Abstract
Place fields of hippocampal pyramidal cells expand asymmetrically when adult rats repeatedly follow the same route. This behaviorally-induced expression of neuronal plasticity utilizes an NMDAR-dependent, LTP-like mechanism and could be used by hippocampal networks to store information. Aged spatial memory-impaired rats exhibit defective experience-dependent place field expansion plasticity. One possible explanation for this aged-associated deficit is alterations in glutamatergic function. In fact, both NMDAR- and AMPAR-mediated field excitatory postsynaptic potentials in CA1 decrease with aging. The current study investigated whether modulation of either AMPA or NDMA receptor activity could restore this experience-dependent plasticity by prolonging AMPAR activity with the ampakine CX516, and modulating the NMDAR with the noncompetitive antagonist memantine. The spatial firing characteristics of multiple CA1 pyramidal cells were monitored under both treatment conditions as aged rats repeatedly traversed a circular track. Compared to the saline baseline condition, acute administration of memantine but not CX516, reinstated experience-dependent place field expansion. Taken together, these data suggest that pharmacological manipulation of the NMDAR can improve the function of hippocampal networks critical to optimal cognition in aging.
Keywords: aging, CA1, hippocampus, place cell, theta phase precession
Introduction
Normal aging is associated with a decline in cognitive function that can be explained by alterations in the brain structures that support these processes. The hippocampus is one region that is vulnerable to the aging process. Because spatial learning and memory require an intact hippocampus (Jarrard, 1993; Morris, Garrud, Rawlins, & O'Keefe, 1982; Sutherland, Kolb, & Whishaw, 1982), it is not surprising that aged humans (Newman & Kasznaik, 2000; Wilkniss, Jones, Korol, Gold, & Manning, 1997) and other animals show deficits on tasks designed to test spatial navigation (Bach et al., 1999; Barnes, 1979; Gallagher & Rapp, 1997; Lai, Moss, Killiany, Rosene, & Herndon, 1995; Markowska et al., 1989; Rapp, Kansky, & Roberts, 1997). Therefore, the study of age-related disruptions in neuronal information processing in the hippocampus, and how normal function could be restored pharmacologically, is of considerable interest.
In adult rats, neuronal recordings from the hippocampus reveal that when a rat explores an environment, pyramidal (O'Keefe & Dostrovsky, 1971) and granule (Jung & McNaughton, 1993) cells show patterned neural activity that is highly correlated with a rat's position in space (that is, the cells are active in distinct locations, or exhibit ‘place fields’). All hippocampal principal cells appear capable of expressing this form of selective activity, but in a typical experimental environment, only a fraction of cells show place fields (approximately 30–50% of dorsal CA1 pyramidal cells) (e.g. Gothard, Skaggs, Moore, & McNaughton, 1996; Muller & Kubie, 1987; Wilson & McNaughton, 1993).
Although aged rats are impaired on many tasks that require an intact hippocampus, basic firing characteristics of rat CA1 pyramidal cells such as spike amplitude, spike width, mean and maximum firing rates, and inter-spike interval (ISI) distributions do not change appreciably in advanced age (e.g. Barnes, McNaughton, & O'Keefe, 1983; Barnes, Suster, Shen, & McNaughton, 1997; Markus, Barnes, McNaughton, Gladden, & Skaggs, 1994; Mizumori, Lavoie, & Kalyani, 1996; Oler & Markus, 2000; Shen, Barnes, McNaughton, Skaggs, & Weaver, 1997). Moreover, upon the initial pass through a place field, or during a task in which the rat's trajectory is not restricted (i.e., random foraging), the place fields of CA1 pyramidal cells in aged rats are just as specific as those of young rats (e.g. Barnes et al., 1997; Markus et al., 1994; Mizumori et al., 1996; Oler & Markus, 2000; Shen et al., 1997; Tanila, Shapiro, Gallagher, & Eichenbaum, 1997). In contrast, the dynamic properties of place fields that require functional plasticity are altered in advanced age.
In young rats, CA1 pyramidal cells exhibit place fields that expand asymmetrically during repeated route following. This experience-dependent backward expansion can be measured in several different ways, including a shift in the center of mass of place fields in the direction opposite to the rat's trajectory (Lee, Rao, & Knierim, 2004; Mehta, Barnes, & McNaughton, 1997; Shen et al., 1997), changes in place field characteristics such as skewness (Mehta, Quirk, & Wilson, 2000), and an increase in the number of spikes fired within a place field (Ekstrom, Meltzer, McNaughton, & Barnes, 2001). The asymmetric expansion is consistent with neural network models dating back to Hebb's (1949) concept of the ‘phase sequence’ of cell assemblies, which have suggested that an associative, temporally-asymmetric synaptic plasticity mechanism could serve to encode sequences or episodes of experience (Hebb, 1949). The formation of such ‘phase sequences’ allows future predictions of neural activity to be made based on current brain states, and provide a means to encode learned routes and goal locations necessary for spatial learning and memory (Abbott & Blum, 1996; Blum & Abbott, 1996).
Interestingly, the behaviorally-induced expansion of CA1 place fields resets after only a day's absence from a familiar environment (Lee et al., 2004; Mehta et al., 1997; Mehta et al., 2000) and it is NMDAR-dependent (Ekstrom et al., 2001). The dependence of place field expansion on the NDMAR suggests that the phenomenon utilizes an LTP-like mechanism (Ekstrom et al., 2001), and it is presumed that uncorrelated neural activity during the intervening hours may enable the behaviorally-induced increase in synaptic strength to either decay or actively depotentiate (Mehta et al., 1997; Mehta et al., 2000).
Consistent with data showing age-related deficits in experimentally-induced LTP (for review, see Burke & Barnes, 2006; Rosenzweig & Barnes, 2003), the magnitude of behaviorally-induced place field expansion, and the resulting changes in the characteristics of place fields, significantly decrease in aged rats (Shen et al., 1997). Because aged rats do not show robust place field expansion, it is possible that pharmacological agents that increase the induction or maintenance of LTP might act to reinstate this experience-dependent plasticity phenomenon. Two agents that have been shown to enhance in vivo LTP are ampakines and memantine.
