Abstract
Purpose
Romidepsin (depsipeptide or FK228) is a member of a new class of antineoplastic agents active in T-cell lymphoma, the histone deacetylase inhibitors. On the basis of observed responses in a phase I trial, a phase II trial of romidepsin in patients with T-cell lymphoma was initiated.
Patients and Methods
The initial cohort was limited to patients with cutaneous T-cell lymphoma (CTCL), or subtypes mycosis fungoides or Sézary syndrome, who had received no more than two prior cytotoxic regimens. There were no limits on other types of therapy. Subsequently, the protocol was expanded to enroll patients who had received more than two prior cytotoxic regimens.
Results
Twenty-seven patients were enrolled onto the first cohort, and a total of 71 patients are included in this analysis. These patients had undergone a median of four prior treatments, and 62 patients (87%) had advanced-stage disease (stage IIB, n = 15; stage III, n= 6; or stage IV, n = 41). Toxicities included nausea, vomiting, fatigue, and transient thrombocytopenia and granulocytopenia. Pharmacokinetics were evaluated with the first administration of romidepsin. Complete responses were observed in four patients, and partial responses were observed in 20 patients for an overall response rate of 34% (95% CI, 23% to 46%). The median duration of response was 13.7 months.
Conclusion
The histone deacetylase inhibitor romidepsin has single-agent clinical activity with significant and durable responses in patients with CTCL.
INTRODUCTION
Histone deacetylase inhibitors (HDIs) cause growth arrest, cellular differentiation, and apoptosis.1–4 Their antitumor effects have been hypothesized to occur through modulation of gene expression; however, acetylation of nonhistone proteins may be more important.5,6 Romidepsin (FK228, FR901228, depsipeptide), (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(1-methyletheyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone, is a potent HDI isolated from Chromobacterium violaceum (Appendix Fig A1, online only).7,8 Dramatic responses observed in patients with T-cell lymphoma9–11 prompted a phase II trial to assess the response rate and toxicity profile. The activity of romidepsin in cutaneous T-cell lymphoma (CTCL) seems to be a class effect, with other HDIs also found to demonstrate activity.12,13
Between 2,000 and 3,000 new cases of CTCL occur in the United States each year, with mycosis fungoides (MF) and the Sézary syndrome (SS) being the predominant subtypes.14 MF is categorized as limited stage (IA, IB, and IIA), characterized as plaques or patches limited to skin, and advanced stage (IIB to IVB), characterized by cutaneous tumors and involvement of the blood, lymph nodes, bone marrow, or visceral organs.15 SS is characterized by generalized erythroderma and abnormal lymphoid cells in the blood.16 Limited-stage disease may effectively be treated with skin-directed therapies including topical nitrogen mustard or psoralen plus ultraviolet A therapy.17 However, in patients with advanced disease, control is often short lived, and the disease is relentlessly progressive. Although response rates to cytotoxic chemotherapy range from 60% to 80% in patients with advanced disease, the median duration of response is usually measured in months.18 Agents with novel mechanisms of action have been pursued, including retinoids, interferon, monoclonal antibodies, and denileukin diftitox; none has been found to be curative.
This trial was initiated to evaluate the efficacy of romidepsin in patients with T-cell lymphoma. Secondary goals included evaluation of long-term safety of romidepsin. This report is limited to patients with MF or SS; patients with peripheral T-cell lymphoma (PTCL) will be reported separately.
PATIENTS AND METHODS
Patient Eligibility
Patients with relapsed, refractory, or advanced CTCL, either as MF or SS, were eligible. The first cohort included patients who had received no more than two systemic cytotoxic chemotherapy regimens. Topical therapies, such as psoralen plus ultraviolet A therapy or topical chemotherapy; systemic therapies, such as corticosteroids, retinoids, interferon, or denileukin diftitox; and nonradiolabeled antibodies, such as alemtuzumab, were not considered cytotoxic chemotherapy; prior therapy with any number of these therapies was allowed. Patients with stage IA, IB, or IIA disease15 were only eligible if they were refractory to, intolerant of, or had reached a 6-month or longer response plateau on at least two prior CTCL therapies. The observed activity led us to open the trial at additional sites and to include patients who had previously received more than two cytotoxic therapies. In addition, after completion of the first cohort, a replicate cohort with the same inclusion criteria was undertaken. The protocol, informed consent, and subsequent amendments were approved by the institutional review boards of all participating institutions. Histologic diagnosis was confirmed by the respective treating institution. All patients signed informed consent. Standard phase II inclusion and exclusion criteria were used (detailed in Appendix Table A1, online only). Patients maintained on a stable dose of corticosteroids at protocol entry were allowed, with tapering to follow initiation of therapy. Effective birth control was required.
Trial Design and Treatment Plan
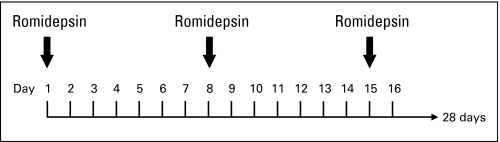
Romidepsin (NSC 630176) was provided by the Cancer Therapy Evaluation Program of the National Cancer Institute (NCI). Romidepsin was administered as a 4-hour infusion at 18 mg/m2 on days 1 and 5 of a 21-day cycle for the first three patients, which was the schedule originally studied at the NCI.9 Subsequently, by amendment, patients were treated on the more tolerable schedule of 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle (Appendix Fig A2, online only).10 Doses were held for absolute neutrophil count less than 0.5 × 109 cells/L, platelet count less than 50 × 109/L, or grade 3 or worse nonhematologic toxicity. Doses were reduced from 14.0 to 10.5 mg/m2 (dose level –1) or from 10.5 to 8.0 mg/m2 (dose level –2) for absolute neutrophil count between 0.5 and 1.0 × 109 cells/L or platelet count between 50 and 75 × 109/L on days 8 or 15. Dose escalation to 17.5 mg/m2 (dose level +1) was allowed in the absence of toxicity. Radiotherapy of nonresponding lesions was allowed for patients with evidence of overall response. Irradiated lesions were not included in response assessment after radiation. Patients who received radiation while on protocol were not categorized as complete responders. The NCI Common Toxicity Criteria, version 2.0, were used.
