Abstract
Purpose
Health disparities exist according to an individual's place of residence. We evaluated the association between primary area of residence (urban v rural) according to treatment provider (university based v community based) and overall survival in patients with lymphoma and determined whether there are patient groups that could benefit from better coordination of care.
Patients and Methods
Population-based, retrospective cohort study of 2,330 patients with centrally confirmed lymphoma from Nebraska and surrounding states and treated by university-based or community-based oncologists from 1982 to 2006.
Results
Among urban residents, 321 (14%) were treated by university-based providers (UUB) and 816 (35%) were treated by community-based providers (UCB). Among rural residents, 332 (14%) were treated by university-based providers (RUB), and 861 (37%) were treated by community-based providers (RCB). The relative risk (RR) of death among UUB, UCB, and RUB were not statistically different. However, RCB had a higher risk of death (RR, 1.37; 95% CI, 1.14 to 1.65; P = .01; and RR, 1.26; 95% CI, 1.06 to 1.49; P = .01) when compared with UUB and RUB, respectively. This association was true in both low- and intermediate-risk patients. Among high-risk patients, UCB, RUB, and RCB were all at higher risk of death when compared with UUB.
Conclusion
Survival outcomes of patients with lymphoma may be associated with place of residence and treatment provider. High-risk patients from rural areas may benefit from better coordination of care.
INTRODUCTION
Health disparities exist according to place of residence.1–8 Rural patients are less likely to receive optimal treatment, and have less favorable outcomes for different types of illnesses.1,2,5,8–10 This disparity can be due to many factors including lack of access to quality health care providers.2,11,12 Rural patients have also been shown to present with more severe disease, have lower socioeconomic status, and have generally poorer health habits.1,4,6,8,13 Despite the predisposition of rural patients for inferior outcomes, studies in some chronic diseases have shown that outcome disparities according to place of residence can be improved through standardization of care using medical outreach teams, implementing practice guidelines, using nurse navigators, and tracking clinician performance.1,2,8,14–16
Outcome disparity also exists according to academic affiliation of health care providers.17 In stage I and II breast cancer, breast conserving surgeries were more likely to be performed on patients treated by university-based providers.9 In colorectal cancer, patients treated by university-based providers were less likely to have local recurrence and death when compared with patients treated by community-based providers.18,19 It is not known whether cancer outcomes differ according to patient's place of residence and treatment provider.
In 1982, the Nebraska Lymphoma Study Group (NLSG) was established as collaboration between community-based and university-based oncologists and pathologists throughout Nebraska and surrounding states. Such collaboration allows for centralized diagnosis and staging, while affording community-based patients therapies according to physician preference, and the expertise and facilities of university-based oncologists as needed. With the rising incidence of lymphoma in the United States, we used the NLSG collected data to evaluate whether a disparity exists in the outcomes of patients with lymphoma according to place of residence and treatment provider. Findings from this study would allow us to identify patients who may benefit from better coordination of care.
PATIENTS AND METHODS
Data Source
Data were obtained from the University of Nebraska Medical Center (UNMC) Oncology Database; a password protected, web-based database of patients with cancer. Data pertaining to patients with lymphoma was reported to the NLSG. A common consent form approved by the institutional review board at UNMC is used for all patients. Patient-, disease-, and treatment-related data are collected, as well as, fresh, frozen, and fixed tissues for pathologic and immunologic studies. Data are sent to a central office in Omaha, NE, where a rigorous data verification and audit process are implemented by trained data specialists. Audits are verified from various medical, pathology, laboratory, and ancillary reports. Follow-up information is obtained every 6 months. Causes of deaths are ascertained from death certificates or medical records and are confirmed by the attending oncologist. The NLSG invites its members to educational meetings and are also informed of ongoing clinical trials at UNMC. The UNMC institutional review board approved this study.
Patients
Patients included are primarily from the state of Nebraska, with some from the states of CO, WY, SD, KS, MO, and IA. A total of 3,619 consented patients were reported to the NLSG between 1982 and 2006. Three hundred twenty-nine patients (9%) had missing residential ZIP codes, while 960 (26%) had incomplete prognostic clinical data. Thus, a total of 2,330 patients were included in this study. Patients included were similar in age and sex distribution to patients with missing data, with a higher rate of Hodgkin's lymphoma (HL). The 5-year overall survival (OS) between the excluded and included patients were similar, 59% versus 57%, respectively. Follow-up information was obtained for all patients until December, 2007.