Ampakines act as positive modulators of the glutamate AMPA receptor to enhance and prolong AMPAR-mediated responses (e.g. Arai et al., 1996a; Arai, Kessler, Rogers, & Lynch, 1996b). One chemical in the ampakine family, CX516, is of special interest, due to its efficiency and safety (Arai, Xia, Rogers, Lynch, & Kessler, 2002). CX516 reaches the brain approximately 5 minutes after intraperitoneal administration. Peak concentration is reached at 10 minutes, after which CX516 is metabolized rapidly, with a half-life in blood of approximately 15-20 minutes (Hampson, Rogers, Lynch, & Deadwyler, 1998). CX516 has been shown to facilitate the induction of in vivo LTP in young rats when a sub-optimal stimulus protocol is applied (Arai et al., 2002), and improves memory performance of young rats in several tasks (e.g. Granger et al., 1993; Larson et al., 1995). It has also been shown that CX516 facilitates old rats' working memory performance in a radial maze (Davis et al., 1997), and improves performance of old humans on a delayed recall of nonsense syllables task (Lynch et al., 1997).
In contrast, memantine is a low-affinity, non-competitive NMDA receptor antagonist that blocks the open pore of the glutamate receptor (Parsons et al., 1995). It is currently used therapeutically to reduce the cognitive impairments associated with Alzheimer's disease (Rogawski & Wenk, 2003). In human clinical trials, memantine has been shown to improve cognitive function and global status significantly over a 24 week period in patients with mild to moderate dementia (Peskind et al., 2006). In animals, memantine has been shown to improve spatial learning in a transgenic mouse model of Alzheimer's disease (Minkeviciene, Banerjee, & Tanila, 2004) and to increase adult rats' retention of the memory for the location of the hidden escape platform on the spatial version of the Morris swim task (Barnes, Danysz, & Parsons, 1996). It is possible that the cognitive enhancing properties of memantine result from an improvement in plasticity mechanisms, which are disrupted by pathology or the normal aging process. In support of this idea, memantine has been shown to increase the maintenance of experimentally-induced LTP in adult rats (Barnes et al., 1996).
While CX516 has been shown to enhance LTP induction and memantine has been shown to improve LTP maintenance, neither agent has been used to enhance behaviorally-induced plasticity. The current experiments were designed to test whether the ampakine CX516 and/or memantine could reinstate experience-dependent plasticity of CA1 place fields in aged rats with known spatial memory impairments.
Methods
Subjects and Behavioral Training
Electrophysiological studies were conducted on twelve Fisher-344 male rats between 24-30 months old. Seven were treated with CX516 (Experiment 1) and five were treated with memantine (Experiment 2). The rats were housed individually, and maintained on a 12:12 light-dark cycle. Before rats were implanted with the hyperdrive recording device all rats were screened for spatial memory impairments and normal vision using the Morris swim task (Morris, 1984). All animals were tested over 4 days with 6 spatial trials on each day. Animals were then screened for visual ability with two days of cued visual trials (6 trials/day) in which the escape platform was above the surface of the water but the position of the platform changed between each trial. This procedure has been described in detail previously (e.g. Barnes et al., 1996; Shen & Barnes, 1996). Rats' performance on the swim task was analyzed off-line with in-house software (WMAZE, M. Williams). Since different release locations and differences in swimming velocity produce variability in the latency to reach the escape platform, a corrected integrated path length (CIPL) was calculated to ensure comparability of the rats' performance across different release locations (Gallagher, Burwell, & Burchinal, 1993).
During electrophysiological recordings, the animals were food deprived to about 85% their ad libitum weight and trained to run on a circular track (286 cm in circumference for experiment 1 and 382 cm in circumference for experiment 2) for food reinforcement. Rats ran uni-directionally in the counterclockwise direction and all electrophysiological recordings took place during the dark phase of the rats' light/dark cycle. Food was given at one position on the track after the completion of each single lap, and all rats were required to run approximately 40 laps or 30 minutes for each recording session, whichever criteria was reached first. Each track running epoch was flanked by 30-40 minutes in which the rat rested in the same room in a pot off of the track. Thus, the activity of CA1 neurons was monitored during an initial rest session (before behavior), during track running, and during a rest session after behavior. Data from the rest periods were used to assess firing stability across the entire recording session (data not shown).
Surgical Procedures
Surgery was conducted according to National Institutes of Health guidelines for rodents and protocols approved by the University of Arizona IACUC. Prior to surgery, the rats were administered bicillin (30,000 units i.m. in each hindlimb) to combat infection. The rats were implanted, under isofluorane or Nembutal anesthesia, with an array of 14 separately moveable tetrode recording probes using a “hyperdrive” manipulator device. This device, implantation methods, and the parallel recording technique have been described in detail elsewhere (Gothard et al., 1996). Briefly, each hyperdrive consisted of 14 drive screws coupled by a nut to a guide cannula. Twelve guide of these cannulae contained tetrodes (McNaughton, O'Keefe, & Barnes, 1983b; Recce & O'Keefe, 1989), four-channel electrodes constructed by twisting together four strands of insulated 13 μm nichrome wire (H. P. Reid, Inc., Neptune, NJ). Two additional tetrodes with their individual wires shorted together served as an indifferent reference and an EEG recording probe. A full turn of the screw advanced the tetrode 318 μm. For all twelve rats, recordings were made from the dorsal CA1 region (3.8 posterior, 2.0 lateral to bregma) and tetrode hippocampal location was verified histologically. The implant was cemented in place with dental acrylic anchored by small screws. Immediately after surgery, all tetrodes were lowered approximately 1 mm into the cortex and rats were orally administered 26 mg of acetaminophen (Children's Tylenol Elixir, McNeil, PA) for analgesia. They also received 2.7 mg/ml acetaminophen in the drinking water for 1-3 days after surgery and oral ampicillin (Bicillin, Wyeth Laboratories, Madison, NJ) on a 10 days on/10 days off regimen for the duration of the experiment.
