Abstract
Purpose
To compare the antitumor efficacy of three different anthracyclines in combination with cytarabine and etoposide in adult patients with newly diagnosed acute myeloid leukemia (AML).
Patients and Methods
We randomly assigned 2,157 patients (age range, 15 to 60 years) to receive intensive induction-consolidation chemotherapy containing either daunorubicin, idarubicin, or mitoxantrone. After achieving complete remission (CR), patients were assigned to undergo either allogeneic or autologous stem-cell transplantation (SCT), depending on the availability of a sibling donor.
Results
The overall CR rate (69%) was similar in the three groups. Autologous SCT was performed in 37% of cases in the daunorubicin arm versus only 29% and 31% in mitoxantrone and idarubicin, respectively (P < .001). However, the disease-free survival (DFS) and survival from CR were significantly shorter in the daunorubicin arm: the 5-year DFS was 29% versus 37% and 37% in mitoxantrone and idarubicin, respectively. The proportion of patients who underwent allogeneic SCT (22%) was equivalent in the three treatment groups, and the outcome was similar as well: the 5-year overall survival rates were 34%, 34%, and 31%, respectively.
Conclusion
In adult patients with AML who do not receive an allogeneic SCT, the use of mitoxantrone or idarubicin instead of daunorubicin enhances the long-term efficacy of chemotherapy.
INTRODUCTION
Approximately 60% to 80% of adults with acute myeloid leukemia (AML) achieve complete remission (CR) with an induction regimen including the anthracycline daunorubicin and the antimetabolite cytarabine.1,2 The precise value of the addition of 6-thioguanine is not known currently,3 whereas limited benefit derives from the addition of etoposide in patients younger than 55 years.4,5 Strategies to improve results have included the use of intercalating agents other than daunorubicin, such as idarubicin or mitoxantrone, while keeping the dose of cytarabine constant. In several randomized trials comparing idarubicin with daunorubicin, idarubicin was associated with a lower incidence of resistant leukemia and higher CR rates, particularly in younger patients.6–8 The anthraquinone derivative mitoxantrone has also been extensively used with cytarabine as part of effective induction regimens.9–15 However, because of the relatively small sample size and/or the relatively short follow-up time, the benefits of these new agents on the long-term outcome of patients with AML have not been definitively established.
The aims of this randomized trial were to compare the relative efficacy and toxicities of the intercalating agents daunorubicin, mitoxantrone, and idarubicin in an intensive treatment program including stem-cell transplantation (SCT) in patients aged 60 years or younger with newly diagnosed AML.
PATIENTS AND METHODS
Patients
Previously untreated patients with a diagnosis of AML according to the criteria of the French-American-British group16,17 were eligible for entry onto the trial provided they met the following requirements (1): age 15 to 60 years (2); diagnosis of primary or secondary AML (including AML after myelodysplastic syndrome) other than French-American-British M3 (3); no evidence of severe concurrent cardiac, pulmonary, neurologic, and metabolic diseases or uncontrolled infections; and (4) adequate liver (serum bilirubin level < 2× upper normal limit) and renal (serum creatinine < 2× upper normal limit) function tests. Exclusion criteria included blast crisis of chronic myeloid leukemia and AML supervening after other chronic myeloproliferative diseases and other progressive malignant diseases. The study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All participants gave their informed consent.
Study Design
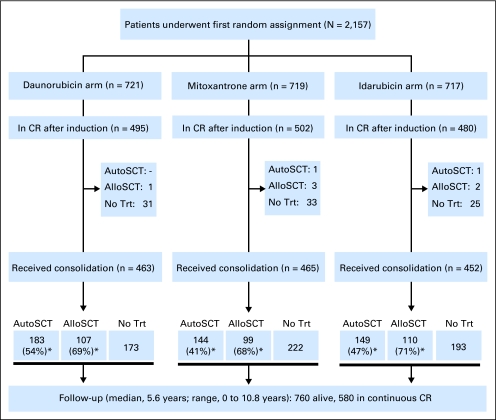
The AML-10 was a randomized phase III study carried out by the European Organisation for Research and Treatment of Cancer leukemia group and Gruppo Italiano Malattie Ematologiche dell'Adulto in 80 European centers. Study design and patient disposition are represented in Figure 1.
Fig 1.
CONSORT diagram: study design and patient disposition. (*) In each arm, percentage of autologous stem-cell transplantation (AutoSCT) is calculated on the total number of patients in complete remission (CR) without an HLA-identical sibling, and percentage of allogeneic stem-cell transplantation (AlloSCT) is calculated on the total number of patients in CR with an HLA-identical sibling. Trt, treatment.
The main objective of the study was to evaluate the relative efficacy and toxicity of an intensive remission induction and consolidation chemotherapy incorporating one of three intercalating agents (daunorubicin, mitoxantrone, or idarubicin) in combination with cytarabine and etoposide in induction and with cytarabine alone in consolidation in patients with newly diagnosed AML. An amendment to the protocol was adopted in 1994, introducing a second randomization to compare the feasibility and results of peripheral-blood versus bone marrow autologous SCT as rescue from myeloablative therapy after remission consolidation in patients without an available HLA-identical sibling donor. Primary end point of the first randomization was overall survival, with secondary end points being the CR rate after induction, the disease-free survival and survival from CR, type and grade of toxicity related to different treatment steps, time to recovery, the feasibility of stem-cell collection after the consolidation course, and the rate of completion of autologous and allogeneic SCT.