Variables Analyzed
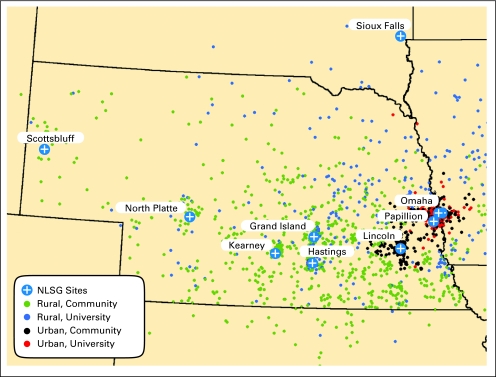
The main variable analyzed classified patients into four groups: urban residents treated by university-based provider (UUB, n = 321), urban residents treated by community-based provider (UCB, n = 816), rural residents treated by university-based provider (RUB, n = 332), and rural residents treated by community-based provider (RCB, n = 861). Place of residence was defined according to the rural urban commuting area code assigned to the ZIP code of the patient's primary residence at diagnosis.20 We dichotomized the rural urban commuting area classification into urban or rural designation. Urban areas consisted of urban commuting and urban core, while rural areas consisted of large rural commuting, large rural core, small rural commuting, and small rural core. Oncologists who treated the patient's lymphoma first were classified according to academic affiliation. University-based consisted of oncologists from UNMC, Creighton University Medical Center, and the Veterans' Administration of Nebraska and Western Iowa and see mainly hematologic malignancies, while community-based consisted of oncologists who do not have any academic affiliations. Community-based oncologists treat a wide range of malignancies, not limited to lymphoma, and can either be in solo or group practice. Figure 1 shows the distribution of the patients according to place of residence and treatment provider.
Fig 1.
Map showing the distribution of patients included in the study according to place of residence and type of treatment provider NLSG, Nebraska Lymphoma Study Group.
Table 1 presents all the variables included in the analyses. The residential ZIP code was used to derive the patient's mean household income using the 2000 US Census (categorized into quartile distribution), as well as the distance from place of residence to treatment provider.21 In addition, the distance from place of residence to UNMC was approximated to provide an index of how far patients need to travel to seek university-based treatment.
Table 1.
Patient Demographic and Disease Characteristics According to Place of Residence and Treatment Provider
| Characteristic | Urban University Based |
Urban Community Based |
Rural University Based |
Rural Community Based |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| No. of patients | 321 | 14 | 816 | 35 | 332 | 14 | 861 | 37 | |
| Median age, years | 54 | 62 | 60 | 66 | < .01 | ||||
| Range | 19-94 | 19-93 | 19-92 | 19-96 | |||||
| Age group | < .01 | ||||||||
| < 40 | 83 | 25 | 139 | 17 | 65 | 20 | 99 | 12 | |
| 40-60 | 115 | 36 | 233 | 29 | 98 | 30 | 222 | 26 | |
| > 60 | 123 | 38 | 444 | 54 | 169 | 51 | 540 | 63 | |
| Male sex | 168 | 52 | 432 | 53 | 177 | 53 | 443 | 51 | .92 |
| Race/ethnicity | < .01* | ||||||||
| White | 299 | 93 | 787 | 96 | 327 | 98 | 850 | 99 | |
| Black | 14 | 4 | 15 | 2 | 0 | 0 | 1 | < 1 | |
| Asian | 3 | 1 | 1 | < 1 | 2 | < 1 | 1 | < 1 | |
| Hispanic | 2 | < 1 | 10 | 1 | 0 | 0 | 8 | 1 | |
| Native American | 1 | < 1 | 1 | < 1 | 3 | 1 | 1 | < 1 | |
| Other | 2 | < 1 | 2 | < 1 | 0 | 0 | 0 | 0 | |
| Median household income, $ | 44,101 | 42,023 | 35,449 | 35,240 | < .01 | ||||
| ≤ 30,000 | 23 | 7 | 60 | 7 | 38 | 11 | 117 | 14 | |
| 30,001-39,999 | 92 | 29 | 263 | 32 | 260 | 78 | 685 | 80 | |
| 40,000-44,999 | 64 | 20 | 162 | 20 | 24 | 7 | 49 | 6 | |
| ≥ 45,000 | 142 | 44 | 331 | 41 | 10 | 3 | 10 | 1 | |
| Karnofsky performance score at diagnosis | .11 | ||||||||
| 80-100 | 286 | 89 | 703 | 86 | 297 | 89 | 732 | 85 | |
| < 80 | 35 | 11 | 113 | 14 | 35 | 11 | 129 | 15 | |
| Disease type | .02 | ||||||||
| Low grade non-Hodgkin's lymphoma† | 51 | 16 | 143 | 18 | 59 | 18 | 159 | 18 | |