Neurophysiology
The tetrodes were lowered after surgery into the hippocampus, allowed to stabilize for several days just above the CA1 hippocampal subregion, and then gradually advanced into the CA1 stratum pyramidale. The neutral reference electrode was located in or near the corpus callosum. The EEG probe was used to record theta field activity from the vicinity of the hippocampal fissure. The four channels of each tetrode were attached to a 50-channel unity-gain headstage (Neuralynx, Inc., Tucson AZ). A multi-wire cable connected the headstage to digitally programmable amplifiers (Neuralynx, Inc, Tucson, AZ). The spike signals were amplified by a factor of 1,000-5,000, bandpass-filtered between 600 Hz and 6 kHz, and transmitted to the Cheetah Data Acquisition system (Neuralynx, Inc., Tucson, AZ.). Signals were digitized at 32 kHz and events that reached a predetermined threshold were recorded for a duration of 1 ms. Spikes were sorted off-line on the basis of the amplitude and principal components from the four tetrode channels, by means of a semi-automatic clustering algorithm (KlustaKwik, author: K.D. Harris, Rutgers-Newark). The resulting classification was corrected and refined manually with custom-written software (MClust, author: A.D. Redish, University of Minnesota; updated by S.L. Cowen and D.R. Euston, University of Arizona), resulting in a spike-train time-series for each of the well-isolated cells. No attempt was made to match cells from one daily session to the next. Therefore, the numbers of recorded cells reported does not take into account possible recordings from the same cells on consecutive days; however, because the electrode positions were adjusted from one day to the next, recordings from the same cell over days were probably relatively infrequent. Putative pyramidal neurons were identified by means of the standard parameters of firing rate, burstiness, and spike waveform (Ranck, 1973). The electroencephalogram (EEG) was recorded from a separate probe that was positioned approximately 0.5mm below the CA1 pyramidal layer, near the hippocampal fissure. This is an optimal location for recording the prominent 6-10 Hz oscillation that occurs during active exploration, referred to as theta activity (Vanderwolf, 1969). The theta signal was band-pass filtered between 1 and 300 Hz and sampled at a frequency of 2.4 kHz.
Several diodes were mounted on the headstage to allow position tracking. The position of the diode array was detected by a TV camera placed directly above the experimental apparatus and recorded with a sampling frequency of 60 Hz. The sampling resolution was such that a pixel was approximately 0.3 cm.
Drug Preparation and Injection
For Experiment 1, in which the effect of ampakines on experience-dependent plasticity was measured, CX516 (Cortex Pharmaceuticals, Irvine, CA) was dissolved in saline (0.9%) to make a 35 mg/ml solution. The injection was given intraperitoneally 10 min before the rats were placed onto the circular track to run. Each rat was administered saline or 35 mg/kg of CX516 on alternate days. This dose was selected because it has been shown that doses of 30-35mg of CX516 enhance short-term memory in rats performing the delayed non-match to sample task (Hampson et al., 1998) and facilitate old rats' working memory performance in a radial maze (Davis et al., 1997). In Expermiment 2, memantine was dissolved in saline to make a 15 mg/ml solution. Ten minutes after the beginning of rest one and 20 minutes prior to track running, each rat was injected subcutaneously with either, 5 mg/kg, 10 mg/kg, 15 mg/kg of memantine or 0.1cc of saline control. The three doses of memantine and the saline control were given on consecutive days in a pseudo-randomized order, such that no drug dose or the saline control was given on more than two consecutive days. In both Experiments 1 and 2, injections were made while the rats were attached to the head stage and spike and EEG data were being collected. Multiple doses of memantine were used because it has been shown that 5 mg/kg of memantine enhances spatial memory in adult rats (Zoladz et al., 2006) and 10 mg/kg of memantine increases recognition memory (Pitsikas & Sakellaridis, 2007). Additionally, higher doses of memantine have been shown to reverse cognitive dysfunction from entorhinal cortex lesions (Zajaczkowski, Quack, & Danysz, 1996).
Analyses and Statistics
Place field diagrams were constructed by plotting the circular trajectories of the animals on a linearized, one-dimensional scale, using a linear interpolation (Maurer, Vanrhoads, Sutherland, Lipa, & McNaughton, 2005). Place field location and size were determined using the criteria that no place field could extend beyond a single cycle of theta phase precession (Maurer, Cowen, Burke, Barnes, & McNaughton, 2006). It is well documented that as a rat passes through a CA1 neuron's place field, the spike timing shows a systematic shift such that the spikes occur at successively earlier phases of the theta cycle until there is a complete cycle of phase advancement (O'Keefe & Recce, 1993; Skaggs, McNaughton, Wilson, & Barnes, 1996).
By using the phase precession definition, the start location of a field was defined as the location at which spikes fired late in theta phase (approximately 360°) and began a cycle of precession. The end location of the place field was determined to be the location of spikes with the largest phase angle. This was accomplished by creating a two dimensional plot of occupancy normalized position (X axis) versus phase of spikes (Y axis), as well as occupancy-normalized position versus phase density plots (Fig 1A). In order to construct the theta phase of firing versus position plots, each spike was assigned a nominal theta phase. Precisely, the phase assigned to a spike at time t was 360° (t - t0) / (t1 − t0), where t0 and t1 are the times of the preceding and following peaks of the filtered reference EEG signal (Skaggs et al., 1996). The phase is always a number between 0 and 360.
Figure 1.

(A) The theta phase (Y axis) by position (X axis) plots which were used to determine place field location and extent. The upper panel shows the place field boundaries (vertical black lines) using the definition of a place field as the distance traversed for the cell to precess no more than 360° with respect to the theta rhythm. Each dot represents a spike and the red dots are spikes that were included in the place field. The bottom panel is the raw data presented as a phase by position density plot using a hanning window of 7cm by 70 degrees. Red areas indicate a higher density of spikes while the dark areas indicate that no spikes were fired in those positions. Note that two cycles of theta phase precession are plotted for both panels. (B) Raster plots of the place field from (A) showing a CA1 pyramidal cell recorded in an aged rat injected with 10mg/kg of memantine. The rat was running from right to left. The top panel shows the firing rate histogram, and the bottom panel shows spikes by lap.
Place field boundaries were manually drawn using the theta phase precession information by an experimenter who was blind to the treatment condition of the recording session (see Fig 1A). Place fields that overlap with a food dish often show an abrupt halt in theta phase precession and can show considerably less than 360° of precession. For this reason place fields that overlapped with a food dish were excluded. Additionally, if a place field had two or more clear cycles of precession that overlapped spatially (see Maurer et al., 2006), it was excluded from the analysis. Furthermore, neurons that did not fire for at least 3/4 of the laps or did not fire within the first four laps were eliminated from the analysis. Based on these exclusion criteria, 10.8% of place fields were excluded from Experiment 1 (24 place fields) and 9.6% of place fields were excluded from Experiment 2 (98 place fields). Figure 1B shows the firing rate by position (top panel) and individual lap raster plot for a representative place field used in the current analysis.
After the place field boundaries were determined, the number of in-field spikes, place field size and normalized center of mass shift were calculated for each place field by lap. Normalized center of mass shift was calculated by finding the mean position of all spikes within the theta phase precession boundary and then subtracting the center of mass of the first lap from the subsequent laps. Averages for each rat were then calculated so that all statistical tests were conducted using the numbers of rats rather than the number of individual place fields. This was done so that statistical power was not inflated by the large number of degrees of freedom from the high number of cells recorded and so that rats in which more cells were recorded from did not contribute disproportionately to the analysis. Finally, paired samples t-tests (Experiment 1) or repeated-measures factorial ANOVAs and simple contrasts, or Tukey HSD post hoc analyses (Experiment 2) were performed using SPSS software (SPSS Inc., Chicago, IL) to assess possible drug effects.