Patients were screened at each center, and those who fulfilled the eligibility criteria were randomly assigned at the European Organisation for Research and Treatment of Cancer Data Center in Brussels, Belgium. Randomization was stratified according to center, age (15 to 45 years v 46 to 60 years), WBC count (< 50 v 50 to 249 v > 250 × 109/L), and WHO performance status (0 to 2 v 3 to 4) using the minimization technique.
Response assessment was planned on day 28 after induction. The Cancer and Leukemia Group B criteria for response to treatment and relapse were used.18 After CR, the availability of histocompatible sibling donors was evaluated. Patients ≤ 45 years of age (or 55 years according to the policy of center) with HLA-matched (or single-antigen mismatched) family donor were offered allogeneic SCT, whereas the remainder were randomly assigned between either peripheral-blood or bone marrow autologous SCT.
Treatment
Remission induction treatment consisted of cytarabine 25 mg/m2 as intravenous bolus followed immediately by 100 mg/m2 given as a continuous infusion daily for 10 days; etoposide 100 mg/m2 in 0.9% saline daily by intravenous infusion (1 hour) on days 1 to 5; and on days 1, 3, and 5, one of the following: daunorubicin 50 mg/m2 as a 5-minute infusion, mitoxantrone 12 mg/m2 as a 30-minute infusion, or idarubicin 10 mg/m2 as a 5-minute infusion. In case of partial remission, a second remission induction course with the same drugs was given. No myeloid growth factor administration was planned by protocol during induction. In case of CR, a single course of consolidation therapy was administered, consisting of intermediate-dose cytarabine (500 mg/m2 every 12 hours in a 2-hour infusion on days 1 through 6) and the same intercalator used during induction, given on days 4 to 6.
Younger patients with a sibling donor were then assigned to undergo an allogeneic SCT. Those without such a donor, as well as older patients, were to receive an unpurged autologous blood or bone marrow SCT. Mobilization and collection of autologous blood stem cells was planned for all these patients irrespective of the assignment of the second randomization and was scheduled during the recovery phase of the consolidation course. Lenograstim (150 mcg/m2) was given by daily subcutaneous injections from day 20 of the consolidation course until completion of the peripheral-blood SCT. The total blood stem-cell collection was considered successful if the CD34+ cells yield was at least 2 × 106/kg body weight. Those patients who had to undergo a bone marrow transplantation underwent a bone marrow collection procedure under general anesthesia.
Statistical Analysis
Overall survival was defined as the time interval from random assignment until death, whatever the cause. Disease-free survival was defined as the time from CR until the first relapse or death from any cause. Survival from CR was defined as the time from CR until death from any cause. The duration of recovery was defined as the time from the first day of the chemotherapy course until neutrophil recovery (neutrophils > 0.5 × 109/L) or platelet recovery (platelets > 20 × 109/L); patients without recovery were censored at day 90. Toxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria version 2.0.
The required number of patients was calculated to detect an increase in the 5-year survival rate from 40% to 50% for each of the two main comparisons, idarubicin versus daunorubicin and mitoxantrone versus daunorubicin (two-tailed test, α = 2.5%, β = 20%). For such comparisons, a total of 744 patients observed until death were required (ie, a minimum of 1,353 patients to be randomly assigned and observed for 5 years). Such number of patients also allowed the detection of a 10% difference (70% v 80%) in the CR rate after induction (α = 2.5%, β = 10%).
The actuarial survival curves were computed using the Kaplan-Meier technique, and the SEs of the estimates were obtained via the Greenwood formula.19 The differences between curves were tested for statistical significance using the two-tailed log-rank test.19 The estimate of the cumulative incidence of relapse and of the incidence of death in CR and their corresponding SEs were obtained by using competing risk methods.19 The Cox's proportional hazards model was used to obtain the estimate and the 97.5% or 99% CI of the hazard ratio of the instantaneous event rate in each experimental group (mitoxantrone or idarubicin) versus the daunorubicin group. This model has also been used to adjust the treatment comparison for stratification factors, except center, disease, and cytogenetics features.
The linear logistic regression model was used to perform the treatment comparisons regarding CR rate after induction.
All the efficacy analyses were performed according to the intention-to-treat-principle (all patients randomly assigned were included). Patients who started the protocol treatment were considered for sensitivity analyses regarding efficacy and for comparisons of toxicities and of time to recovery. The database was frozen on October 5, 2006. The SAS 9.1 software (SAS Institute Inc, Cary, NC) was used for the statistical analyses.