| High grade non-Hodgkin's lymphoma‡ | 183 | 57 | 506 | 62 | 206 | 62 | 555 | 64 | |
| Hodgkin's lymphoma§ | 87 | 27 | 167 | 20 | 67 | 20 | 147 | 17 | |
| Ann Arbor stage at diagnosis | < .01 | ||||||||
| I-II | 123 | 38 | 373 | 46 | 119 | 36 | 387 | 45 | |
| III-IV | 198 | 62 | 443 | 54 | 213 | 64 | 474 | 55 | |
| Presence of B symptoms | 83 | 26 | 217 | 27 | 99 | 30 | 234 | 27 | .66 |
| Tumor bulk, cm | .04 | ||||||||
| < 5 | 132 | 41 | 384 | 47 | 130 | 39 | 362 | 42 | |
| ≥ 5 | 189 | 59 | 432 | 53 | 202 | 61 | 499 | 58 | |
| Nodal involvement | .09 | ||||||||
| None | 29 | 9 | 108 | 13 | 41 | 12 | 125 | 15 | |
| At least 1 | 292 | 91 | 708 | 87 | 291 | 88 | 736 | 85 | |
| Extranodal involvement | .47 | ||||||||
| None | 137 | 43 | 383 | 47 | 150 | 45 | 376 | 44 | |
| At least 1 | 184 | 57 | 433 | 53 | 182 | 55 | 485 | 56 | |
| Elevated lactate dehydrogenase | 125 | 39 | 258 | 32 | 140 | 42 | 299 | 35 | < .01 |
| Risk group‖ | .08 | ||||||||
| Low | 144 | 45 | 394 | 48 | 131 | 39 | 361 | 42 | |
| Intermediate | 119 | 37 | 293 | 36 | 139 | 42 | 335 | 39 | |
| High | 58 | 18 | 129 | 16 | 62 | 19 | 165 | 19 | |
| No. of chemotherapy cycles | .07 | ||||||||
| 1-3 | 94 | 29 | 195 | 24 | 96 | 29 | 200 | 23 | |
| > 3 | 191 | 60 | 553 | 68 | 207 | 62 | 583 | 68 | |
| Missing | 36 | 11 | 68 | 8 | 29 | 9 | 78 | 9 | |
| Radiation as part of treatment | 104 | 32 | 222 | 27 | 79 | 24 | 229 | 27 | < .01 |
| Transplantation as part of treatment | < .01 | ||||||||
| Yes | 61 | 19 | 88 | 11 | 54 | 16 | 85 | 10 | |
| No | 260 | 81 | 728 | 89 | 278 | 84 | 776 | 90 | |
| Year of treatment¶ | < .01 | ||||||||
| 1982-1988 | 65 | 20 | 192 | 24 | 133 | 40 | 209 | 24 | |
| 1989-1993 | 50 | 16 | 228 | 28 | 53 | 16 | 256 | 30 | |
| 1994-1998 | 69 | 22 | 234 | 29 | 52 | 16 | 236 | 27 | |
| 1999-2006 | 137 | 43 | 162 | 20 | 94 | 28 | 160 | 18 | |
| Distance to treatment center | |||||||||
| Median | 8 | 5 | 107 | 48 | < .01 | ||||
| Range | < 1-472 | < 1-469 | 25-491 | < 1-456 | |||||
| Distance to UNMC | |||||||||
| Median | 8 | 51 | 107 | 138 | |||||
| Range | < 1-473 | < 1-665 | 25-491 | 22-602 | < .01 | ||||
NOTE. Bold font indicates statistical significance.
Abbreviation: UNMC, University of Nebraska Medical Center.
P value applies to comparison between white and non-white.
Low-grade lymphomas included: diffuse follicular center grade 1, diffuse follicular center grade 2, extranodal marginal zone, lymphoplasmacytic, nodal marginal zone, splenic marginal zone, diffuse small cleaved, extramedullary plasmacytoma, follicular mixed, follicular small cleaved, mucosa-associated lymphoid tissue, monocytoid B, non-Hodgkin's not otherwise specified, natural killer cell granular lymphocytic proliferation, and small lymphocytic.
High-grade lymphomas included: precursor T lymphoblastic, lymphoblastic, small noncleaved non-Burkitt's, peripheral gamma delta T cell, anaplastic large T/null cell, peripheral T cell, follicular large, Burkitt's, precursor B lymphoblastic, B-cell unclassifiable, composite, diffuse large B cell, diffuse mixed, and Burkitt-like.
Types of Hodgkin's disease included: not otherwise specified, interfollicular, lymphocyte depleted, lymphocyte predominate, lymphocyte rich, mixed cellularity, and nodular sclerosis.
Risk factors included: age ≥ 60 years, Karnofsky performance score < 80, Ann Arbor stage III or IV, presence of B symptoms, elevated lactate dehydrogenase, tumor bulk ≥ 5.0 cm, at least one nodal involvement, at least one extranodal involvement (low risk: ≤ 3 risk factors present; intermediate risk: 4 to 5 risk factors present; high risk: ≥ 6 risk factors present).
Cut points determined by quartile distribution of patients by year of treatment in the entire Nebraska Lymphoma Study Group database.