For drug conditions that resulted in a significant difference in running speed (see results) the effect of the drug on firing rate and running speed was examined further. In order to calculate the effect of drug condition on the relationship between firing rate and running speed, the position of the rat's head in two dimensions, derived from the video tracker data, was converted to angular coordinates and projected onto a one dimensional line corresponding to the center of the track. Speed was calculated by numerically differentiating the one dimensional position data, and for each recording session, speed occupancy distributions were computed as the number of speed samples per 6 cm/s bin, with 0 speeds in the first bin. For each recorded neuron, the number of spikes that occurred within each speed interval was computed. The firing rate vs. speed function was computed from the ratios of these two distributions. Because the animal's average running speed varied systematically with position on the track, the individual tuning functions for pyramidal cells do not accurately reflect the hypothetical true firing rate vs. speed function, because firing rate is a function of both speed and location. For example, a cell with a place field in a region of low average speed would appear to have a bias to fire at slower speeds; however, the average of the rate vs. speed distributions over all cells should accurately reflect the ensemble firing rate vs. speed function. This is the desired relationship, and is used for the present analysis.
Results
Morris swim task
The mean performance of the aged rats on the spatial version of the Morris swim task was significantly worse than the performance of ten representative adult rats. Every aged rat was able to learn the visual version of the task, however. Figure 2A shows the mean corrected integrated path length (CIPL, Gallagher et al., 1993) for the twelve aged rats used in this analysis, compared to the CIPL of ten young rats (9 months) that were tested on the Morris swim task at the same time or within several weeks of the testing of the aged rats. All animals were tested over 4 days with 6 spatial trials on each day. As reported previously (e.g. Barnes et al., 1997), the old rats had a significantly longer CIPL, compared to the young rats, on both days 3 and 4 (F[7,68] = 10.78, p < 0.001 for both comparisons; ANOVA). In fact, all but one aged rat had a CIPL that was greater than 2 standard deviations from the mean CIPL score of the young rats on day 4, not allowing for a separation of these animals into age-impaired and age-unimpaired groups. The old rats, however, did show a modest, but significant improvement, in finding the hidden platform between day 1 and day 4 (F[1,11] = 6.34, p < 0.05, repeated-measures ANOVA). This improvement did not significantly differ between the animals that were administered memantine and the animals that were given CX516 (F[1,11] = 0.16, p = 0.7).
Figure 2.
(A) The mean and standard error of the mean (SEM) for the corrected integrated path length (CIPL) of the twelve old rats used in the current study (diagonal bars) and ten young rats (clear bars) on the spatial version of the Morris swim task. The old rats were significantly impaired at learning the location of the hidden platform. (B) The CIPL of the young (clear bars) and old (diagonal bars) rats for the visual version of the water maze in which the escape platform was no longer hidden below the surface of the water. The CIPL was significantly shorter on the second day, indicating that the old rats were able to use the visual cues to find the platform. Additionally, on day 2, the mean behavioral score did not differ significantly between the young and aged rats. Error bars indicate ± one SEM. (C) The mean and SEM CIPL values of the aged rats separated by experiment 1 (light grey) versus experiment 2 (dark grey). The aged rats in experiments 1 and 2 show similar degrees of spatial impairment.
To test whether the spatial impairments of the aged rats were due to visual problems, the aged rats also performed the visual version of the Morris swim task in which the platform was raised above the surface of the water for two days and six visual trials were performed on each day. Figure 2B shows the CIPL for the visual trials of the Morris swim task for the trials on day 1 and day 2 for the old rats and control young animals. The aged rats showed a significant decrease in the CIPL on the second day compared to the first day (T[9] = 4.03, p<0.01; paired-samples t test). Additionally, the performance of the old rats used in the current experiments was not significantly different from young animals on day 2 of the visual trials (p = 0.68, Tukey HSD). These data indicate that aged rats used for electrophysiological recordings were able to use the visual cues to swim to the platform.
Finally, the aged rats used in Experiment 1 and the aged rats used in Experiment 2 showed similar degrees of spatial impairment. Figure 2C shows the mean CIPL values for the 6 aged rats used in experiment 1 compared to the five aged rats used in experiment 2. Performance between the two groups of rats was not significantly different (F[7, 28] = 0.5, p = 0.8) suggesting that the two groups of rats showed similar amounts of hippocampal dysfunction.
Experiment 1: CX516
Single unit firing characteristics and running speed
The activity of 524 pyramidal cells in the CA1 region of seven aged rats was recorded in this experiment. A summary of the database from which the following results are derived is given in Table 1.
Table 1.
Summary of the database used in experiment 1
| Rat | Saline | CX516 35mg/kg | ||
|---|---|---|---|---|
| cells | place fields | cells | place fields | |
| 6978 | 72 | 23 | 36 | 13 |
| 6981 | 31 | 16 | 56 | 20 |
| 7009 | 14 | 8 | 30 | 11 |
| 7091 | 41 | 9 | 42 | 14 |
| 7096 | 45 | 15 | 16 | 8 |
| 7097 | 17 | 6 | 12 | 6 |
| 7144 | 47 | 18 | 65 | 30 |
| total | 267 | 95 | 257 | 102 |
Table 2 shows the effect of CX516 on the single-unit firing characteristics of neurons with place fields and the rats' running speed compared to the saline control. There was no significant difference between the running speed, firing rate, or in-field firing rate when rats were injected with CX516 versus saline.
Table 2.
The effect of CX516 on the single unit firing characteristics and running speed.
| Saline | CX516 35mg/kg | P value | |
|---|---|---|---|
| Mean firing rate for all behavior (Hz) | 0.64 ± 0.14 | 0.61 ± 0.06 | T[33] = 0.24 p = 0.81 |
| Mean in-field firing rate (Hz) | 23.69 ± 3.41 | 21.41 ± 2.34 | T[33] = 0.56 p = 0.58 |
| Mean running speed (cm/s) | 8.10 ± 0.32 | 9.05 ± 0.48 | T[33] = 1.64 P = 0.11 |
CX516 and experience-dependent place field expansion plasticity
In order to assess the effects of CX516 on the place field characteristics over time, place fields were identified based on the neuron's spike timing relative to the theta rhythm, as described in the methods. Once place fields were identified, the effect of multiple traversals through a cell's place field was assessed by measuring the number of spikes that occurred in a single place field for laps 1-20 individually for all of the drug conditions. This measure of experience-dependent place field change was used, in addition to other measures, because it is less dependent on running speed. Specifically, the relationship between running speed and firing rate tends to keep the number of spikes in a place field constant even when the animal's speed changes. Moreover, it has been shown previously that, as the place field expands, the number of spikes also increases (Ekstrom et al., 2001). Firing rate by lap was not used because firing rate is proportional to running speed and the significantly faster running speeds on the first lap would confound these results.