RESULTS
Patient Characteristics
Between November 1993 and December 1999, a total of 2,157 patients were randomly assigned in the trial. Their median age was 44 years (range, 15 to 60 years); 50.3% of patients were male. The median WBC count was 16.3 × 109/L (range, 0.4 to 590 × 109/L). The three treatment groups were evenly matched with respect to various baseline characteristics (Table 1). A total of 65 patients were considered ineligible: 19 patients in the daunorubicin arm, 26 patients in the mitoxantrone arm, and 20 patients in the idarubicin arm. Reasons for ineligibility included concomitant diseases in 29 patients, insufficient data in 20 patients, prior chemotherapy for AML in six patients, and other causes in 10 patients.
Table 1.
Patient Characteristics by Randomized Treatment Group
| Characteristic | Treatment Arm |
|||||
|---|---|---|---|---|---|---|
| DNR |
MXR |
IDA |
||||
| No. | % | No. | % | No. | % | |
| Total | 721 | 719 | 717 | |||
| Cooperative group | 309 | 42.9 | 295 | 41.0 | 311 | 43.4 |
| EORTC | 309 | 42.9 | 295 | 41.0 | 311 | 43.4 |
| GIMEMA | 412 | 57.1 | 424 | 59.0 | 406 | 56.6 |
| Age at diagnosis, years | ||||||
| 15-25 | 96 | 13.3 | 104 | 14.5 | 83 | 11.6 |
| 26-45 | 308 | 42.7 | 294 | 40.9 | 314 | 43.8 |
| 46-60 | 317 | 44.0 | 321 | 44.6 | 320 | 44.6 |
| Male sex | 354 | 49.1 | 358 | 49.8 | 373 | 52.0 |
| Performance status* at random assignment | ||||||
| 0 | 305 | 42.3 | 308 | 42.8 | 312 | 43.5 |
| 1 | 307 | 42.3 | 310 | 43.1 | 301 | 42.0 |
| 2 | 97 | 13.5 | 89 | 12.4 | 92 | 12.8 |
| 3-4 | 12 | 1.6 | 12 | 1.7 | 12 | 1.7 |
| Missing | 16 | 2.2 | 19 | 2.6 | 13 | 1.8 |
| Type of AML | ||||||
| De novo | 693 | 96.1 | 688 | 95.7 | 683 | 95.3 |
| Therapy-related | 15 | 2.1 | 15 | 2.1 | 17 | 2.4 |
| Secondary | 13 | 1.8 | 16 | 2.2 | 17 | 2.4 |
| FAB subtype† | ||||||
| M0 | 30 | 4.2 | 31 | 4.3 | 31 | 4.3 |
| M1 | 115 | 16.0 | 141 | 19.6 | 131 | 18.3 |
| M2 | 233 | 32.3 | 231 | 32.1 | 226 | 31.5 |
| M3 | 2 | 0.3 | 4 | 0.6 | 3 | 0.4 |
| M4 | 163 | 22.6 | 139 | 19.3 | 147 | 20.5 |
| M5 | 136 | 18.9 | 133 | 18.5 | 133 | 18.5 |
| M6 | 22 | 3.1 | 24 | 3.3 | 23 | 3.2 |
| M7 | 8 | 1.1 | 5 | 0.7 | 6 | 0.8 |
| Missing/unknown | 12 | 1.7 | 11 | 1.5 | 17 | 2.3 |
| WBC count at random assignment, ×109/L | ||||||
| < 50 | 525 | 72.8 | 520 | 72.3 | 522 | 72.8 |
| 50-249.9 | 184 | 25.5 | 185 | 25.7 | 182 | 25.4 |
| ≥ 250 | 12 | 1.7 | 14 | 1.9 | 13 | 1.8 |
| Cytogenetics‡ | ||||||
| Favorable | 82 | 11.3 | 61 | 8.5 | 79 | 11.0 |
| Intermediate | 158 | 21.9 | 146 | 20.3 | 151 | 21.1 |
| Unfavorable | 50 | 6.9 | 63 | 8.8 | 66 | 9.2 |
| Other | 108 | 15.0 | 99 | 13.8 | 101 | 14.1 |
| Missing/unknown | 323 | 44.8 | 350 | 48.7 | 320 | 44.6 |
Abbreviations: DNR, daunorubicin; MXR, mitoxantrone; IDA, idarubicin; EORTC, European Organisation for Research and Treatment of Cancer; GIMEMA, Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto; AML, acute myeloid leukemia; FAB, French-American-British.
WHO scale.
Based on the opinion of local cytologist or of the review, if available. Six patients were scored as M3 by the local cytologist: one was confirmed by the reviewer, one was not confirmed, and three were not reviewed. Five additional patients, initially considered as non-M3, were considered thereafter as M3 by the reviewer.
Abnormalities 16q22, and t(8;21) were considered favorable risk abnormalities, whether other abnormalities were present or not; eight patients with t(15;17), ineligible for this trial, have been randomly assigned and considered in this favorable group as well. NN karyotypes (ie no abnormalities seen in a minimum of 20 mitoses) or those with –Y only were classified as intermediate risk. Deletions of the long arm of chromosomes 5 (5q−) and/or 7 (7q−), or of the entire chromosomes (−5,−7) and complex abnormalities (> three abnormalities) were considered of unfavorable prognosis. Patients with other abnormalities were pooled into a separate cytogenetic risk group (“other”). Patients with unknown, not done, or unsuccessful cytogenetics were grouped together as unknown risk.