Supportive Care
Patients with CTCL are at risk for hypomagnesemia.19 Because hypomagnesemia and hypokalemia are associated with T-wave and ST segment abnormalities and QT interval prolongation, findings also associated with HDI therapy, the protocol was amended to mandate supplementation of electrolytes to achieve serum magnesium and potassium levels of greater than 0.85 mmol/L and 4.0 mmol/L, respectively, before romidepsin administration.20 The protocol was also amended to exclude medications known to either prolong the QTc or interfere with CYP3A4 metabolism. The latter exclusion was added after it was found that romidepsin may be metabolized in part by CYP3A4.21,22 Antiemetics were administered to prevent nausea.
Response Evaluation
Response assessment used a rigorous composite approach. Disease in skin or viscera was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria,23 and lymph node disease was assessed using International Working Group guidelines.24 Bone marrow involvement, as recommended by International Working Group criteria, was scored as present or absent. Generalized erythroderma was scored as present or absent. Although the presence of circulating tumor cells is considered prognostically significant, the change in quantity of cells during therapy has not been demonstrated to be predictive of response.25 Thus, flow cytometry of blood was assessed as present or absent. Complete response required clearing of known sites of disease. Partial response required documented response in skin or lymph nodes.
Pharmacokinetic Analysis
Blood samples were collected with the first dose before drug administration, at the end of infusion (4 hours), and at 6, 11, 13, 15, 18, and 22 hours after start of infusion. Samples were centrifuged for 5 minutes at 1,200 × g, and collected plasma was stored at −80°C. Samples were analyzed using a sensitive analytic liquid chromatography-mass spectrometry assay validated for the range of 2 to 1,000 ng/mL.26 Noncompartmental pharmacokinetic data analysis was performed using WINNonlin v.5 (Scientific Consultant, Apex, NC). The area under the curve (AUC) from time zero to time of final quantifiable sample was calculated using the linear trapezoidal method, whereas AUC extrapolated to infinity (AUCinf) was calculated by extrapolation to infinity. Volume of distribution was estimated during the terminal phase, and systemic clearance was calculated as dose divided by AUCinf.
Statistical Methods
The trial began as a single-institution analysis of romidepsin in patients with CTCL or PTCL after no more than two prior cytotoxic therapies evaluated in separate cohorts. The Simon two-stage design27 for the first cohort required a response in one of nine patients to accrue the full cohort of 24 patients to target a response rate of 25% and rule out a 5% response rate, with 10% probabilities of accepting a poor agent and of rejecting a good agent. Duration of response and time to progression were determined using the Kaplan-Meier method.
RESULTS
Patient Characteristics
Seventy-one patients with MF or SS were enrolled onto the phase II trial of romidepsin for patients with T-cell lymphoma as of the cutoff date for this analysis. Twenty-seven patients were enrolled onto the original cohort for patients who had not received more than two systemic cytotoxic chemotherapeutic regimens. Subsequently, by amendment, an additional 44 patients with CTCL were enrolled, 12 of whom had previously received more than two systemic cytotoxic chemotherapeutic regimens. Patient characteristics are listed in Table 1. At enrollment, 25 patients in cohort 1 (93%) and 62 (87%) of the 71 patients overall had advanced disease (stages IIB to IVB). Furthermore, 30 patients had elevated LDH, 43 patients had low albumin, and 51 patients were greater than 50 years old, which are all features associated with poor outcome.28,29 These three prognostic factors were entirely absent in only three patients. All patients tested negative for human T-lymphotropic virus. Patients had received a median of four prior regimens (Table 1). Prior therapies included topical treatments (61%), biologic agents (68%), cytotoxic chemotherapy (65%), and radiation therapy (56%).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Cohort 1 (n = 27) |
All Patients (N = 71) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Sex | ||||
| Male | 20 | 48 | ||
| Female | 7 | 23 | ||
| Age, years | ||||
| Median | 57 | 57 | ||
| Range | 31-77 | 28-84 | ||
| < 50 | 5 | 20 | ||
| ≥ 50 | 22 | 51 | ||
| Disease stage at time of enrollment | ||||
| IA | 0 | 1 | ||
| IB | 2 | 6 | ||
| IIA | 0 | 2 | ||
| IIB | 5 | 15 | ||
| IIIA | 2 | 3 | ||
| IIIB | 0 | 3 | ||
| IVA | 14 | 28 | ||
| IVB | 4 | 13 | ||
| ECOG performance status | ||||
| 0 | 5 | 16 | ||
| 1 | 16 | 45 | ||
| 2 | 6 | 10 | ||
| Elevated LDH | 10 | 30 | ||
| Low albumin | 8 | 43 | ||
| No. of prior therapies* | ||||
| Median | 3 | 4 | ||
| Range | 1-11 | 0-14 | ||
| Previous topical therapies | 17 | 63 | 43 | 61 |
| PUVA | 16 | 59 | 39 | 55 |
| Topical NM | 7 | 26 | 16 | 23 |
| Topical bexarotene | 3 | 11 | 4 | 6 |
| Topical steroids | 4 | 15 | 11 | 15 |
| Previous radiation therapy† | 15 | 56 | 40 | 56 |
| Localized radiotherapy | 12 | 44 | 32 | 45 |
| TSEB | 6 | 22 | 13 | 18 |
| Previous extracorporeal photopheresis | 4 | 15 | 16 | 23 |
| Previous biologic therapies | 22 | 81 | 48 | 68 |
| IFN | 9 | 33 | 23 | 32 |
| Denileukin diftitox | 6 | 22 | 14 | 20 |
| Alemtuzumab | 1 | 4 | 4 | 6 |
| Anti-Tac antibody | 1 | 4 | 5 | 7 |
| Oral corticosteroids | 6 | 22 | 18 | 25 |
| Retinoid: oral bexarotene | 12 | 44 | 32 | 45 |
| Retinoid: other‡ | 5 | 19 | 10 | 14 |
| Previous systemic chemotherapy regimens§ | 13 | 48 | 46 | 65 |
| 0 | 14 | 52 | 25 | 35 |
| 1 | 11 | 41 | 20 | 28 |
| 2 | 2 | 7 | 14 | 20 |
| > 2 | 0 | 0 | 12 | 17 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PUVA, psoralen plus ultraviolet A therapy; NM, nitrogen mustard; TSEB, total skin electron beam; IFN, interferon.
Other treatments not listed include cyclosporine, tacrolimus, azathioprine, remicade, dendritic cell vaccine, and peldesine.
Some patients had both localized radiation and TSEB.
Other retinoids include isotretinoin, acitretin, and etretinate. Three patients received bexarotene as well as another retinoid.