Eight clinical risk factors were identified and used to categorize patients according to prognostic risk groups (bottom of Table 1). Type of lymphoma was categorized into low grade, high grade, and HL as has been used in other observational studies22–25 by the NLSG principal pathologist with expertise in lymphoma (D.D.W.); details of classification are presented as footnote in Table 1. Because no universally accepted way of classifying the risk for all kinds of lymphoma is available, we identified these risk factors from the data available. Alternatively, the International Prognostic Index (IPI) score was computed for patients with non-Hodgkin's lymphoma (NHL).26 The distribution of patients according to IPI risk groups were dichotomized into low risk (low and low-intermediate risk IPI) or high risk (high-intermediate and high IPI) categories to have adequate statistical power. The Hasenclever prognostic index for HL was not computed because of missing hemoglobin levels and serum albumin for majority of the patients.
Outcomes Evaluated
The primary outcome of interest was OS defined as death from any cause. Time to event was computed from date of lymphoma diagnosis to time of death or last contact. We also evaluated progression-free survival (PFS) defined as presence of progression or death. Time to event for PFS was from the date of lymphoma diagnosis to time of progression, death, or last contact. We also evaluated whether death was related to lymphoma or due to disease progression.
Statistical Analysis
Univariate comparisons of all characteristics according to the four groups were done using the χ2 test for categorical data and Kruskal-Wallis test for continuous data. Time-specific probability estimates of PFS and OS were obtained through the Kaplan-Meier estimation method and compared using the log-rank test.27 The multivariate Cox proportional hazards regression analysis were used: to identify which pretreatment risk factors predict OS, to delineate the number of risk factors that have unique risk level thresholds, and to elucidate the urban-rural and treatment provider effect. All model building tested for the assumption of proportionality using time-dependent covariates.28 The first set of Cox models were done to identify which clinical risk factors are associated with OS. Pretreatment covariates listed in Table 1 were evaluated using a stepwise approach. Covariates with an α of at least .05 were retained in the models. All Cox models were stratified for type of lymphoma (low grade, high grade, and HL). Eight factors were found to be associated with OS. The sum of risk factors per patient was determined and tested for statistical difference using stratified Cox regression analysis. The best cut point for number of risk factors was selected from the model with the largest partial likelihood.29 We determined the following categories: low risk (one to three risk factors), intermediate risk (four to five risk factors), and high risk (≥ six risk factors). The final set of stratified Cox regression models were done to evaluate the association of our four level main effects (UUBs as reference group, UCB, RUB, and RCB) with OS and PFS as a whole and then separately according to risk levels. In addition, we tested whether adding income, distance traveled, and year of treatment altered the association between the main variable and outcomes. Because of the exploratory nature of our study, we used an α of .05 to decide on statistical significance despite performing multiple comparisons. All analyses were performed using SAS software, version 9.1 (SAS Institute, Cary NC).
RESULTS
Patient and Disease Characteristics
Table 1 presents the patient characteristics. RCB patients were more likely to be older (median age, 66 years) compared with UUB, UCB and RUB patients (median age, 54 v 62 v 60, respectively). Both RUB and RCB patients were also more likely to be white, have lower median household income, and travel greater distance to seek lymphoma-related treatment when compared with UUB and UCB. Median distance traveled by UCB patients to obtain treatment was 5 miles, and increases to 51 miles if treatment was sought from a university-based provider. Conversely, among RCB treated patients, the median distance traveled was 48 miles, and increases to 138 miles when treatment was sought from university-based provider. A slightly higher proportion of aggressive lymphomas in the RCB cohort (64%) compared with UUB (57%), UCB (62%), and RUB (62%) were noted, although no differences in the proportion of individual types of NHL were found across the groups. There was no difference in the distribution of patients according to risk level. There was also no difference in the distribution of patients according to risk level over time. More patients from rural areas and treated by community-based providers was noted in the earlier time period, while there are more patients from urban areas treated by university-based providers in the more recent period.
Treatment-Related Characteristics
A total of 23 community-based treatment sites representing 65 physicians and three university-based treatment sites representing eight physicians were included in the study. Although UUB patients were more likely to receive radiation as part of treatment (32%) compared with UCB (27%), RUB (24%), and RCB (27%), this difference was no longer noted after stratifying for type of lymphoma. The number of chemotherapy cycles patients received was also not different across groups. The use of hematopoietic cell transplantation (HCT), a common treatment for relapsed lymphoma and shown to be curative for diffuse large cell and Hodgkin's lymphoma, was significantly higher in patients treated by university-based providers (UUB = 19%; RUB = 16%) compared with patients treated by community-based providers (UCB = 11%; RCB = 10%, P < .01). The use of rituximab in diffuse large cell lymphoma from the year 2000, was significantly higher in UUB (64%) and RUB (73%) compared with patients treated by community-based providers (UCB = 40%; RCB 51%, P = .04).