Remission Induction and Consolidation Chemotherapy
A CR after one or two courses of induction chemotherapy was achieved in 1,477 (68.5%) of 2,157 patients, with no significant difference observed between the treatment arms, mitoxantrone versus daunorubicin (P = .63) and idarubicin versus daunorubicin (P = .49). In most cases (93%), this response was obtained after the first induction cycle. Additional details regarding the response rates and types of failure are reported in Table 2 and Figure 1.
Table 2.
Response to Induction Chemotherapy by Assigned Treatment Group
| Response | Treatment Arm |
|||||
|---|---|---|---|---|---|---|
| DNR |
MXR |
IDA |
||||
| No. | % | No. | % | No. | % | |
| No. of patients | 721 | 719 | 717 | |||
| Overall complete response | 495 | 68.7 | 502 | 69.8 | 480 | 66.9 |
| Partial response | 16 | 2.2 | 14 | 1.9 | 21 | 2.9 |
| Resistant disease | 98 | 13.6 | 83 | 11.5 | 102 | 14.2 |
| Persistent hypoplasia | 5 | 0.7 | 7 | 1.0 | 3 | 0.4 |
| Early death* | 23 | 3.2 | 21 | 2.9 | 24 | 3.3 |
| Death in hypoplasia | 64 | 8.9 | 72 | 10.0 | 74 | 10.3 |
| Unknown/missing data | 20 | 2.8 | 20 | 2.8 | 13 | 1.8 |
Abbreviations: DNR, daunorubicin; MXR, mitoxantrone; IDA, idarubicin.
Early death was defined as death before the completion of the first cycle of induction therapy, and death in hypoplasia was defined as death after the completion of the induction cycle (1 or 2) before hematologic recovery.
One consolidation course was administered to 1,380 (93.4%) of 1,477 complete responders, evenly distributed in the three arms. Consolidation was not given to the other 97 patients for various reasons: severe toxicity from induction chemotherapy (n = 57), early relapse (n = 8), withdrawal of consent/protocol violation (n = 12), and other causes (n = 20). Among these 97 patients, two patients underwent an autologous and six patients underwent an allogeneic transplantation in first CR.
Transplantation
Only five of the 1,477 patients who reached CR were not typed. An HLA-matched sibling donor was found in 465 (31.6%) of 1,472 typed patients in CR. Among these, allogeneic SCT was eventually performed in 322 (69.2%; in six patients without consolidation cycle). The rate of patients who underwent allogeneic SCT (SCT performed of patients with an HLA-identical sibling available) was similar in the three intercalator arms.
Among the remaining 1,007 patients in CR—typed but without a donor—autologous SCT was successfully performed in a total of 513 patients: 194 patients in the daunorubicin arm, 161 patients in the mitoxantrone arm, and 158 in the idarubicin arm. In particular, cell collection was completed by peripheral blood in 357 (70%) of 513 patients: 132 patients in the daunorubicin arm, 116 patients in the mitoxantrone arm, and 109 patients in the idarubicin arm. Additional details about this step of the treatment program have been published previously.20
Autograft was successfully performed in 478 cases (476 after the consolidation plus two in patients who did not receive the consolidation course). The distribution of autologous SCT among the three arms was uneven: 183 (54.3%) of 337 patients in the daunorubicin arm, 145 (41.4%) of 350 patients in the mitoxantrone arm, and 150 (46.9%) of 320 patients in the idarubicin arm (P < .001). This significant difference was due to a higher rate of withdrawals from toxicity and/or to a lower success rate of sufficient stem-cell collection in the mitoxantrone and idarubicin arms: 194 (58%) of 337 patients in the daunorubicin arm, as compared with 161 (46%) of 350 patients in the mitoxantrone arm and 158 (49%) of 320 patients in the idarubicin arm (Hematopoietic Recovery and Adverse Effects).
Details and results regarding the second randomization will be published separately.
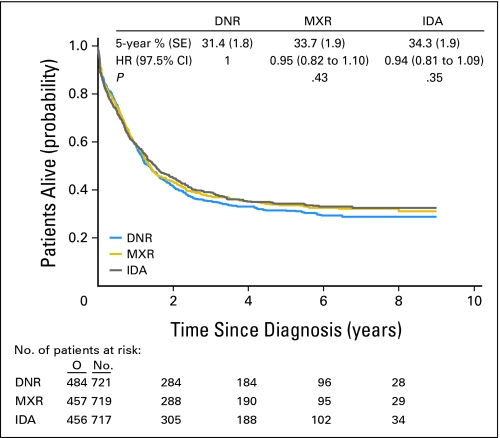
Survival
At the time of data analysis the median follow-up was 5.6 years. The median overall survival was 1.4 years, with no significant differences among the three treatment arms (Fig 2). A Cox model showed that the results remained unchanged after the adjustment for several presenting factors (disease, age, cytogenetics, WBC count).
Fig 2.
Global population of patients: duration of overall survival from diagnosis, according to the treatment arm. DNR, daunorubicin; MXR, mitoxantrone; IDA, idarubicin; HR, hazard ratio; O, observed number of deaths.