Chemotherapies included monotherapy such as chlorambucil, cladribine, fludarabine, gemcitabine, liposomal doxorubicin, methotrexate, or pentostatin and combination therapy such as cyclophosphamide, doxorubicin, vincristine, and prednisone; infusional etoposide, vincristine, and doxorubicin with bolus cyclophosphamide; and cyclophosphamide, vincristine, and prednisone.
Patients received a median of four cycles (range, one to 72 cycles) and 12 doses (range, two to 141 doses; Table 2). Among the 1,462 doses administered over 538 cycles, 1,110 (76%) were full doses, 102 (7%) were escalated doses, and 250 (17%) were reduced doses. Eight doses were held; three doses in three patients were held as a result of thrombocytopenia (< 50 × 109/L), two were held for persistent grade 3 nausea, and three were held as a result of persistent grade 3 fatigue. Protocol-mandated dose reductions were required for 42 doses in 20 patients for the following reasons: 33 dose reductions were a result of thrombocytopenia (> 50 but < 75 × 109/L), four were a result of granulocytopenia (> 0.5 but < 1 × 109 cells/L), three were a result of persistent nausea, and two were a result of fatigue. The remainder of the doses less than 14 mg/m2 (n = 208) were administered as permanent dose reductions in patients who previously had a dose held or had one or more protocol-mandated dose reductions.
Table 2.
Administered Therapy
| Treatment | Cohort 1 (n = 27) | All Patients (N = 71) |
|---|---|---|
| Total No. of cycles | 293 | 538 |
| Cycles per patient, No. of cycles | ||
| Median | 5 | 4 |
| Range | 1-72 | 1-72 |
| Cycles per patient, No. of patients | ||
| ≤ 2 | 4 | 13 |
| 3-5 | 10 | 31 |
| ≥ 6 | 13 | 27 |
| No. of doses per patient | ||
| Median | 15 | 12 |
| Range | 2-141 | 2-141 |
| No. of doses | ||
| Total | 756 | 1,462 |
| Full dose | 554 | 1,110 |
| Dose escalated | 39 | 102 |
| Reduced, total | 163 | 250 |
| Reduced as a result of toxicity | 22 | 42 |
| Held* | 2 | 8 |
| Dose administered | ||
| Cumulative dose, mg/m2 | ||
| Median | 210 | 168 |
| Range | 28-2,538 | 28-2,538 |
| Cumulative dose, mg | ||
| Median | 386 | 306 |
| Range | 43-5,681 | 43-5,681 |
According to protocol criteria.
First-dose pharmacokinetics were evaluable in a total of 64 patients, three of whom received romidepsin 18 mg/m2 and 61 of whom received romidepsin 14 mg/m2. Full pharmacokinetic parameters are listed in Table 3. AUCinf variation is plotted in Appendix Figure A3 (online only). The pharmacokinetic data were incorporated into a larger population pharmacokinetic analysis.30
Table 3.
Pharmacokinetics of Romidepsin
| Parameter | 14 mg/m2 |
18 mg/m2 |
||||
|---|---|---|---|---|---|---|
| No. of Patients* | Geometric Mean | 95% CI | No. of Patients | Geometric Mean | 95% CI | |
| Half-life, hours | 42 | 2.95† | 2.49 to 3.49 | 3 | 2.56 | 1.62 to 4.05 |
| Cmax, ng/mL‡ | 61 | 361.52 | 313.49 to 416.92 | 3 | 722.18 | 366.93 to 1,421.38 |
| AUClast, h·ng/mL | 61 | 1,214.23 | 1,044.16 to 1,412.01 | 3 | 2,571.05 | 1,258.64 to 5,251.92 |
| AUCinf, h·ng/mL | 42 | 1,456.54 | 1,250.74 to 1,696.21 | 3 | 2,582.65 | 1,263.23 to 5,280.17 |
| Vzobs, L/m2 | 42 | 40.89 | 33.40 to 50.06 | 3 | 25.75 | 13.16 to 50.36 |
| Clobs, L/h/m2§ | 42 | 9.61 | 8.25 to 11.19 | 3 | 6.97 | 3.40 to 14.27 |
Abbreviations: Cmax, maximum plasma concentration; AUClast, area under the curve from time zero to time of final quantifiable sample; AUCinf, area under the curve extrapolated to infinity; Vz obs, volume of distribution during the terminal phase; Clobs, observed clearance.
Pharmacokinetic analysis was not possible in seven patients, and a full analysis was not possible in 19 patients.
The median half-life was 2.64 hours (range, 1.0 to 10.9 hours).
Cmax is reported as observed value.
Clearance is expressed as L/hour/m2 because of the body-surface area dosing used.
Toxicities
Toxicities that were commonly observed were similar to toxicities observed in the phase I trials of romidepsin and reported for other HDIs.31 Cycle 1 toxicities are listed in Table 4. Common nonhematologic adverse effects (any grade) included fatigue (41%), nausea (52%), vomiting (20%), and anorexia (21%). Hematologic abnormalities included leukopenia (31%), granulocytopenia (37%), lymphopenia (21%), thrombocytopenia (39%), and anemia (37%). Transient elevations of liver function tests, AST or ALT, were observed in 13 patients; two additional patients had isolated grade 1 hyperbilirubinemia. Hyperuricemia was noted in 11 patients (eight patients with grade 1 and three patients with grade 3), and hypophosphatemia was noted in six different patients. As previously described, ECG changes were noted consisting of asymptomatic T-wave flattening (71%) or ST segment depression (9%). Toxicities in later cycles mirrored those observed in the first cycle. Infections occurred in 38 patients (54%) over 58 cycles (11%), including bacterial infections of the skin and upper respiratory, pulmonary, GI, and urinary tracts; bacteremia; and sepsis, and were not related to neutropenia. Neutropenia was noted in only 25 of the 538 cycles, and only one episode of neutropenic fever was noted in a patient while on protocol, occurring with progression of his bone marrow disease. Supportive care included prophylactic antiemetics for all patients and intravenous hydration in the occasional patient with marked nausea, fever, or hypotension.
Table 4.