Clinical Outcomes
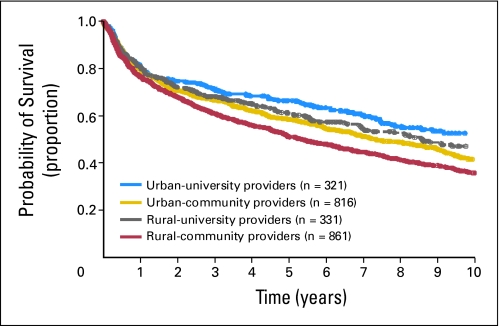
Figure 2 shows the plot of OS probability according to patient groups. There was no statistically significant difference in the 5-year probability of OS among UUB, UCB, and RUB patients (66% v 59% v 61%), but patients in the RCB group had a significantly inferior 5-year OS (51%; P < .001). Table 2 presents the multivariate analyses evaluating the risk of death. Using all 2,330 patients, the relative risk (RR) of death among UUB, UCB, and RUB were not statistically different. However, RCB had a higher risk of death when compared with UUB (RR, 1.37; 95% CI, 1.14 to 1.65; P = .01), as well as when compared with RUB (RR, 1.26; 95% CI, 1.06 to 1.49; P = .01). Income and distance traveled were not statistically associated with the outcomes evaluated. The year of treatment was associated with risk of death, with a significant improvement noted over time. The association noted between main variable and outcome remained with year of treatment in the model. We failed to detect any significant differences in the risk of progression or death across groups.
Fig 2.
Probability of overall survival according to place of residence and treatment provider (all patients).
Table 2.
Multivariate Analysis on Risk of Death According to Primary Area of Residence and Treatment Provider
| Main Effect | Risk of Death* |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients |
Low† |
Intermediate‡ |
High§ |
|||||||||||||
| No. | RR | 95% CI | P | No. | RR | 95% CI | P | No. | RR | 95% CI | P | No. | RR | 95% CI | P | |
| Urban/university-based | 321 | 1.0 | 144 | 1.0 | 119 | 1.0 | 58 | 1.0 | ||||||||
| Urban/community-based | 816 | 1.16 | 0.97 to 1.40 | .11 | 394 | 1.27 | 0.90 to 1.77 | .17 | 293 | 1.27 | 0.95 to 1.69 | .10 | 129 | 1.51 | 1.06 to 2.16 | .02 |
| Rural/university-based | 332 | 1.09 | 0.88 to 1.36 | .44 | 131 | 0.99 | 0.65 to 1.49 | .96 | 139 | 1.0 | 0.71 to 1.39 | .98 | 62 | 1.75 | 1.16 to 2.64 | .01 |
| Rural/community-based | 861 | 1.37 | 1.14 to 1.65 | .01 | 361 | 1.56 | 1.12 to 2.18 | .01 | 335 | 1.43 | 1.08 to 1.89 | .01 | 165 | 1.54 | 1.09 to 2.17 | .01 |
| Rural/university-based | 332 | 1.0 | 131 | 1.0 | 139 | 1.0 | 62 | 1.0 | ||||||||
| Rural/community-based | 861 | 1.26 | 1.06 to 1.49 | .01 | 361 | 1.58 | 1.15 to 2.17 | < .01 | 335 | 1.44 | 1.12 to 1.86 | < .01 | 165 | 0.90 | 0.65 to 1.24 | .52 |
NOTE. Bold font indicates statistical significance.
Model stratified by type of lymphoma (low grade, high grade, and Hodgkin's disease).
Low risk: ≤ 3 risk factors present.
Intermediate risk: 4 to 5 risk factors present.
High risk: ≥ 6 risk factors present.
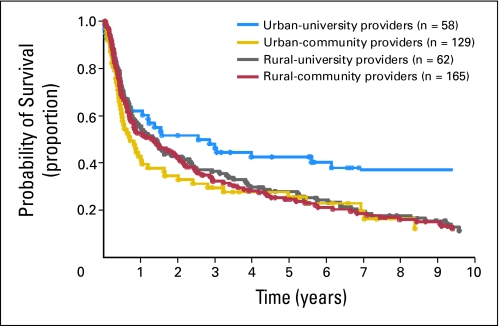
Subset analysis of patients according to risk level showed that among low-risk patients (Table 2), RCB was at 56% higher risk of death than UUB (RR, 1.56; 95% CI, 1.12 to 2.18; P = .01). Among intermediate-risk patients (Table 2), RCB was at 43% higher risk of death than UUB (RR, 1.43; 95% CI, 1.08 to 1.89; P = .01). Using RUB as reference group, RCB had a higher risk of death (RR, 1.58; 95% CI, 1.15 to 2.17; P < .01; RR, 1.44; 95% CI, 1.12 to 1.86; P < .01) for low- and intermediate-risk groups, respectively. Among high-risk patients (Table 2, Fig 3), the risks of death were all higher in the UCB, RUB, and RCB cohorts compared with the UUB cohort. Subset analysis among patients with NHL and with high risk IPI scores showed RCB patients were 40% more at higher risk of death than UUB (RR, 1.40; 95% CI, 1.08 to 1.82; P < .01), while UCB and RUB were not significantly different than UUB. No statistically significant difference in the risk of death among patients with low or low-intermediate IPI scores by patient groups was found. In addition, analyses of risk of progression or death showed no statistically significant differences by place of residence and treatment provider according to the study risk level and IPI scores.