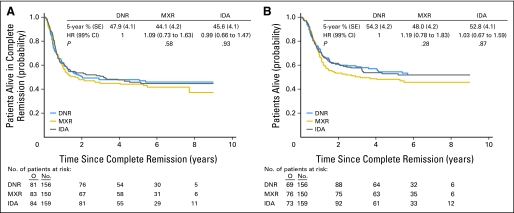
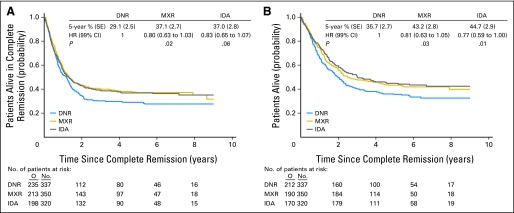
Disease-free survival and survival from CR were similar in the three intercalator arms. Separate treatment comparisons were performed in patients with and in those without a donor. In patients with a donor (n = 465), the use of different intercalators had no impact on the long-term outcome (Figs 3A and 3B). In those without a donor (n = 1,007), the disease-free survival (Fig 4A) and survival from CR (Fig 4B) were longer in the mitoxantrone and idarubicin arms than in the daunorubicin arm.
Fig 3.
Patients with an HLA sibling donor available. (A) Disease-free survival from complete remission according to treatment arm. (B) Survival from complete remission according to treatment arm. DNR, daunorubicin; MXR, mitoxantrone; IDA, idarubicin; HR, hazard ratio; O, observed number of events.
Fig 4.
Patients without an HLA sibling donor available. (A) Disease-free survival from complete remission according to treatment arm. (B) Survival from complete remission according to treatment arm. DNR, daunorubicin; MXR, mitoxantrone; IDA, idarubicin; HR, hazard ratio; O, observed number of events.
In all patients who reached CR, censoring the follow-up at the moment of allogeneic SCT, the comparison of mitoxantrone versus daunorubicin yielded a hazard ratio of 0.82 (97.5% CI, 0.67 to 1.00; P = .025), and idarubicin versus daunorubicin yielded a hazard ratio of 0.85 (97.5% CI, 0.70 to 1.04; P = .07). Regarding survival from CR, the results were 0.86 (97.5% CI, 0.69 to 1.05; P = .09) and 0.81 (97.5% CI, 0.65 to 1.00; P = .025), respectively.
Hematopoietic Recovery and Adverse Effects
The times to a neutrophil count of 0.5 × 109/L and a platelet count of 20 × 109/L after induction treatment were similar in the three arms, whereas after consolidation, the hematopoietic recovery was significantly shorter in the daunorubicin group than in the two other intercalator groups (Table 3).
Table 3.
Time to Hematopoietic Recovery and Incidence of WHO Grade 3 to Grade 4 Main Adverse Events After Induction and Consolidation Treatments
| Time to Recover/Adverse Event | Treatment Arm |
||
|---|---|---|---|
| DNR | MXR | IDA | |
| Time to neutrophil count > 0.5 × 109/L, days* | |||
| First induction | 26 | 27 | 26 |
| Consolidation† | 22 | 26 | 26 |
| Time to platelet count > 20 × 109/L, days* | |||
| First induction | 24 | 25 | 24 |
| Consolidation‡ | 20 | 26 | 26 |
| Grade 3 or 4 infections, % | |||
| First induction | 29.8 | 33.2 | 32.8 |
| Consolidation§ | 13.6 | 24.3 | 22.3 |
| Grade 3 or 4 adverse effects other than infections, % | |||
| First induction | 44.5 | 45.9 | 46.0 |
| Consolidation‖ | 16.8 | 20.4 | 24.0 |
Abbreviations: DNR, daunorubicin; MXR, mitoxantrone; IDA, idarubicin.
Recovery times of patients who achieved a complete remission. Results are presented as median.
P value, overall comparison: < .001.
P value, overall comparison: < .001.
P value, overall comparison: .001 (MXR v DNR, P < .001; IDA v DNR, P = .001).
P value, overall comparison: .008 (MXR v DNR, P = .20; IDA v DNR, P = .01).
The frequencies of various grade 3 or grade 4 adverse effects after induction chemotherapy were similar between the three groups. After consolidation, both severe infections and severe noninfectious toxicities were more frequent in the mitoxantrone and idarubicin arms than in the daunorubicin arm (Table 3). There were no differences regarding the frequency of bacterial and fungal infections (data not shown).
DISCUSSION
This randomized trial, the largest study in adults with AML published to date, attempted to eliminate the possible biases of previous trials comparing intercalating agents: insufficient numbers of patients involved to demonstrate a small but clinically meaningful advantage for one of the treatment arms and insufficient duration of follow-up to demonstrate the impact of treatment on the long-term outcome. The results indicate that mitoxantrone and idarubicin are not superior to daunorubicin regarding the CR rate and overall survival. However, in patients without an HLA-identical sibling donor, the disease-free survival and survival from CR were significantly longer in the mitoxantrone and idarubicin arms than in the daunorubicin arm. This dismal outcome in the daunorubicin group of patients without a donor become more evident after a longer follow-up, because it was mainly related to an higher incidence of late relapse.