Drug-Related Cycle 1 Toxicities
| Toxicity | % of Patients |
|||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematologic | ||||
| Leukopenia | 4 | 14 | 11 | 1 |
| Granulocytopenia | 8 | 14 | 10 | 4 |
| Lymphopenia | 21 | |||
| Thrombocytopenia | 27 | 7 | 6 | |
| Anemia | 20 | 11 | 6 | |
| Constitutional/GI | ||||
| Fatigue | 27 | 7 | 6 | 1 |
| Headache | 4 | 3 | ||
| Nausea | 34 | 15 | 3 | |
| Vomiting | 11 | 7 | 1 | |
| Anorexia | 13 | 7 | 1 | |
| Dysgeusia | 18 | 1 | ||
| Constipation | 3 | 4 | ||
| Diarrhea | 7 | 1 | ||
| ECG | ||||
| T-wave or ST changes | 71 | 9 | ||
| QTc prolongation | 9 | |||
| Cardiac | ||||
| Hypotension | 3 | |||
| Supraventricular arrhythmia | 1 | 1 | ||
| Ventricular arrhythmia | 3 | |||
| Laboratory | ||||
| Hypoalbuminemia | 14 | 6 | ||
| Hyperbilirubinemia | 3 | |||
| AST | 8 | 1 | 3 | |
| ALT | 4 | 1 | 3 | |
| Hyperglycemia | 11 | 7 | ||
| Hypermagnesemia | 7 | |||
| Hyperuricemia | 11 | 4 | ||
| Hypocalcemia | 10 | 31 | 1 | |
| Hypoglycemia | 4 | 1 | ||
| Hypokalemia | 8 | 1 | ||
| Hypomagnesemia | 15 | |||
| Hyponatremia | 8 | |||
| Hypophosphatemia | 1 | 3 | 4 | |
NOTE. Drug related was defined as possibly, probably, or definitely related to the drug.
Three deaths occurred among patients with CTCL while on study, and three deaths occurred within 30 days of removal from study. Deaths on study included a 70-year-old man with hypertension and severe valvular heart disease who had a partial response to romidepsin. After nine cycles of therapy, he developed atrial fibrillation and was placed on warfarin and digoxin. Romidepsin was restarted, and he was found without pulse 1 day after receiving the second dose of the subsequent cycle. Autopsy revealed hypertrophic cardiac disease with significant valvular pathology but no evidence of acute infarction or myocyte injury. Two patients died from sepsis 10 and 12 days after administration of romidepsin, one patient with Escherichia coli and another with methicillin-resistant Staphylococcus aureus. Each of the three patients who died within 30 days of study removal had been removed as a result of progression of disease and died after receiving cytotoxic chemotherapy.
Responses
In the initial cohort of 27 patients who received no more than two prior cytotoxic regimens, three patients achieved a complete response and eight patients achieved a partial response, for an overall response rate of 41% (95% CI, 22% to 61%), thus exceeding the fraction required to declare the regimen of further interest based on the two-stage design. Detailed response data are listed in Tables 5 and 6 and Appendix Tables A2 and A3 (online only). The overall response rate for the group of 71 patients was 34% (95% CI, 23% to 46%). Representative responses are shown in Figure 1 and Appendix Figure A4 (online only).
Table 5.
Responses
| Best Response | Cohort 1 (n = 27) |
All Patients (N = 71) |
||||
|---|---|---|---|---|---|---|
| Response |
Response Duration (months) | Response |
Response Duration (months) | |||
| No. | % | No. | % | |||
| CR | 3 | 11 | 6, 14*, 63† | 4 | 7 | 6, 14*, 26‡, 63† |
| PR | 8 | 30 | 2§, 2, 5, 10, 14, 20§, 32, 76‡ | 20 | 26 | 1, 1, 2, 2, 2, 2§, 3, 4‡, 5, 5, 6‖, 7§, 10, 11‡, 14, 14, 15, 20§, 32, 76‡ |
| SD | 10 | 37 | 3, 3, 4, 4, 4, 6, 6, 7, 7, 8 | 26 | 38 | 3, 3, 3, 3, 3, 4, 4, 4, 4, 4, 4, 4, 4, 4‖, 5, 6, 6, 6, 6, 6, 7, 7, 8, 8, 10, 11 |
| PD | 3 | 11 | 15 | 17 | ||
| NE | 3 | 11 | 6 | 12 | ||
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
After 14 months in CR, this patient developed several small plaques but did not require additional therapy for over 43 months.
Patient completed therapy 58 months prior to cut off date for this report.
Patients with continued responses to therapy at time of this report.
Patient self-withdrew from study.
Death on study.
Table 6.
Responses Divided by Stage
| Stage | No. of Patients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 27) |
All Patients (N = 71) |
|||||||||||
| Total | CR | PR | SD | PD | NE | Total | CR | PR | SD | PD | NE | |
| IA | 1 | 1 | ||||||||||
| IB | 2 | 1 | 1 | 6 | 4 | 1 | 1 | |||||
| IIA | 2 | 1 | 1 | |||||||||
| IIB | 5 | 3 | 1 | 1 | 15 | 1 | 6 | 6 | 1 | 1 | ||
| IIIA | 2 | 1 | 1 | 3 | 1 | 1 | 1 | |||||
| IIIB | 3 | 1 | 1 | 1 | ||||||||
| IVA | 14 | 2 | 2 | 7 | 1 | 2 | 28 | 2 | 3 | 13 | 7 | 3 |
| IVB | 4 | 1 | 1 | 2 | 13 | 1 | 3 | 4 | 5 | |||
| Total | 27 | 3 | 8 | 10 | 3 | 3 | 71 | 4 | 20 | 26 | 15 | 6 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
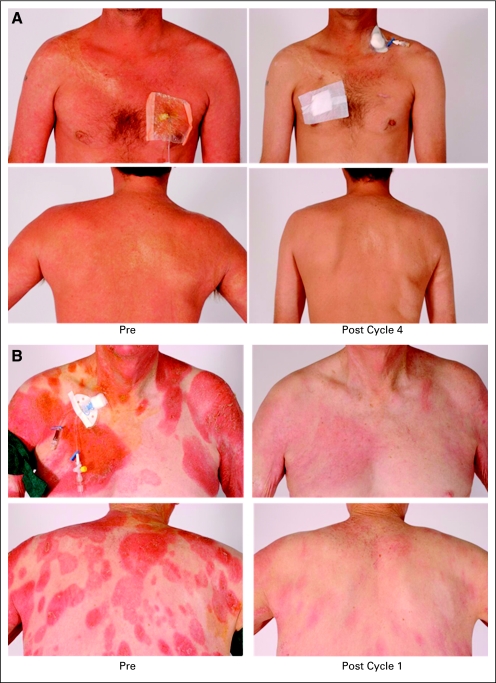
Fig 1.