Figure 3A shows the mean number of spikes within a place field for CX516 and the saline control for the first through the twentieth laps. Overall, for the seven rats, the mean number of spikes that occurred during a single pass through a place field was not significantly different between the CX516 and the saline control conditions (T[6] = 0.35, p = 0.74; paired-samples t test). Figure 3B shows the difference in the number of in-field spikes between lap 1 and lap 20 for the saline and CX516 conditions. The number of spikes did not increase between lap 1 and lap 20 for either the saline control or CX516 treatment (F[1,10] = 1.14, p = 0.31; repeated measures ANOVA). Moreover, in both cases the increase in spike number was not significantly different from zero.
Figure 3.
(A) The mean and SEM of number of spikes averaged for each rat across laps 1 through 20 for the saline control (black) and 35 mg/kg of the ampakine CX516 (grey). The number of spikes was similar between the saline and the CX516 conditions. (B) The difference in the mean number of spikes for lap 20 minus lap 1 for saline (black) and CX516 (grey). The increase in the number of spikes between lap 1 and lap 20 was similar between the ampakine and the saline conditions and not significantly different from zero for both saline and CX516. Error bars indicate ± one standard error of the mean (SEM).
In addition to spike number, the measures of place field size and center of mass shift were also used to assess whether CX516 could restore experience-dependent place field expansion plasticity. Place field boundaries were determined as described in the methods (see Fig 1B), and then place field size for each lap was measured by calculating the difference between the location of the first spike and the last spike within the theta phase precession boundary. Figure 4A shows the mean size of the place fields for saline and CX516 across laps 1 through 20. In contrast to the analysis of spike number by lap, there was a significant increase in place field size between the first and the twentieth lap (F[1, 10] = 6.74, p < 0.05; repeated measures ANOVA) but the interaction between lap (1 versus 20) and drug condition (saline versus CX516) was not significant (F[1,10] = 0.20, p = 0.67; repeated measures ANOVA). This suggests that CX516 did not increase place field size beyond that observed in the saline control condition. Figure 4B shows the mean change in the size of place fields between lap 1 and 20 for both drug conditions. Overall, the mean size of place fields was similar between saline and CX516 conditions (T[5] = 0.42, p = 0.69; paired-samples t-test).
Figure 4.
(A) The effects of ampakines on average place field size across laps 1 through 20 for the saline control (black), and 35 mg/kg of CX516 (grey). Both conditions showed a similar increase in place field size. (B) The average change in place field size for the saline control (black), and 35 mg/kg of CX516 (grey) between lap 1 and lap 20. Error bars indicate ± one standard error of the mean (SEM).
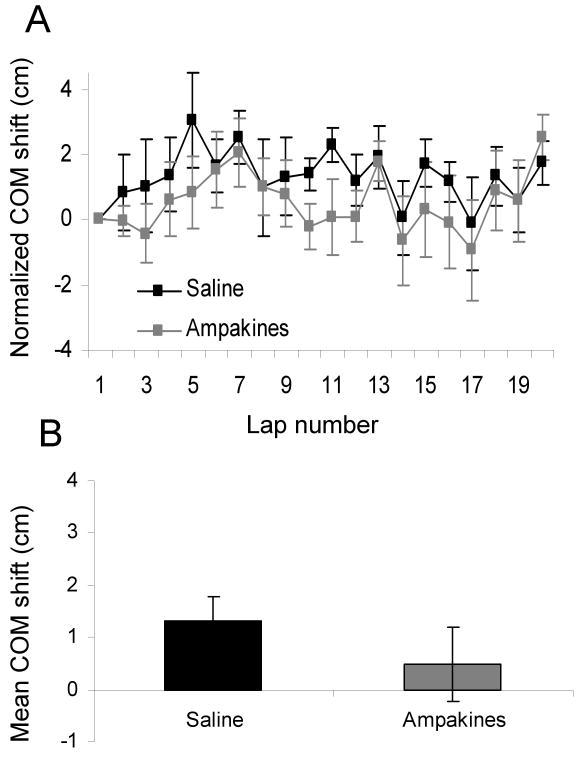
As a final measure of place field expansion, normalized center of mass shift was calculated by finding the mean position of all spikes within the theta phase precession boundary and then subtracting the center of mass of the first lap from the subsequent laps. Figure 5A shows the normalized center of mass shift across laps 1 through 20. There was no significant effect of drug condition on center of mass shift in these old rats (T[5] = 2.0, p = 0.1; paired samples t-test). Figure 5B shows that the mean center of mass shift for saline and CX516 conditions was similar.
Figure 5.
(A) Shows the mean and SEM for normalized center of mass (COM) shift of place fields from laps one to twenty for the saline control (black), and 35 mg/kg of CX516 (grey). (B) The average normalized COM shift over all laps for the saline control (black) and CX516 (grey). The COM shift was similar between the two drug and the control conditions. Error bars indicate ± one SEM.
Experiment 2: Memantine
Single unit firing characteristics and running speed
The activity of 1995 pyramidal cells in the CA1 region of five aged rats was recorded. A summary of the database from which the following results are derived is given in Table 3.
Table 3.