The interpretation of these findings requires careful attention. Whereas the three induction regimens seemed comparable both in terms of efficacy and toxicity, the longer duration of neutrophil and platelet recovery after consolidation seem to indicate a more pronounced cumulative hematopoietic toxicity of the mitoxantrone and idarubicin arms. These two arms showed also a significantly higher rate of severe infections and other adverse effects than in the daunorubicin arm, resulting in a lower rate of successful collection of peripheral stem cells and, accordingly, a reduced feasibility of autologous SCT.
Considering the significantly higher rate of SCT performed in the daunorubicin arm, and the related possible stronger antileukemic activity, one could have expected better outcomes in terms of disease-free survival in this group of patients; however, this was not the case. Patients treated with mitoxantrone or idarubicin actually had a significantly lower relapse rate and longer survival, possibly suggesting a more prominent antileukemic effect exerted by these two drugs, who could be able to produce a better in vivo purging, resulting in fewer residual normal and leukemic stem cells and “better quality” remissions. These data confirm results previously reported in the detailed analysis of peripheral-blood stem-cell collection and transplantation outcomes.20
With regard to the mechanism of action, both mitoxantrone and idarubicin are known to be active against leukemia cell lines resistant to daunorubicin.21,22 Whether these drugs exert a differential activity on normal hematopoietic stem cells is not clear. However, the comparable toxicity of the three drugs in combination with conventional-dose cytarabine and etoposide during induction does not necessarily imply that the same doses of the drugs have equivalent effects with the combination of intermediate-dose cytarabine during postremission chemotherapy. Unfortunately, no other randomized study has made this direct comparison.
In patients with a sibling donor, most of whom underwent an allogeneic SCT, the beneficial effect of mitoxantrone and idarubicin was not apparent, probably due to the additional graft-versus-leukemia effect, which is likely drug-independent.
Comparison of our trial with previous trials can hardly be performed because of the different study designs and/or patient populations. A randomized study of the Eastern Cooperative Oncology Group in older patients (age > 55 years) comprising 362 patients only did not show any difference in remission rates, disease-free survival, overall survival, or toxicity among patients receiving one of these three agents during the induction phase.13 Aside from that, no other study has directly compared the three intercalators.
A meta-analysis of randomized trials comparing idarubicin with daunorubicin reported similar early induction failure rates (20% for idarubicin v 18% for daunorubicin; P = .4), but fewer late (after day 40) induction failures with idarubicin (53% v 62%, P = .002).23 Among patients achieving CR, fewer patients assigned to idarubicin experienced relapse (P = .008) but somewhat more died in CR, resulting in no significant benefit in disease-free survival (P = .07). Furthermore, overall survival was better with idarubicin compared with daunorubicin, with 13% versus 9%, respectively, alive at 5 years (P = .03).
In the Medical Research Council AML-12 trial,15 1,243 patients 15 to 59 years of age (26 patients between 60 and 65 years of age) were randomly assigned to either daunorubicin or mitoxantrone, each given with cytarabine for one or two courses for induction. All patients achieving CR subsequently received multiple courses of consolidation chemotherapy. There were no significant differences in CR, percentage of patients dying in CR, resistant disease, relapse, disease-free survival, or overall survival between the two induction regimens.
Another systematic overview of chemotherapy effects in AML has not shown conclusive evidence in favor of the use of mitoxantrone.24
In summary, our results indicate that in adults with AML without an HLA-compatible sibling donor, the use of mitoxantrone or idarubicin during both induction and consolidation reduces the risk of relapse. Either of these drugs should be considered to replace daunorubicin in future trials for the treatment of AML, provided that allogeneic SCT is not considered as postremission therapy.
Acknowledgment
The following members of the GIMEMA participated in this study: Drs Mandelli, Petti and Meloni (La Sapienza, Roma, IT); Drs Resegotti and Pileri (Torino, IT); Dr Giustolisi (Ferrarotto, Catania, IT); Drs Liso and Specchia (Bari, IT); Drs Ferrara and Mettivier (Cardarelli, Napoli, IT); Dr Rotoli (Federico II, Napoli, IT); Drs Amadori and Venditti (S Eugenio, Roma, IT); Dr Martelli and Tabilio (Perugia, IT); Dr Fioritoni (Pescara, IT); Drs Leone and Sica (Gemelli, Roma, IT); Dr Rossi Ferrini (Firenze, IT); Drs Caronia and Mirto (Cervello, Palermo, IT); Drs Carotenuto and Melillo (S Giovanni Rotondo, IT); Drs Cajozzo and Mariani (Giaccone, Palermo, IT); Dr Volpe (Avellino, IT); Dr Broccia (Cagliari, IT); Drs Damasio, Carella and Bacigalupo (San Martino, Genova, IT); Dr Leoni (Ancona, IT); Drs Alberti and Peta (Catanzaro, IT); Dr Miraglia (Nuovo Pellegrino, Napoli, IT); Dr Nobile (Reggio Calabria, IT); Dr De Riu (Latina, IT); Dr Gallamini (Cuneo, IT); Dr Ricciuti (Potenza, IT); Dr Rizzoli (Parma, IT); Dr Gabbas (Nuoro, IT); Drs Iori and Gugliotta (Reggio Emilia, IT); Dr Lucarelli (Pesaro, IT); Dr Bordignon (S Raffaele, Milano, IT); Drs De Laurenzi and Pacilli (S Camillo, Roma, IT); Drs Porcellini and Morandi (Cremona, IT); Dr Monaco (Foggia, IT); Dr Saglio (Orbassano, IT); Dr Mozzana (Gallarate, IT); Dr Citarrella (Università, Palermo, IT); Dr Torelli (Modena, IT); Dr Mazza (Taranto, IT); Dr Morra (Niguarda, Milano, IT); Dr Monfardini (Aviano, IT); Dr Nalli (Lodi, IT); Dr Avanzi (Novara, IT); Dr Levis (Alessandria, IT); Dr Quarta (Brindisi, IT); Dr Gobbi (Università, Genova, IT); Dr Bonanno (S Gennaro, Napoli, IT); Dr Pastorini (Sondalo, IT).