Fig 3.
Probability of overall survival according to place of residence and treatment provider (high-risk patients).
Causes of Death
Patients from rural areas (RUB and RCB) were slightly more likely to die from primary disease (lymphoma) than patients from urban areas (80% v 75%; P = .04). Sixty percent of the patients in the RCB group died from lymphoma-related causes, while 82%, 72%, and 56% of the patients in the UUB, UCB, and RUB groups, respectively, died from lymphoma-related causes. Deaths due to disease progression was, however, not significantly different according to patient groups.
DISCUSSION
To our knowledge, this is the first study to show that patients with lymphoma from rural areas treated by community-based providers have inferior OS than UUB, UCB and RUB patients. This relationship was seen in different patient risk levels. In addition, our study showed that among high-risk patients, OS was inferior in all other patient groups when compared with UUB patients.
Generally, university-based physicians practice evidenced-based medicine that has superior outcomes. Various phase II and III clinical trials thought to have a positive impact on patients' outcomes are also more likely to be offered to patients.9,17 The inferior results we found in RCB patients may be explained by the disparity in the type of treatment offered. For instance, our study showed that HCT and rituximab, although shown to have superior outcomes30–37 in lymphoma, were less likely to be extended to patients treated by community-based providers. Alternatively, this can be explained by the systematic differences in the type of patients treated according to place of residence and treatment provider. However, this was not true in our study sample. Our study was able to consistently show the inferior outcome of RCB patients regardless of risk level, but for rural patients in the high-risk level outcomes are inferior regardless of where treatment is performed. This suggests that patient's area of residence in itself may be an independent risk factor for outcome. Our previous study in patients with lymphoma who underwent autologous HCT have demonstrated this.7 We think this may be related to a rural patient's logistical support structure that prevents them from acquiring consistent quality care, probably because of longer distance traveled.
Our findings may suggest that regionalization of lymphoma treatment to university-based providers is a reasonable option. But there are also advantages to acquiring treatment from community-based providers. Care in the community may be less expensive as it precludes having to make strenuous trips to a distant provider in an unfamiliar city.5,38 These factors are not trivial because cancer treatment in general when delivered closer to home has been shown to maintain or improve the patient's quality of life through the support of family and friends.39–47 If interventions are to be designed toward improving outcomes, distance must not be a barrier to one's ability to obtain or be provided evidence-based treatments. However, it should be noted that the decision-making process on where to obtain treatment is intrinsically less complex for patients from urban areas than for patients from rural areas. Studies have shown that rural patients would prefer to receive treatment from community-based providers.48,49 This has profound implications as to what kind of intervention can be designed to improve outcomes among rural patients.
There are several limitations to our study. One third of the patients were excluded due to unknown ZIP codes and incomplete prognostic clinical data. However, the survival outcome was similar between the included and excluded patients. Our study is retrospective and therefore cannot with certainty establish causality. Our study sample consisted of residents within the Midwest, and may have limited generalizability. However, since the central issue behind rural health care is based on dispersion and isolation of its population, we believe our findings can be generalized to other regions in the United States. It may actually be worst in other US regions where there are more racial or ethnic minorities. An analysis performed on specific types of lymphoma that received similar treatments would have been ideal; however, we did not have adequate statistical power to perform these analyses. Our study did not account for physician factors (training, experience, practice type, volume case load) that may have an impact on outcomes. This analysis requires physician level information and need to account for correlated data. Finally, we classified the treatment provider at the time of initial treatment of lymphoma. The complex patterns of shifting care, referrals, and level of interactions between the university- and community-based providers are not captured in this retrospective study. Despite all of these limitations, our study is still the largest population-based study conducted in this area of research and may serve as basis for further studies.
Our study highlights that despite the increase in new and innovative treatments for lymphoma, the issue of how these treatments are extended to patients in rural areas remain unmet. Outreach programs designed to improve availability of effective treatments especially to those in the rural areas should be implemented especially in high-risk patients with lymphoma.