Summary of the database used in experiment 2
| Rat | Saline | 5 mg/kg memantine | 10 mg/kg memantine | 15 mg/kg memantine | ||||
|---|---|---|---|---|---|---|---|---|
| cells | place fields | cells | place fields | cells | place fields | cells | place fields | |
| 8092 | 68 | 30 | 50 | 17 | 67 | 22 | NA | NA |
| 8116 | 92 | 41 | 88 | 48 | 82 | 46 | 60 | 35 |
| 8117 | 134 | 69 | 195 | 83 | 215 | 102 | 191 | 108 |
| 8118 | 82 | 31 | 123 | 59 | 68 | 28 | 109 | 39 |
| 8237 | 115 | 58 | 108 | 40 | 68 | 30 | 80 | 40 |
| total | 491 | 229 | 564 | 247 | 500 | 228 | 440 | 222 |
On different days, the same rats were injected with saline, or 5 mg/kg, 10 mg/kg, or 15 mg/kg of memantine. Table 4 shows the effects of the 3 different doses of memantine on the single-unit firing characteristics of neurons with place fields and the rats' running speed. Overall, the effect of drug condition on firing rate during track running was significant (F[3, 44] = 6.00, p < 0.01; ANOVA). The 10mg/kg and 15 mg/kg doses of memantine significantly increased the overall mean firing rate of pyramidal cells during the entire behavioral epoch compared to the saline control condition (p < 0.01 for both comparisons; simple contrast), while the 5 mg/kg dose had no effect. None of the doses of memantine, however, had a significant effect on the mean in-field firing rate (F[3, 44] = 1.59, p = 0.21; ANOVA). Memantine also significantly increased the mean running speed of the aged rats (F[3,43] = 4.13, p < 0.05; ANOVA) but only at the 15 mg/kg dose (p < 0.05), while the 5 mg/kg (p = 0.97) and 10 mg/kg (p = 0.98) doses had no significant effect on running speed. This is consistent with a previous report, which shows that systemic injections of high doses of memantine can increase locomotor activity (Bubser, Keseberg, Notz, & Schmidt, 1992).
Table 4.
The effect of memantine on the single unit firing characteristics and running speed. *Indicates that mean the mean is significantly different from the saline control condition at the p < 0.05.
| Saline | 5 mg/kg memantine | 10 mg/kg memantine | 15 mg/kg memantine | P value | |
|---|---|---|---|---|---|
| Mean firing rate for all behavior (Hz) | 1.04 ± 0.07 | 1.05 ± 0.06 | 1.34* ± 0.08 | 1.32* ± 0.04 | F[3, 44] = 6.00 p < 0.01 |
| Mean in-field firing rate (Hz) | 18.41 ± 0.96 | 17.60 ± 1.48 | 18.62 ± 1.96 | 22.27 ± 2.00 | F[3,44] = 1.59 p = 0.21 |
| Mean running speed (cm/s) | 4.12 ± 0.68 | 3.59 ± 0.54 | 4.63 ± 0.74 | 7.33 ± 0.46* | F[3,44] = 4.13 p < 0.05 |
Because the firing rate of hippocampal neurons has been shown to be proportionally related to an animal's running speed (Czurko, Hirase, Csicsvari, & Buzsaki, 1999; Maurer et al., 2005; McNaughton, Barnes, & O'Keefe, 1983a; Shen et al., 1997), the relationship between running speed on the track and firing rate was investigated. Figure 6A shows the effects of memantine on the mean running speed for the first 20 laps. For all drug conditions and the saline control, rats had the fastest running speeds on the first lap and then slowed their running speed during subsequent laps (F[19,860] = 2.33, p < 0.01; ANOVA, deviation contrast). This is distinct from the observation in young rats that running speed is similar over 20 laps after saline injection but running speed increases over laps 1-10 after administration of the NMDA receptor antagonist CPP (Ekstrom et al., 2001). As shown previously in young (Czurko et al., 1999; Maurer et al., 2005; McNaughton et al., 1983a), and aged rats (Shen et al., 1997), there was an overall effect of running speed on pyramidal cell firing rate. Figure 6B shows the firing rate by running speed for all drug conditions. Similar to the previous observations, there is an approximate linear increase in firing rate with increasing running speeds up to 35 cm/s (Czurko et al., 1999; Maurer et al., 2005; Shen et al., 1997). This effect was consistent across all drug conditions (F[8, 10288] = 21.84, p < 0.001; ANOVA). Moreover, there was not a significant interaction between running speed and drug condition (F[24, 10288] = 1.46, p = 0.07; ANOVA), indicating that firing rate was modulated by running speed similarly for the control condition and the three doses of memantine. At running speeds faster than 35 cm/s there was increasing variability in the firing rate, which is likely due to the small sample size of faster running speeds in the aged animals.
Figure 6.
(A) The mean running speed by lap for laps 1 through 20 for the saline control (black), 5 mg/kg (red), 10 mg/kg (yellow), and 15 mg/kg (blue) doses of memantine. For all four conditions, the rats ran fastest on the first lap and then speeds were consistent for the subsequent nineteen laps. (B) The relationship between running speed and firing rate for all doses of memantine and the saline control. Firing rate increased similarly for all conditions for speeds up to 35 cm/s. Error bars indicate ± one standard error of the mean (SEM).
Memantine and experience-dependent place field expansion plasticity
In order to assess the effects of the three doses of memantine on the place field characteristics over time, place fields were identified based on the neuron's spike timing relative to the theta rhythm as in Experiment 1 (see methods). Again, three measures of place field expansion plasticity were used: spike number, place field size, and center of mass.
Figure 7A shows the mean number of spikes within a place field for the 3 doses of memantine and the saline control for the first through the twentieth laps. There was a significant effect of drug condition on the mean number of within field spikes over all 20 laps (F[3,376] = 8.94, p < 0.001; ANOVA) with both the 5 mg/kg and 10 mg/kg doses of memantine leading to an increase in the number of in-field spikes compared to the saline control (p < 0.05, for all comparisons; simple contrast). Figure 7B shows the difference in the number of spikes between lap 1 and lap 20 for all four drug conditions. There was a significant effect of drug condition on the increase in spikes between lap 1 and lap 20 (F[3,9] = 25.46, p < 0.01; repeated-measures ANOVA) with both the 5 mg/kg dose and the 10 mg/kg doses resulting in a significant increase in spikes compared to the saline control (p < 0.05 for both comparisons; simple contrast).
Figure 7.
(A) The mean and SEM for number of spikes across laps 1 through 20. (B) The difference in the mean number of spikes within place fields between lap 1 and lap 20 for the saline control (black), 5 mg/kg (red), 10 mg/kg (yellow), and 15 mg/kg (blue) doses of memantine on lap 1 and lap 20. For all four conditions there was a significant increase in spike number but this increase was largest for the 5 mg/kg and 10 mg/kg doses of memantine. Error bars indicate ± one SEM.
