention arms. The ICER associated with this intervention was $21,124 per percentage point increase in CRC screening by colonoscopy.
The ICER for this intervention is markedly higher than all but one of the previously reported ICERs for CRC screening promotion interventions (Table 1). As noted earlier, prior cost-effectiveness analyses were based on studies conducted in large academic clinical settings or among practitioners who worked in eight large inner city medical clinics.4,7,10,11 The ICERs per extra percentage increased in screening for these prior low-intensity interventions ranged from $131 to $1,161 for patient-directed or infrastructural interventions and $9,639 for a physician-directed intervention involving feedback CRC screening rates.4,7,10,11 By contrast, a highly intensive patient-directed intervention involving patient-specific mailings combined with telephone reminders to delinquent patients was associated with an ICER of $38,600 per percentage point increase in CRC screening rate.7 Taken together, these findings suggest that low-intensity interventions that can be shown to improve CRC screening may be more cost-effective than high-intensity interventions, even if clinically effective. Given that 75% of medical visits in the United States are made to small group practices of four or fewer physicians, and 39% of these visits are to solo practitioners, interventions that can optimally target a broad range of small practice settings in an economically feasible manner have yet to be described.15
Our findings can be interpreted in the context of key elements for evaluating the cost effectiveness of cancer screening promotional studies suggested by Andersen et al.16 These elements include basing interventions around screening tests that are known to be cost-effective; targeting average-risk individuals who have not previously participated in screening efforts; evaluating quality-of-life concerns of participants involved in cancer screening programs; focusing on interventions that are likely to be cost-effective; formally assessing the cost effectiveness of the promotion efforts during a randomized intervention; recognizing that the unique characteristics of the patient population, physicians, and health care system play an important role in the promotional efforts; and addressing the sustainability of the promotional program. This health care provider–directed intervention addresses all of the proposed lessons, with the exception of including prospective quality-of-life assessments and cost-effectiveness data in terms of quality-adjusted life years. As the purpose of this analysis was not to elucidate the cost effectiveness of the screening technology (which has been repeatedly investigated), but rather to study the promotion strategy itself in the context of previously reported CRC screening promotion efforts, the present study uses the much more relevant cost-effectiveness metric of cost per percentage point increase in screening rate.12
Some limitations to our study should be noted. First, cost-effectiveness analyses of CRC screening promotion interventions are based on a wide range of cost inputs, as operational aspects of each intervention differ. Second, the high costs of the intervention were attributed in large part to the time costs for the academic detailer to travel between practices. In sensitivity analyses where the average practice size was twice as large as in the actual intervention, the estimated ICER remained higher than $13,000 per percentage point increase in CRC screening rate. However, when there were more than 16 physicians per practice group, the estimated ICER decreased to less than $10,000 per percentage point increase in CRC screening rates, similar to our previously reported low-intensity physician-directed intervention.11 Third, the metric used in this analysis, cost per percentage point increase in screening rate, has not routinely been used in describing interventions to promote CRC screening; this metric, however, is more applicable at the level of managed care organizations in which quality measures are based on percentages and populations rather than individuals. Fourth, the intervention was associated with an increase in uptake of screening by colonoscopy, but not FOBT or flexible sigmoidoscopy. It should be noted that during the time period for the intervention, the New York City Department of Health identified colonoscopy as the preferred CRC screening approach in the metropolitan New York area.17 The baseline screening rates for colonoscopy in both Middle Manhattan (28%) and Upper Manhattan/Bronx (10.3%), however, were significantly lower compared with the national colonoscopy screening average (approximately 45%).1
In conclusion, an academic detailing intervention consisting of physician education and addressing barriers to CRC screening resulted in a 7% increase in CRC screening in two socioeconomically and ethnically disparate Manhattan communities. The academic detailing intervention is associated with an ICER of $21,124 per percentage point increase in screening. The need for dissemination of cost-effective interventions to improve CRC screening in small practices is crucial, as few small practices have the ability to create organized processes to proactively improve CRC detection.18 Future randomized trials of CRC screening promotion efforts in small community practice settings should focus on low-intensity interventions. High-intensity interventions involving medical practices with small numbers of physicians, even if clinically effective, are unlikely to be affordable.
Supplementary Material
Acknowledgment
We thank Grace Hillyer, MPH, Ashfaque Hossain, MBBS, MPH (deceased), and Mary Riley-Jacome, MA, Sharon Guilfoyle, MPH (deceased), and Gylynthia Trotman, MPH, field staff, for their contributions to the collection of the data; Julia E. Heck, PhD, and Chenbo Zhu, MS, for their initial analyses of the data; the members of our clinical advisory group, notably Drs Alfred Neugut, MD, PhD, Columbia College of Physicians and Surgeons and the Mailman School of Public Health, Andrew Dannenberg, MD, Cornell Weill Medical College, Jonathan David, MD, Harlem Hospital Center, Ana Natale, MD, MPH, and Diana DeCosimo, MD, University of Medicine and Dentistry of New Jersey, for their contributions to the oversight of this study; and the primary care physicians who participated in this study.