Footnotes
Supported in part by Grants No. USPHS CA36727 from the National Cancer Institute, Department of Health and Human Services, and P30 CA 036727 from the Eppley Cancer Center Support Fund.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Presented at the Annual Meeting of the American Society of Hematology, San Francisco, CA, December 8, 2008.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Fausto R. Loberiza Jr, James O. Armitage
Financial support: James O. Armitage
Administrative support: Matt J. Moehr, Martin A. Bast
Provision of study materials or patients: Julie M. Vose, Philip J. Bierman, R. Gregory Bociek, James O. Armitage
Collection and assembly of data: Fausto R. Loberiza Jr, Anthony J. Cannon, Matt J. Moehr, Martin A. Bast
Data analysis and interpretation: Fausto R. Loberiza Jr, Anthony J. Cannon, Dennis D. Weisenburger, Julie M. Vose, Matt J. Moehr, Philip J. Bierman, R. Gregory Bociek, James O. Armitage
Manuscript writing: Fausto R. Loberiza Jr, Anthony J. Cannon, Dennis D. Weisenburger, Julie M. Vose, Philip J. Bierman, R. Gregory Bociek, James O. Armitage
Final approval of manuscript: Fausto R. Loberiza Jr, Anthony J. Cannon, Dennis D. Weisenburger, Julie M. Vose, Matt J. Moehr, Martin A. Bast, Philip J. Bierman, R. Gregory Bociek, James O. Armitage
REFERENCES
- 1.Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: The eastern North Carolina experience. Diabetes Educ. 2005;31:712–718. doi: 10.1177/0145721705280830. [DOI] [PubMed] [Google Scholar]
- 2.Johnson EA, Webb WL, McDowall JM, et al. A field-based approach to support improved diabetes care in rural states. Prev Chronic Dis. 2005;2:A08. [PMC free article] [PubMed] [Google Scholar]
- 3.Larson SL, Fleishman JA. Rural-urban differences in usual source of care and ambulatory service use: Analyses of national data using urban influence codes. Med Care. 2003;41:III65–III74. doi: 10.1097/01.MLR.0000076053.28108.F2. [DOI] [PubMed] [Google Scholar]
- 4.Lutfiyya MN, Lipsky MS, Wisdom-Behounek J, et al. Is rural residency a risk factor for overweight and obesity for U.S. children? Obesity (Silver Spring) 2007;15:2348–2356. doi: 10.1038/oby.2007.278. [DOI] [PubMed] [Google Scholar]
- 5.Onega T, Duell E, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 6.Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California 1988-2000. Cancer. 2006;107:1189–1195. doi: 10.1002/cncr.22016. [DOI] [PubMed] [Google Scholar]
- 7.Rao K, Darrington D, Schumacher J, et al. Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. Biol Blood Marrow Transplant. 2007;13:1508–1514. doi: 10.1016/j.bbmt.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Siminerio L, Piatt G, Zigbor J. Implementing the chronic care model for improvements in diabetes care and education in a rural primary care practice. Diabetes Educator. 2005;31:225–234. doi: 10.1177/0145721705275325. [DOI] [PubMed] [Google Scholar]
- 9.Ayanian J, Guadagnoli E. Variation in breast cancer treatment by patient and provider characteristics. Breast Cancer Res Treat. 1996;40:65–74. doi: 10.1007/BF01806003. [DOI] [PubMed] [Google Scholar]
- 10.Wiklund O, Haversen L, Pettersson C, et al. How can we prevent cardiovascular disease in diabetes? J Intern Med. 2007;262:199–207. doi: 10.1111/j.1365-2796.2007.01828.x. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SS, Richards TB, Thompson T, et al. Rural/nonrural differences in colorectal cancer incidence in the United States, 1998-2001. Cancer. 2006;107:1181–1188. doi: 10.1002/cncr.22015. [DOI] [PubMed] [Google Scholar]
- 12.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: A review of barriers to quality care. Cancer. 1999;86:2378–2390. [PubMed] [Google Scholar]
- 13.Higginbotham JC, Moulder J, Currier M. Rural v. urban aspects of cancer: First-year data from the Mississippi Central Cancer Registry. Fam Community Health. 2001;24:1–9. doi: 10.1097/00003727-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 14.McCullough D, Price M, Hindmarsh M, et al. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract. 1998;1:12–22. [PubMed] [Google Scholar]
- 15.Montori VM, Dinneen SF, Gorman CA, et al. The impact of planned care and a diabetes electronic management system on community-based diabetes care: The Mayo Health System Diabetes Translation Project. Diabetes Care. 2002;25:1952–1957. doi: 10.2337/diacare.25.11.1952. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox B. How do you make a difference in patients' lives through your role as a nurse navigator? ONS Connect. 2007;22:12. [PubMed] [Google Scholar]
- 17.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93:501–515. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- 19.Holm T, Johansson H, Cedermark B, et al. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg. 1997;84:657–663. [PubMed] [Google Scholar]
- 20.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95:1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinott R. Virtues of the Haversine. Sky and Telescope. 1984;68:159. [Google Scholar]