A linear regression indicated that there was a significant increase in spikes over the first 20 laps, for the 5 mg/kg dose of memantine (slope = 0.64 ± 0.12 spikes/lap, F[1, 98] = 27.98, p < 0.001), the 10 mg/kg dose (slope = 0.46 ± 0.12 spikes/lap, F[1, 98] = 13.85, p < 0.001), and the 15 mg/kg dose of memantine (slope = 0.40 ± 0.11 spikes/lap, F[1, 98] = 14.63, p < 0.001). The r coefficient was not significant for the control condition, indicating that the spike number did not continue to increase linearly over the first 20 laps when memantine was not administered. In fact, Figure 7A shows that the largest increase in spike number for the control condition was between the first and the second lap. In contrast, the effect of memantine on spike number in the aged rats is similar to what has been observed in young rats with normal place field expansion plasticity (Ekstrom et al., 2001).
In addition to spike number, the measures of place field size and center of mass shift were used to assess the effects of memantine on experience-dependent place field expansion plasticity. Place field size was measured as described in Experiment 1 (see Fig 1B). Figure 8A shows the mean size of the place fields for each drug condition across laps 1 through 20. When place field size was analyzed across all laps there was a significant effect of drug condition (F[3, 376] = 14.40, p < 0.001; repeated-measures ANOVA), with all doses of memantine resulting in significantly larger place fields when compared to the saline control condition (p < 0.001 for all comparisons; simple contrasts). Figure 8B shows the change in the mean size of place fields between lap 1 and lap 20 for all drug conditions. There was a significant effect of drug condition on the increase in place field size between laps 1 and 20 (F[3,9] = 7.78, p < 0.05; repeated-measures ANOVA) with the 5 mg/kg and the 10 mg/kg doses of memantine resulting in larger place fields compared to the saline control condition (p < 0.05 for all comparisons, simple contrast).
Figure 8.
(A) The effects of memantine on place field size across laps 1 through 20 for the saline control (black), 5 mg/kg (red), 10 mg/kg (yellow), and 15 mg/kg (blue) doses of memantine. All four conditions showed an increase in place field size, but the increase was larger for all memantine conditions. (B) The average increase in the size of place fields between lap 1 and lap 20 for the saline control (blue), 5 mg/kg (red), 10 mg/kg (green), and 15 mg/kg (yellow) doses of memantine. Place fields were significantly larger when the rats had been given any dose of memantine compared to the saline control. Error bars indicate ± one standard error of the mean (SEM).
In order to estimate over how many laps the size of place fields continued to expand, the relationship between lap number and place field size was fit with a third-order polynomial and the lap at which the first derivative was equal to zero was calculated for each dose of memantine. This analysis revealed that for the 5 mg/kg dose of memantine, dynamic place field expansion occurred until lap 10. For the 10 mg/kg and 15 mg/kg doses, the increase in place field size stopped at lap 9. This is similar to what has been observed in young rats, where place fields dynamically expand with experience over approximately 10 laps (estimate from Mehta et al., 1997).
Figure 9A shows the normalized center of mass shift for each lap through lap 20. Across laps 1 through 20 there was a significant effect of drug condition of mean center of mass shift (F[3,237] = 58.69, p < 0.001; repeated-measures ANOVA), with all of the doses of memantine leading to a significant increase in the center of mass shift (p < 0.05 for all comparisons; simple contrast). Figure 10B shows the mean center of mass shift for all drug conditions.
Figure 9.
(A) Shows the normalized center of mass (COM) shift of place fields from laps 1 though 20 for the saline control (black), 5 mg/kg (red), 10 mg/kg (yellow), and 15 mg/kg (blue) doses of memantine. (B) The average normalized COM shift over all laps for the three doses of memantine and the saline control. There was a significant increase in the COM shift compared to saline control for the 5 mg/kg, 10 mg/kg and 15 mg/kg doses of memantine. Error bars indicate ± one SEM.
Figure 10.
The mean and SEM for the rate of theta phase precession for the saline control (blue), 5 mg/kg (red), 10 mg/kg (green), and 15 mg/kg (yellow) doses of memantine. The rate was significantly less for all doses of memantine compared to the saline control. This is consistent with the observation that memantine reinstated experience-dependent place field expansion plasticity.
Due to the observation that memantine appeared to reinstate place field expansion in aged rats, a final analysis was conducted to corroborate these findings. Multiple studies have reported that place field expansion plasticity is accompanied by a decrease in the rate of theta phase precession (Ekstrom et al., 2001; Shen et al., 1997). This observation occurs because the in-field spikes precess a total of 360° degrees with respect to the theta rhythm regardless of the size of the place field. Therefore, as the place field gets larger the number of degrees that the spikes need to precess per centimeter that rat moves decreases. In fact, aged rats show a faster rate of theta phase precession compared to young animals with normal experience-dependent place field expansion plasticity (Shen et al., 1997). Therefore, as a final measure of memantine's potential to reinstate place field expansion, we also computed the rate of theta phase precession for the different doses of memantine compared to the saline control. The rate of phase precession was estimated as previously described (O'Keefe & Recce, 1993). Specifically, for each place field a linear regression was applied to the phase by position plot and the rate was defined as the slope of the line of best fit. There was a significant effect of dose of memantine on the rate of theta phase precession (F[3,9] = 4.28, p < 0.05; repeated-measures ANOVA) with all doses of memantine leading to a decrease in the rate (p < 0.05 for all comparisons; simple contrasts). Figure 10 shows the mean rate of theta phase precession for all drug conditions.
Discussion
Aged rats have functional alterations in the dynamics of CA1 place fields such that when a rat repeatedly takes the same path, the increase in place field size does not occur to the same degree as that observed in young rats (Shen et al., 1997). The main finding that emerges from the current study is that modulation of NMDAR activity in aged rats with memantine leads to an increase in the size of CA1 place fields. This conclusion was derived from three different parameters of dynamic place field expansion that are normally absent in old rats: an increase in spike number, increase in place field size, and a center of mass shift. Additionally, treatment with memantine lead to a decrease in the rate of theta phase precession, which occurs under conditions in which place fields are larger (Ekstrom et al., 2001; Maurer et al., 2006; Shen et al., 1997). In contrast, intraperitoneal injection of 35 mg/kg of the ampakine CX516 failed to alter place field expansion plasticity in old rats.