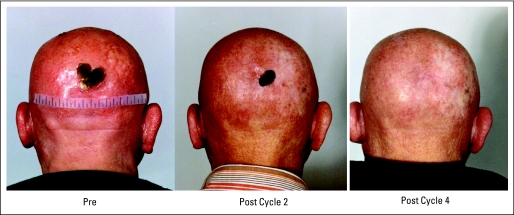
(A) This patient with Sézary syndrome had progression of disease after denileukin diftitox and alemtuzumab. He remains in complete response after 63 months. (B) This patient with mycosis fungoides had progression of disease after psoralen with ultraviolet A therapy, etretinate, interferon alfa, and methotrexate. The patient demonstrated a good response that lasted 8 months.
Complete responses were observed in four patients (6%); response was documented in all sites of disease. The complete responders included one patient with MF and three patients with SS who had complete clearing of generalized erythroderma, including follow-up skin biopsies without evidence of disease. Involved sites, including lymph nodes and bone marrow, also had response documented in those sites. Two patients remain without evidence of disease at 26 and 63 months. One patient developed progression of disease in his skin after 8 months and was taken off protocol. One patient developed small thin patches of disease after 14 months in complete response. Romidepsin was discontinued, and no further therapy was needed for another 43 months.
Partial responses were observed in 20 patients (28%). Seven patients had skin-only disease, and response was documented by measurement of skin lesions using RECIST criteria. Twelve patients had response documented in skin as well as in their other sites of disease, with documentation of response in lymphadenopathy in seven patients, visceral lesions in three patients, and complete clearing of blood as determined by flow cytometry in seven patients. One patient with minor response of skin disease had primary response determined by lymph node response. Three patients continuing in partial response are being observed on protocol after 11, 13, and 82 months. Of the other 17 patients, 10 developed progression of disease, three self-withdrew from protocol, three came off study after experiencing an adverse event of fatigue, infection, or hypotension, and one died on study (discussed earlier).
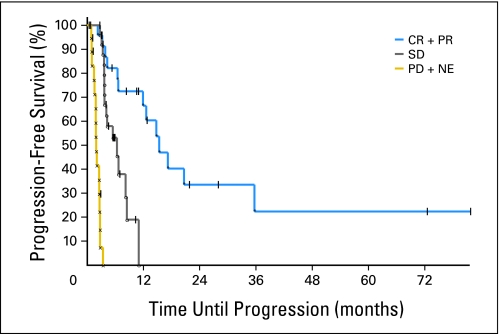
Stable disease was noted in 26 patients. Sixteen of these patients developed progression of disease; seven patients withdrew from study, mainly to seek alternative therapy; two patients withdrew as a result of adverse events of infection and fatigue, and one patient died on study from sepsis with methicillin-resistant S aureus (discussed earlier). Progression of disease without evidence of response was noted in 15 patients. Six patients were nonevaluable; reasons included intercurrent medical illness of pituitary macroadenoma with associated endocrinopathy, the discovery of an intracardiac mass later shown to be the patient's lymphoma,32 one death on study as a result of infection (discussed earlier), one patient on study for 2 months who moved and was lost to follow-up, and one patient who refused further therapy after one cycle. The sixth patient, who was taken off protocol as a result of worsening of generalized erythroderma, completely cleared after antibiotic therapy. Two patients, one with partial response and one with stable disease, withdrew from study after reviewing informed consent revised to include discussion of sudden death reported in patients treated with romidepsin.20 Among the patients with a major response (complete or partial), the median time to response was 2 months (range, 1 to 6 months), and the median duration of overall response was 13.7 months (Appendix Fig A5, online only). The median time to progression was 15.1 months for patients with a major response (complete or partial), 5.9 months for patients with stable disease, and 1.9 months for patients who had disease progression as best response or who were nonevaluable.
DISCUSSION
This phase II trial was initiated after responses were observed in patients with CTCL and PTCL treated on a phase I trial of romidepsin.11 Although patients with CTCL may have multiple therapeutic options, responses to those agents are seldom durable. Our patients had prior regimens that included topical therapies, radiation, biologic agents, and systemic chemotherapy. The overall response rate was 34%, with a median duration of response of 13.7 months. Among 62 patients with stage IV disease, 18 (29%) had a complete or partial response. Although it is generally recognized that patients with SS are more refractory to available therapies, three patients with SS achieved a complete response to romidepsin, with one patient continuing in complete remission at 63 months, more than 55 months after romidepsin discontinuation.
Overall, romidepsin was well tolerated. The toxicities observed were similar to those previously reported, including fatigue, nausea, and vomiting.9,10 Laboratory abnormalities noted included transient granulocytopenia and thrombocytopenia, with values returning to baseline by the next cycle. These toxicities seem to be a class effect among the HDIs.31 Infectious complications are common in patients with CTCL, who have impaired cellular immunity,33 and are a significant cause of morbidity and mortality.34 In addition to compromised integument, the presence of CTCL in the skin contributes to colonization with S aureus, which in turn stimulates the growth of lymphoma in the skin.35
Because asymptomatic T-wave flattening and ST segment depression were observed in phase I testing,9,10 cardiac evaluation was incorporated into the study. Analysis of these results in the first 42 patients with CTCL or PTCL treated at the National Institutes of Health Clinical Center (25 of whom are included in this cohort of 71 patients) has been reported.20 This testing revealed no evidence of acute or cumulative cardiac damage based on serial troponin I values, multiple-gated acquisition scans, or echocardiograms.20 When ECGs were evaluated for QT interval changes, a median increase of 14 milliseconds was observed, and 0.2% of the 2,051 ECGs evaluated had a QTcB (Bazett's correction) interval of more than 500 milliseconds.20 Although these studies were evidence of the safety of romidepsin administration, it should be noted that one patient among the 71 died unexpectedly, as discussed in the Results and the Appendix (online only). This patient had severe valvular heart disease, and the protocol was amended to exclude patients at risk for sudden death and to avoid concomitant use of medications that prolong the QT interval or inhibit CYP3A4. A summary of events noted in the patients reported here is found in the Appendix. A detailed review of the cardiac monitoring is in preparation.