- 22.Arora NK, Hamilton AS, Potosky AL, et al. Population-based survivorship research using cancer registries: A study on non-Hodgkin's lymphoma survivors. J Cancer Surviv. 2007;1:49–63. doi: 10.1007/s11764-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 23.Bierman PJ, Sweetenham JW, Loberiza FR, et al. Syngeneic hematopoietic stem cell transplantation for non-Hodgkin's lymphoma: A comparison with allogeneic and autologous transplantation. J Clin Oncol. 2003;15:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 24.Navarro WH, Loberiza FR, Bajorunaite R, et al. Impact of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:541–551. doi: 10.1016/j.bbmt.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having non-Hodgkin lymphoma from the 1990s to the early 21st century. Arch Intern Med. 2008;10:469–476. doi: 10.1001/archinternmed.2007.125. [DOI] [PubMed] [Google Scholar]
- 26.The International Non-Hodgkin's Lymphoma Prognostic Factors Project: A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 28.Cox D. Regression models and life tables. J R Stat Soc Ser A. 1972;34:187–220. [Google Scholar]
- 29.Klein J. Chapter 2: Discretizing a continuous covariate in survival studies. In: Balakrishnan N, Rao CR, editors. Handbook of Statistics. Amsterdam, the Netherlands: Elsevier Science; 2008. pp. 27–42. [Google Scholar]
- 30.Andre M, Henry-Amar M, Pico JL, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin's disease induction failure: A case-control study: Societe Francaise de Greffe de Moelle. J Clin Oncol. 1999;17:222–229. doi: 10.1200/JCO.1999.17.1.222. [DOI] [PubMed] [Google Scholar]
- 31.Gianni AM, Bregni M, Siena S, et al. High-dose chemotherapy and autologous bone marrow transplantation compared with MACOP-B in aggressive B-cell lymphoma. N Engl J Med. 1997;336:1290–1297. doi: 10.1056/NEJM199705013361804. [DOI] [PubMed] [Google Scholar]
- 32.Gisselbrecht C, Lepage E, Molina T, et al. Shortened first-line high-dose chemotherapy for patients with poor-prognosis aggressive lymphoma. J Clin Oncol. 2002;20:2472–2479. doi: 10.1200/JCO.2002.02.125. [DOI] [PubMed] [Google Scholar]
- 33.Haioun C, Lepage E, Gisselbrecht C, et al. Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: Final analysis of the prospective LNH87-2 protocol: A Groupe d'Etude des Lymphomes de l'Adulte study. J Clin Oncol. 2000;18:3025–3030. doi: 10.1200/JCO.2000.18.16.3025. [DOI] [PubMed] [Google Scholar]
- 34.Philip T, Armitage JO, Spitzer G, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med. 1987;316:1493–1498. doi: 10.1056/NEJM198706113162401. [DOI] [PubMed] [Google Scholar]
- 35.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 36.Vose JM, Rizzo DJ, Tao-Wu J, et al. Autologous transplantation for diffuse aggressive non-Hodgkin lymphoma in first relapse or second remission. Biol Blood Marrow Transplant. 2004;10:116–127. doi: 10.1016/j.bbmt.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Yuen AR, Rosenberg SA, Hoppe RT, et al. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin's disease. Blood. 1997;89:814–822. [PubMed] [Google Scholar]
- 38.Probst JC, Laditka SB, Wang JY, et al. Effects of residence and race on burden of travel for care: Cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;7:40. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bass EB, Jenckes MW, Fink NE, et al. Use of focus groups to identify concerns about dialysis: Choice Study. Med Decis Making. 1999;19:287–295. doi: 10.1177/0272989X9901900307. [DOI] [PubMed] [Google Scholar]
- 40.Coiffier B, Herbrecht R, Tilly H. GELA study comparing CHOP and R-CHOP in elderly patients with DLBCL: 3-year median follow-up with an analyis according to co-morbidity factors. Proc Am Soc Clin Oncol. 2003;22:596. abstr 2395. [Google Scholar]
- 41.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 42.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 43.Krouse R, Grant M, Ferrell B, et al. Quality of life outcomes in 599 cancer and non-cancer patients with colostomies. J Surg Res. 2007;138:79–87. doi: 10.1016/j.jss.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 44.Moist LM, Bragg-Gresham JL, Pisoni RL, et al. Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;51:641–650. doi: 10.1053/j.ajkd.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Pandey M, Thomas BC, SreeRekha P, et al. Quality of life determinants in women with breast cancer undergoing treatment with curative intent. World J Surg Oncol. 2005;3:63. doi: 10.1186/1477-7819-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vose JM, Link BK, Grossbard ML, et al. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2001;19:389–397. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 47.Voutilainen P, Backman K, Isola A, et al. Family members' perceptions of the quality of long-term care. Clin Nurs Res. 2006;15:135–149. doi: 10.1177/1054773805285697. [DOI] [PubMed] [Google Scholar]
- 48.Adams EK, Houchens R, Wright GE, et al. Predicting hospital choice for rural Medicare beneficiaries: The role of severity of illness. Health Serv Res. 1991;26:583–612. [PMC free article] [PubMed] [Google Scholar]
- 49.Adams EK, Wright GE. Hospital choice of Medicare beneficiaries in a rural market: Why not the closest? J Rural Health. 1991;7:134–152. doi: 10.1111/j.1748-0361.1991.tb00715.x. [DOI] [PubMed] [Google Scholar]