Drs Tabilio, Carotenuto and Rotoli deceased.
The following members of the EORTC Leukemia Group participated in this study: Drs de Witte, Muus (Nijmegen, NL); Drs Zittoun, Marie and Vekhouff (Hotel Dieu, Paris, FR); Drs Archimbaud, Belhabri, Chelghoum, Thomas, Fière (Lyon, FR); Dr Labar (Zagreb, HR), Dr Willemze (Leiden, NL); Dr Varet (Necker-Institut Curie, Paris, FR); Dr Jehn (Muenchen, DE); Dr Bourhis (Villejuif, FR); Dr Fillet (Liège, BE); Drs Selleslag and Louwagie (Brugge, BE); Drs Bron, Stryckmans and Feremans (Bordet-Erasme, Brussels, BE); Dr Beksac (Ankara, TR); Drs Gastl and Stauder (Innsbruck, AT); Dr Sinnige ('S Hertogenbosch, NL); Dr Dreyfus (Cochin, Paris, FR); Dr Gandra (Porto, PT); Dr Jaksic (Zagreb, HR); Drs Berneman and Peetermans (University Hospital, Antwerpen, BE); Dr Cermak (Prague, CZ); Dr De Bock (Middelheim, Antwerpen, BE); Dr Vreugdenhil (Veldhoven, NL); Dr Sousa (Coimbra, PT), Dr Vermeulen (Verviers, BE); Dr Indrak (Olomouc, CZ); Teixeira (Coimbra, PT); Dr De Valk (Amsterdam, NL); Dr Schaafsma (Enschede, NL); Dr Thyss (Nice, FR); Dr Efira (St. Pierre, Brussels, BE); Dr Gerhartz (Duisburg, DE), Dr Baumelou (Suresnes, FR).
Drs Archimbaud and Stryckmans deceased.
We thank the cytogeneticists of the different institutions, in particular Drs Bernheim (Villejuif), Mancini (Rome), Olde-Weghuis (Nijmegen) and Hagemeijer (Leuven), for the review of karyotypes. We thank the data-managers who took care of this study: Mr G. Solbu, Mrs M. Dardenne, Mrs U. Morjaria, Mr S. Lejeune, Mrs S De Simone, Mrs F Cotugno and Mrs E Criado.
We acknowledge St Jude Children's Research Hospital for providing an SAS macro allowing the computation of the cumulative incidences of relapse and of death in CR.
Footnotes
Supported in part by Grants No. 2U10-CA11488-23 through 2U10-CA11488-36 from the National Cancer Institute, Bethesda, MD; and by grants from the Italian Cancer League and by the Italian Association Against Leukemias, Lymphoma, and Myeloma.
The contents of this study are solely the responsibility of the authors and do not represent the official views of the National Cancer Institute.