Footnotes
Supported by Grant No. ACS TIOG CPC-99783 from the American Cancer Society (S.S.G., principal investigator). The funder played no role in the study's design, conduct, or reporting of the trial. This work was also supported by a postdoctoral grant from the Veterans Affairs Administration Center for Management of Complex Chronic Care (V.S.) and the National Institute of Health K Award, Grant No. 1K01CA134554-01 (J.M.M.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00441311.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Veena Shankaran, Thanh Ha Luu, June M. McKoy, Joshua Graff Zivin, Rafael Lantigua, Charles L. Bennett, Sherri Sheinfeld Gorin
Financial support: Sherri Sheinfeld Gorin
Administrative support: Elizabeth Richey, Rafael Lantigua, Sherri Sheinfeld Gorin
Provision of study materials or patients: Rafael Lantigua, Sherri Sheinfeld Gorin
Collection and assembly of data: Veena Shankaran, Thanh Ha Luu, Elizabeth Richey, June M. McKoy, Marc Scoppettone, Charles L. Bennett, Sherri Sheinfeld Gorin
Data analysis and interpretation: Veena Shankaran, Thanh Ha Luu, Narissa Nonzee, Elizabeth Richey, June M. McKoy, Joshua Graff Zivin, Alfred Ashford, Rafael Lantigua, Charles L. Bennett, Sherri Sheinfeld Gorin
Manuscript writing: Veena Shankaran, Thanh Ha Luu, Narissa Nonzee, Elizabeth Richey, June M. McKoy, Charles L. Bennett, Sherri Sheinfeld Gorin
Final approval of manuscript: Veena Shankaran, Thanh Ha Luu, Narissa Nonzee, Elizabeth Richey, June M. McKoy, Joshua Graff Zivin, Alfred Ashford, Harold Frucht, Charles L. Bennett, Sherri Sheinfeld Gorin
REFERENCES
- 1.American Cancer Society. Cancer Prevention and Early Detection Facts & Figures 2007. http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf.
- 2.Anderson WF, Guyton KZ, Hiatt RA, et al. Colorectal cancer screening for persons at average risk. J Natl Cancer Inst. 2002;94:1126–1133. doi: 10.1093/jnci/94.15.1126. [DOI] [PubMed] [Google Scholar]
- 3.Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20:989–995. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirikos TN, Christman LK, Hunter S, et al. Cost-effectiveness of an intervention to increase cancer screening in primary care settings. Prev Med. 2004;39:230–238. doi: 10.1016/j.ypmed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Denberg TD, Coombes JM, Byers TE, et al. Effect of a mailed brochure on appointment-keeping for screening colonoscopy: A randomized trial. Ann Intern Med. 2006;145:895–900. doi: 10.7326/0003-4819-145-12-200612190-00006. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira MR, Dolan NC, Fitzgibbon ML, et al. Health care provider-directed intervention to increase colorectal cancer screening among veterans: Results of a randomized controlled trial. J Clin Oncol. 2005;23:1548–1554. doi: 10.1200/JCO.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 7.Lairson DR, Dicarlo M, Myers RE, et al. Cost-effectiveness of targeted and tailored interventions on colorectal cancer screening use. Cancer. 2008;112:779–788. doi: 10.1002/cncr.23232. [DOI] [PubMed] [Google Scholar]
- 8.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 9.Roetzheim RG, Christman LK, Jacobsen PB, et al. A randomized controlled trial to increase cancer screening among attendees of community health centers. Ann Fam Med. 2004;2:294–300. doi: 10.1370/afm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankaran V, McKoy JM, Dandade N, et al. Costs and cost-effectiveness of a low-intensity patient-directed intervention to promote colorectal cancer screening. J Clin Oncol. 2007;25:5248–5253. doi: 10.1200/JCO.2007.13.4098. [DOI] [PubMed] [Google Scholar]
- 11.Wolf MS, Fitzner KA, Powell EF, et al. Costs and cost effectiveness of a health care provider-directed intervention to promote colorectal cancer screening among Veterans. J Clin Oncol. 2005;23:8877–8883. doi: 10.1200/JCO.2005.02.6278. [DOI] [PubMed] [Google Scholar]
- 12.Pignone M, Saha S, Hoerger T, et al. Cost-effectiveness analyses of colorectal cancer screening: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA, Farmer MM, Belman MJ, et al. Results of a randomized controlled trial to increase colorectal cancer screening in a managed care health plan. Cancer. 2005;104:2072–2083. doi: 10.1002/cncr.21434. [DOI] [PubMed] [Google Scholar]
- 14.United States Department of Labor. U.S. Bureau of Labor Statistics. http://www.bls.gov/
- 15.Cherry DK, Hing E, Woodwell DA, et al. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report. 2008:1–39. [PubMed] [Google Scholar]
- 16.Andersen M, Urban N, Ramsey S, et al. Examining the cost-effectiveness of cancer screening promotion. Cancer. 2004;101:1229–1238. doi: 10.1002/cncr.20511. [DOI] [PubMed] [Google Scholar]
- 17.New York City Department of Health and Mental Hygiene CCCC: 2008 Citywide Colon Cancer Control Coalition (C5) Summit. http://www.nyc.gov/html/doh/html/cancer/cancercolon.shtml.
- 18.Casalino LP, Devers KJ, Lake TK, et al. Benefits of and barriers to large medical group practice in the United States. Arch Intern Med. 2003;163:1958–1964. doi: 10.1001/archinte.163.16.1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.