The observed responses and duration of response to romidepsin in CTCL are noteworthy and compare favorably to those seen after chemotherapy, bexarotene, or denileukin diftitox. The activity of romidepsin in CTCL has proven to be a class effect, with other HDIs demonstrating activity in this disease.11–13 With differing structures, potencies, enzyme affinities, and schedules of administration, it is expected that differences in outcome or adverse effects with the HDIs will emerge. The biologic basis of the responsiveness of T-cell lymphoma to HDI therapy remains to be elucidated. It has been postulated that malignancies with an alteration in histone deacetylase or histone acetyltransferase activity may be susceptible to HDIs; however, no such alteration has been described in T-cell lymphomas. Approaches to increase efficacy include combination with agents that have activity in CTCL, particularly denileukin diftitox, retinoids, and cytotoxic agents, that may be potentiated by an HDI.36
Acknowledgment
The following individuals also contributed to this work: Laura Hutchins (Arkansas Cancer Research Center, Little Rock, AR), Michael Craig (Mary Babb Randolph Cancer Center, Morgantown, WV), Xiaohong Chen (Center for Cancer Research, National Cancer Institute, Bethesda, MD), and Alexander Ling (Diagnostic Radiology Department, National Institutes of Health Clinical Center, Bethesda, MD). We acknowledge Douglas Rosing, Mark Raffeld, and the nurses, fellows, and patients for their willingness to participate in investigational trials. We also acknowledge the contributions of Wyndham Wilson, Lynn Sorbara, John Janik, Sam Hwang, Edward Cowen, Lyudmila Kalnitskaya, and Susan Bakke.
Appendix
Cardiac Events Observed As Part of Intensive Cardiac Monitoring
Intensive cardiac monitoring was performed on this protocol to evaluate any potential effects of romidepsin on cardiac function.20 These studies focused on ECG changes, evaluating myocardial integrity, cardiac function, and evidence of potential dysrhythmia. Cardiac studies included serial ECGs, cardiac enzymes, echocardiograms, baseline 24-hour Holter analysis, and telemetry monitoring during the first dose of the first cycle. A first analysis of this testing in 42 patients has been reported and revealed no evidence of acute or cumulative cardiac damage based on serial troponin I values, multiple-gated acquisition scans, or echocardiograms.20
Cardiac events of any attribution reported for the 71 patients of this report are listed in Appendix Table A4. QT interval (QTc) prolongation, greater than 480 milliseconds using the Bazett's correction formula, was noted in 16 patients; prior to starting protocol, eight of these patients had an abnormal QTcB of greater than 450 milliseconds, and four of these patients had a QTcB of greater than 480 milliseconds. Using the Fridericia correction formula instead of Bazett's, six patients had QTc greater than 480 milliseconds, three of whom had prolonged QTc prior to starting protocol. Notably, one patient was found to have an intracardiac lesion,32 and another patient, discussed later, had severe multivalvular dysfunction and left ventricular hypertrophy (QRS intraventricular conduction delay with QRS of 150 milliseconds).
Additional events were rhythm disturbances. One patient was found to have a junctional rhythm while on protocol. This patient was taking felodipine, a calcium channel blocker metabolized through CYP3A4. The patient's rhythm returned to normal after discontinuation of felodipine and counseling to avoid grapefruit juice. There is a marked increase of bioavailability of felodipine when taken with grapefruit juice, which may lead to the development of a junctional rhythm. Interestingly, felodipine was the first agent to be noted for the CYP3A4 effects of grapefruit juice.22
An additional three patients developed atrial fibrillation (Appendix Table A5). The first patient, noted in our previous report, had a history of chronic obstructive pulmonary disease and significant supraventricular ectopy on a 24-hour Holter monitor obtained before starting therapy. The second patient, also noted in our previous report, with structural heart abnormalities and left ventricular hypertrophy, received digoxin and died after restarting romidepsin. The third patient, a 69-year-old man with aortic valve replacement 11 years before starting protocol, was noted to be in atrial fibrillation 3 weeks after the last dose of the first cycle. In addition, because patients treated at the National Institutes of Health Clinical Center were observed by telemetry after the first administration of romidepsin, clinically insignificant supraventricular and ventricular ectopy was noted in three patients. These patients were noted to have considerable ectopy on Holter monitor obtained before starting therapy. Of these six patients, four had intraventricular conduction delay, with QRS of greater than 100 milliseconds or prolonged QTc of greater than 450 milliseconds before starting protocol. A detailed review of the results of the cardiac monitoring and ECG changes is in preparation.
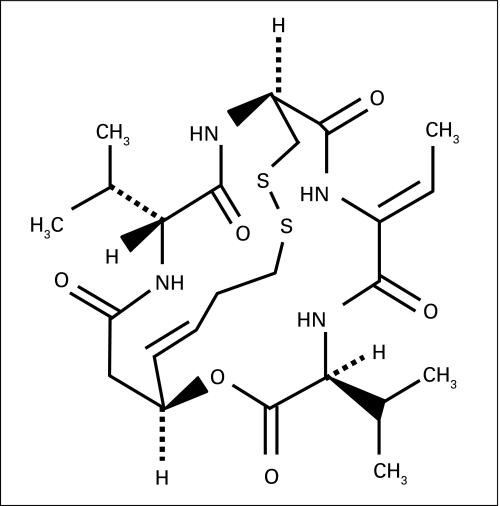
Fig A1.
Structure of romidepsin (FK228).
Fig A2.
Schedule of administration.
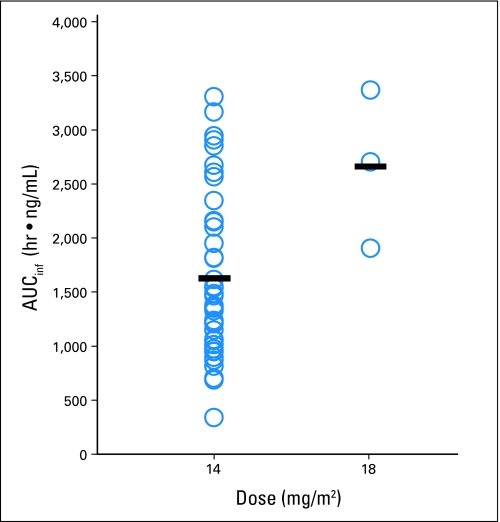
Fig A3.
Plot of area under the curve extrapolated to infinity (AUCinf) by dose level. Each circle represents the romidepsin exposure (AUCinf) for a single patient after the first treatment (42 patients receiving 14 mg/m2 and three patients receiving 18 mg/m2). The black line represents the mean for each dose level. Significant interindividual variability was observed, with AUCinf ranging from 333.15 to 3301.37 hr × ng/mL.
Fig A4.
This patient experienced progression of disease after combination therapy with cyclophosphamide, vincritine, and prednisone and had a partial response on romidepsin that lasted 2 months.