Presented in part at the 45th Annual Meeting of the American Society of Hematology, December 6-9, 2003, San Diego, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002549.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Franco Mandelli, Stefan Suciu, Petra Muus, Roel Willemze, Robert Zittoun, Sergio Amadori, Theo de Witte
Administrative support: Marco Vignetti
Provision of study materials or patients: Franco Mandelli, Maria-Concetta Petti, Giovanna Meloni, Petra Muus, Filippo Marmont, Jean-Pierre Marie, Boris Labar, Xavier Thomas, Francesco Di Raimondo, Roel Willemze, Vincenzo Liso, Felicetto Ferrara, Robert Zittoun, Sergio Amadori, Theo de Witte
Collection and assembly of data: Marco Vignetti, Stefan Suciu, Boris Labar, Liliana Baila, Paola Fazi
Data analysis and interpretation: Franco Mandelli, Marco Vignetti, Stefan Suciu, Roberto Stasi, Maria-Concetta Petti, Giovanna Meloni, Petra Muus, Liliana Baila, Paola Fazi, Robert Zittoun, Theo de Witte
Manuscript writing: Franco Mandelli, Marco Vignetti, Stefan Suciu, Roberto Stasi, Maria-Concetta Petti, Petra Muus, Roel Willemze, Theo de Witte
Final approval of manuscript: Franco Mandelli, Marco Vignetti, Stefan Suciu, Roberto Stasi, Maria-Concetta Petti, Giovanna Meloni, Petra Muus, Filippo Marmont, Jean-Pierre Marie, Boris Labar, Xavier Thomas, Francesco Di Raimondo, Roel Willemze, Vincenzo Liso, Felicetto Ferrara, Paola Fazi, Robert Zittoun, Sergio Amadori, Theo de Witte
REFERENCES
- 1.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332:217–223. doi: 10.1056/NEJM199501263320403. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Goldstone AH, Stevens RM, et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC AML 10 trial. UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet. 1998;351:700–708. doi: 10.1016/s0140-6736(97)09214-3. [DOI] [PubMed] [Google Scholar]
- 4.Bishop JF, Lowenthal RM, Joshua D, et al. Etoposide in acute nonlymphocytic leukemia: Australian Leukemia Study Group. Blood. 1990;75:27–32. [PubMed] [Google Scholar]
- 5.Bishop JF, Matthews JP, Young GA, et al. Intensified induction chemotherapy with high dose cytarabine and etoposide for acute myeloid leukemia: A review and updated results of the Australian Leukemia Study Group. Leuk Lymphoma. 1998;28:315–327. doi: 10.3109/10428199809092687. [DOI] [PubMed] [Google Scholar]
- 6.Berman E, Heller G, Santorsa J, et al. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666–1674. [PubMed] [Google Scholar]
- 7.Wiernik PH, Banks PL, Case DC, Jr, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–319. [PubMed] [Google Scholar]
- 8.Vogler WR, Velez-Garcia E, Weiner RS, et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: A Southeastern Cancer Study Group Study. J Clin Oncol. 1992;10:1103–1111. doi: 10.1200/JCO.1992.10.7.1103. [DOI] [PubMed] [Google Scholar]
- 9.Arlin Z, Case DC, Jr, Moore J, et al. Randomized multicenter trial of cytosine arabinoside with mitoxantrone or daunorubicin in previously untreated adult patients with acute nonlymphocytic leukemia (ANLL): Lederle Cooperative Group. Leukemia. 1990;4:177–183. [PubMed] [Google Scholar]
- 10.Wahlin A, Hornsten P, Hedenus M, et al. Mitoxantrone and cytarabine versus daunorubicin and cytarabine in previously untreated patients with acute myeloid leukemia. Cancer Chemother Pharmacol. 1991;28:480–483. doi: 10.1007/BF00685827. [DOI] [PubMed] [Google Scholar]
- 11.Pavlovsky S, Gonzalez Llaven J, Garcia Martinez MA, et al. A randomized study of mitoxantrone plus cytarabine versus daunomycin plus cytarabine in the treatment of previously untreated adult patients with acute nonlymphocytic leukemia. Ann Hematol. 1994;69:11–15. doi: 10.1007/BF01757342. [DOI] [PubMed] [Google Scholar]
- 12.Löwenberg B, Suciu S, Archimbaud E, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy: The value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly—Final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J Clin Oncol. 1998;16:872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 13.Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: A trial by the Eastern Cooperative Oncology Group. Blood. 2004;103:479–485. doi: 10.1182/blood-2003-05-1686. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: The results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 15.Burnett AK, Goldstone AH, Milligan DW. Daunorubicin versus mitoxantrone as induction for AML in younger adults given intensive chemotherapy: Preliminary results of MRC AML-12 Trial. Br J Haematol. 1999;105(suppl 1):67a. [Google Scholar]
- 16.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia: A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JM, Catovsky D, Daniel MT, et al. Proposal for the recognition of minimally differentiated acute myeloid leukaemia (AML-MO) Br J Haematol. 1991;78:325–329. doi: 10.1111/j.1365-2141.1991.tb04444.x. [DOI] [PubMed] [Google Scholar]
- 18.Yates J, Glidewell O, Wiernik P, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: A CALGB study. Blood. 1982;60:454–462. [PubMed] [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. ed 2. Hoboken, NJ: Wiley Inter-Science; 2002. [Google Scholar]
- 20.Keating S, Suciu S, de Witte T, et al. The stem cell mobilizing capacity of patients with acute myeloid leukemia in complete remission correlates with relapse risk: Results of the EORTC-GIMEMA AML-10 trial. Leukemia. 2003;17:60–67. doi: 10.1038/sj.leu.2402782. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto S, Ogawa M. Antitumor activity of mitoxantrone against murine experimental tumors: Comparative analysis against various antitumor antibiotics. Cancer Chemother Pharmacol. 1982;8:157–162. doi: 10.1007/BF00255476. [DOI] [PubMed] [Google Scholar]
- 22.Berman E, McBride M. Comparative cellular pharmacology of daunorubicin and idarubicin in human multidrug-resistant leukemia cells. Blood. 1992;79:3267–3273. [PubMed] [Google Scholar]
- 23.The AML Collaborative Group. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. Br J Haematol. 1998;103:100–109. [PubMed] [Google Scholar]
- 24.Kimby E, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in acute myeloid leukaemia. Acta Oncol. 2001;40:231–252. doi: 10.1080/02841860151116321. [DOI] [PubMed] [Google Scholar]