Fig A5.
Kaplan-Meier curves of progression-free survival of patients with best response of complete response (CR) or partial response (PR), patients with stable disease (SD), and patients with progressive disease (PD) or nonevaluable (NE) patients.
Table A1.
Inclusion and Exclusion Criteria
| Inclusion/Exclusion Criteria |
| Inclusion criteria |
| ≥ 18 years old |
| Measurable disease |
| ECOG performance status 0-2 |
| Life expectancy of > 12 weeks |
| > 4 weeks between prior chemotherapy, 6 weeks in the case of nitrosoureas or mitomycin |
| Laboratory values (unless impairment is a result of organ involvement by lymphoma): AGC ≥ 1,000/μL, platelets ≥ l00,000/μL, bilirubin < 1.5× ULN, AST < 3× ULN, creatinine < 1.5× ULN |
| Exclusion criteria |
| CNS lymphoma |
| HIV |
| Patients pregnant or breast feeding |
| Uncontrolled infection |
| Prior therapy with HDAC inhibitor |
| MI within previous 6 months |
| EF < 45% |
| QTc > 500 milliseconds |
| Active cardiovascular disease |
| Protocol was later amended to incorporate additional cardiac exclusion criteria |
| MI within previous 12 months |
| QTc > 480 milliseconds or are known to have congenital long QT syndrome |
| Cardiomyopathy |
| Uncontrolled hypertension |
| History of sustained VT, VF, torsades de pointes, or cardiac arrest |
| Cardiac arrhythmia requiring antiarrhythmic medication other than beta-blocker or calcium channel blocker |
| Patients taking digitalis |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; AGC, absolute granulocyte count; ULN, upper limit of normal; HDAC, histone deacetylase; MI, myocardial infarction; EF, ejection fraction; VT, ventricular tachycardia; VF, ventricular fibrillation.
Table A2.
Responses Observed in Patients Not in Cohort 1
| Best Response | Combined Cohorts (n = 44) |
||
|---|---|---|---|
| Response |
Response Duration (months) | ||
| No. | % | ||
| CR | 1 | 2 | 26 |
| PR | 12 | 27 | 1, 1, 2, 2, 3, 4*, 5, 6†, 7‡, 11*, 14, 15 |
| SD | 16 | 36 | 3, 3, 3, 4, 4, 4, 4, 4, 4†, 5, 6, 6, 6, 8, 10, 11 |
| PD | 12 | 27 | |
| NE | 3 | 7 | |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
Patients with continued responses to therapy at time of this report.
Death on study.
Patient self-withdrew from study.
Table A3.
Responses Observed in Patients Other Than Cohort 1 Divided by Stage (n = 44)
| Stage | Combined Cohorts (No. of patients) |
|||||
|---|---|---|---|---|---|---|
| Total | CR | PR | SD | PD | NE | |
| IA | 1 | 1 | ||||
| IB | 4 | 3 | 1 | |||
| IIA | 2 | 1 | 1 | |||
| IIB | 10 | 1 | 3 | 5 | 1 | |
| IIIA | 1 | 1 | ||||
| IIIB | 3 | 1 | 1 | 1 | ||
| IVA | 14 | 1 | 6 | 6 | 1 | |
| IVB | 9 | 2 | 2 | 5 | ||
| Total | 44 | 1 | 12 | 16 | 12 | 3 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
Table A4.
Cardiac Events (n = 20)
| Event | No. of Patients | Grade | Attribution |
|---|---|---|---|
| QTc prolongation | 16 | 1 | Possible or probable |
| Junctional rhythm | 1 | 1 | Possible |
| Sinus bradycardia | 2 | 1 | Unlikely |
| Atrial fibrillation | 3 | See Appendix Table A5 | Possible |
| Supraventricular and ventricular ectopy | 3 | See Appendix Table A5 | Possible |
Table A5.
Details of Ectopy Observed
| Patient No. | SVT Grade | VT Grade | Description | Preprotocol Findings |
|---|---|---|---|---|
| 1 | 3 | Atrial fibrillation | Preprotocol Holter demonstrated significant supraventricular ectopy and some ventricular ectopy | |
| 2 | 1 | Atrial fibrillation | Preprotocol patient had significant valvular heart disease and ECG demonstrated intraventricular conduction delay with QRS of 150 milliseconds and QTc of 488 milliseconds | |
| 3 | 3 | Atrial fibrillation | Preprotocol ECG demonstrated ectopy and first-degree atrioventricular block; patient was 11 years status post–aortic valve replacement | |
| 4 | 1 | 1 | Supraventricular and ventricular ectopy | Preprotocol Holter demonstrated similar ectopy |
| 5 | 3 | 1 | Supraventricular and ventricular ectopy | Preprotocol Holter demonstrated SVT and VT |
| 6 | 1 | 1 | Supraventricular tachycardia of 18 beats and ventricular tachycardia of 4 beats | Preprotocol Holter demonstrated supraventricular ectopy, SVT, and significant ventricular ectopy |
Abbreviations: SVT, supraventricular tachycardia; VT, ventricular tachycardia; QTc, QT interval.
Footnotes
See accompanying article on page 5459
Supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute, Center for Cancer Research and by a Cooperative Research and Development Agreement with Gloucester Pharmaceuticals, Cambridge, MA; also supported in part by federal funds from the National Cancer Institute, NIH, under Grant No. N01-CO-12400 (E.R.G.).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services; mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00020436.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: John P. Leonard, Gloucester Pharmaceuticals (C) Stock Ownership: None Honoraria: Mark H. Kirschbaum, Gloucester Pharmaceuticals; Jasmine Zain, Gloucester Pharmaceuticals Research Funding: Susan E. Bates, through CRADA between National Cancer Institute and Gloucester Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Richard L. Piekarz, Maria Turner, John J. Wright, William D. Figg, Seth M. Steinberg, A. Tito Fojo, Susan E. Bates
Administrative support: Robin Frye, John J. Wright
Provision of study materials or patients: Richard L. Piekarz, Robin Frye, Maria Turner, Steven L. Allen, Mark H. Kirschbaum, Jasmine Zain, H. Miles Prince, John P. Leonard, Larisa J. Geskin, Craig Reeder, Maryalice Stetler-Stevenson, Stephen Lade, A. Tito Fojo
Collection and assembly of data: Richard L. Piekarz, Robin Frye, Maria Turner, Steven L. Allen, Mark H. Kirschbaum, H. Miles Prince, John P. Leonard, Larisa J. Geskin, David Joske, William D. Figg, Erin R. Gardner, Elaine S. Jaffe, Maryalice Stetler-Stevenson, Stephen Lade, Susan E. Bates
Data analysis and interpretation: Richard L. Piekarz, Maria Turner, John J. Wright, David Joske, William D. Figg, Erin R. Gardner, Seth M. Steinberg, Elaine S. Jaffe, Stephen Lade, A. Tito Fojo, Susan E. Bates
Manuscript writing: Richard L. Piekarz, John J. Wright, Steven L. Allen, H. Miles Prince, William D. Figg, Erin R. Gardner, Seth M. Steinberg, Susan E. Bates
Final approval of manuscript: Maria Turner, Steven L. Allen, Mark H. Kirschbaum, Jasmine Zain, H. Miles Prince, John P. Leonard, Larisa J. Geskin, Craig Reeder, David Joske, William D. Figg, Erin R. Gardner, A. Tito Fojo, Susan E. Bates
REFERENCES
- 1.Richon VM, Webb Y, Merger R, et al. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci U S A. 1996;93:5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer SY, Meng SF, Shei A, et al. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci U S A. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandor V, Senderowicz A, Mertins S, et al. P21-dependent G(1) arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer. 2000;83:817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 5.Peart MJ, Smyth GK, van Laar RK, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: Is transcription the primary target? Cancer Cell. 2003;4:13–18. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Nakajima H, Hori Y, et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968: I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiot (Tokyo) 1994;47:301–310. doi: 10.7164/antibiotics.47.301. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Kim YB, Terano H, et al. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp Cell Res. 1998;241:126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- 9.Sandor V, Bakke S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 10.Marshall JL, Rizvi N, Kauh J, et al. A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J Exp Ther Oncol. 2002;2:325–332. doi: 10.1046/j.1359-4117.2002.01039.x. [DOI] [PubMed] [Google Scholar]
- 11.Piekarz RL, Robey R, Sandor V, et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: A case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 12.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIB multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 13.Ellis L, Pan Y, Smyth GK, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 14.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 15.Bunn PA, Lamberg SI. Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treat Rep. 1979;63:725–728. [PubMed] [Google Scholar]
- 16.Vonderheid EC, Bernengo MG. The Sézary syndrome: Hematologic criteria. Hematol Oncol Clin North Am. 2003;17:1367–1389. doi: 10.1016/s0889-8588(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 17.Zackheim HS, Amin S, Kashani-Sabet M, et al. Prognosis in cutaneous T-cell lymphoma by skin stage: Long-term survival in 489 patients. J Am Acad Dermatol. 1999;40:418–425. doi: 10.1016/s0190-9622(99)70491-3. [DOI] [PubMed] [Google Scholar]
- 18.Bunn PA, Jr, Hoffman SJ, Norris D, et al. Systemic therapy of cutaneous T-cell lymphomas (mycosis fungoides and the Sézary syndrome) Ann Intern Med. 1994;121:592–602. doi: 10.7326/0003-4819-121-8-199410150-00007. [DOI] [PubMed] [Google Scholar]
- 19.Morgan M, Maloney D, Duvic M. Hypomagnesemia and hypocalcemia in mycosis fungoides: A retrospective case series. Leuk Lymphoma. 2002;43:1297–1302. doi: 10.1080/10428190290026367. [DOI] [PubMed] [Google Scholar]
- 20.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 21.Shiraga T, Tozuka Z, Ishimura R, et al. Identification of cytochrome P450 enzymes involved in the metabolism of FK228, a potent histone deacetylase inhibitor, in human liver microsomes. Biol Pharm Bull. 2005;28:124–129. doi: 10.1248/bpb.28.124. [DOI] [PubMed] [Google Scholar]
- 22.Bailey DG, Spence JD, Munoz C, et al. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 25.Introcaso CE, Hess SD, Kamoun M, et al. Association of change in clinical status and change in the percentage of the CD4(+)CD26(-) lymphocyte population in patients with Sézary syndrome. J Am Acad Dermatol. 2005;53:428–434. doi: 10.1016/j.jaad.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Chen XH, Gardner ER, Figg WD. Determination of the cyclic depsipeptide FK228 in human and mouse plasma by liquid chromatography with mass-spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;865:153–158. doi: 10.1016/j.jchromb.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 28.Diamandidou E, Colome M, Fayad L, et al. Prognostic factor analysis in mycosis fungoides/Sézary syndrome. J Am Acad Dermatol. 1999;40:914–924. doi: 10.1016/s0190-9622(99)70079-4. [DOI] [PubMed] [Google Scholar]
- 29.Sausville EA, Eddy JL, Makuch RW, et al. Histopathologic staging at initial diagnosis of mycosis fungoides and the Sézary syndrome: Definition of three distinctive prognostic groups. Ann Intern Med. 1988;109:372–382. doi: 10.7326/0003-4819-109-5-372. [DOI] [PubMed] [Google Scholar]
- 30.Woo S, Gardner ER, Chen XH, et al. Population pharmacokinetics of romidepsin in patients with cutaneous T-cell lymphoma and relapsed peripheral T-cell lymphoma. Clin Cancer Res. 2009;15:1496–1503. doi: 10.1158/1078-0432.CCR-08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piekarz R, Bates S. A review of depsipeptide and other histone deacetylase inhibitors in clinical trials. Curr Pharm Des. 2004;10:2289–2298. doi: 10.2174/1381612043383980. [DOI] [PubMed] [Google Scholar]
- 32.O'Mahony D, Peikarz RL, Bandettini WP, et al. Cardiac involvement with lymphoma: A review of the literature. Clin Lymphoma Myeloma. 2008;8:249–252. doi: 10.3816/CLM.2008.n.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sézary syndrome. JAMA. 1992;267:1354–1358. [PubMed] [Google Scholar]
- 35.Tokura Y, Yagi H, Ohshima A, et al. Cutaneous colonization with staphylococci influences the disease activity of Sézary syndrome: A potential role for bacterial superantigens. Br J Dermatol. 1995;133:6–12. doi: 10.1111/j.1365-2133.1995.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 36.Piekarz RL, Sackett DL, Bates SE. Histone deacetylase inhibitors and demethylating agents: Clinical development of histone deacetylase inhibitors for cancer therapy. Cancer J. 2007;13:30–39. doi: 10.1097/PPO.0b013e31803c73cc. [DOI] [PubMed] [Google Scholar]