A number of interesting issues are raised by the observation that modulation of AMPAR function via ampakine administration does not affect naturally occurring NMDAR-dependent hippocampal plasticity. Compared to young rats, aged, learning-impaired rats show reduced evoked field EPSP amplitudes recorded at the Schaffer collateral - CA1 pyramidal cell synapse (Barnes, Rao, Foster, & McNaughton, 1992; Deupree, Bradley, & Turner, 1993; Landfield, Pitler, & Applegate, 1986), and reduced post-synaptic density size of Schaffer collateral perforated synapses (Nicholson, Yoshida, Berry, Gallagher, & Geinisman, 2004), which contain more AMPA receptors compared with non-perforated ones (Baude, Nusser, Molnar, McIlhinney, & Somogyi, 1995; Desmond & Weinberg, 1998; Ganeshina, Berry, Petralia, Nicholson, & Geinisman, 2004). Although it is known that AMPA receptors play an important role in stimulation-induced LTP (e.g. Malenka, 2003; Malenka & Nicoll, 1999), it is possible that CX516 treatment does not optimize conditions for the membrane remodeling processes required for behavior-induced plasticity. This would include selective trafficking of specific AMPAR subtypes (e.g. Chowdhury et al., 2006; Ju et al., 2004), appropriate modulation of AMPA receptors for endosomal recycling (e.g. Park, Penick, Edwards, Kauer, & Ehlers, 2004), or other processes critical for synapse modification. Thus, while it is still possible that CX516 may increase synaptic efficacy, it may not target the mechanisms necessary to restore plasticity in aged rats. On the other hand, it cannot be ruled out that a different dose of CX516 than the one used, could have engaged processes that resulted in improved place field expansion plasticity.
Efficacy of the doses of memantine and CX516
In the current study, all three doses of memantine lead to some increase in place field size compared to the saline control condition. This is not surprising considering that injections of 5 mg/kg and 7.5 mg/kg of memantine have been shown to improve spatial memory (Zoladz et al., 2006). Moreover, injections of 10 mg/kg and 20 mg/kg of memantine improve recognition memory in adult rats (Pitsikas & Sakellaridis, 2007), and 20 mg/kg of memantine has been shown to facilitate spontaneous object recognition in aged rats (Pieta Dias et al., 2007). These data suggest that memantine may be effective at improving behaviorally-induced plasticity at doses ranging from 5 mg/kg to 20 mg/kg.
In contrast, at a dose of 35 mg/kg, CX516 had no affect on place field expansion plasticity. Although CX516 did not alter place field dynamics, it is likely that the drug did affect AMPAR-mediated synaptic transmission in the aged animals at this dose. There are several reasons for this assertion. The range of the behaviorally-effective dose of CX516 is 12-50 mg/kg (personal communication, Dr. Ursula Staubli, Cortex Pharmaceuticals), and a mid-level dose (35 mg/kg) was used in the present study. In addition, 30 mg/kg CX516 was shown to affect spatial working memory performance in 19 month-old Long-Evens rats (Davis et al., 1997). Furthermore, AMPAR binding sites in the CA1 region do not decline with age (Wenk & Barnes, 2000), nor do the numbers of synaptic contacts decline in the stratum radiatum (Geinisman et al., 2004). Although there is a reduction (approximately 50%) in AMPA binding in the dentate gyrus, this occurs between the age of 3 and 12 months, and is stable thereafter (Wenk & Barnes, 2000). Thus, it is unlikely that a lack of AMPAR target sites can explain these results. More importantly, intraperitoneal injection of 35 mg/kg CX516 has been shown to alter the performance of old rats of the same age and strain in the radial arm maze task, suggesting that the drug is active centrally at this dose (Yang, Houston, & Barnes, 2003).
Possible mechanism for memantine's reinstatement of experience-dependent place field expansion plasticity in aged rats
Given that the NMDA antagonist CPP blocks place field expansion, it seems somewhat paradoxical that a different NMDAR antagonist could facilitate plasticity. In contrast to other NMDA receptor antagonists, however, memantine has fast offset kinetics and relatively strong functional voltage-dependency due to its positive charge. This allows memantine to rapidly leave the NMDA channel upon transient physiological activation by glutamate (for review, see Parsons, Stoffler, & Danysz, 2007). Thus, memantine may act to increase signal-to-noise ratios under conditions in which normal synaptic transmission has been disrupted. In old animals this may be particularly important as it has been observed that aged animals show disrupted Ca2+ regulation in CA1 of the hippocampus that has been associated with age-related impairments in plasticity (e.g. Foster & Norris, 1997; Landfield, 1988). Moreover, this Ca2+ dysregulation and the related plasticity deficits in old animals could be responsible for the decline in behaviorally-induced place field expansion plasticity.
In young animals, rapid behaviorally-induced potentiation of feedforward synapses from CA3 to CA1 has been hypothesized to mediate the experience-dependent expansion of CA1 place fields (Mehta et al., 2000). During the first pass through a place field, a dorsal CA1 neuron may inherit its place-specific firing from neurons in layer III of the medial entorhinal cortex (Brun et al., 2002), and input to the CA1 neuron from CA3 would be relatively symmetric. After repeated traversals, in which both directionally selective CA3 and CA1 neurons are activated, a spike-timing dependent plasticity mechanism might be expected to strengthen synapses from those CA3 neurons that had a place field just before that of a CA1 neuron (Lee et al., 2004; Mehta et al., 2000). The theoretically predicted and empirically verified result is a backward, asymmetric shift of CA1 place fields, resulting in larger place fields, a center of mass shift, and an increase in spike number.
If behaviorally-induced plasticity of the CA3 to CA1 Schaffer collateral synapses is the primary mechanism for place field expansion, then it may not be surprising that aged rats fail to show this phenomenon under normal conditions, since a number of aged-associated changes occur at this synapse. For example, aged CA1 pyramidal cells have increased Ca2+ conductances due to a higher density of L-type Ca2+ channels (Thibault & Landfield, 1996). As stated previously, this may lead to disruptions in Ca2+ homeostasis (for review, see Toescu, Verkhratsky, & Landfield, 2004) that ultimately contribute to age-related plasticity deficits (Foster & Norris, 1997; Landfield, 1988). Additionally, at the CA3 to CA1 Schaffer collateral synapse aged rats show impairments in both the induction and maintenance of experimentally induced LTP (for review, see Burke & Barnes, 2006; Rosenzweig & Barnes, 2003). Additionally, the Schaffer collateral synapses of aged rats are more susceptible to LTD (Norris, Korol, & Foster, 1996) and to the reversal of LTP (Foster & Norris, 1997). Thus, an overall shift in the synaptic modification window of CA3 to CA1 Schaffer collateral synapses in aged rats could account for the decline in experience-dependent place field expansion plasticity. This decline may then be reinstated via a modification of the Ca2+ influx through the NMDAR by memantine.
The increase in experience-dependent place field expansion plasticity by memantine administration may also be related to changes within CA3 neurons. In addition to the observed age-related effects on the Schaffer collateral synapse, a number of age-associated changes occur within the CA3 network (for review, see Wilson, Gallagher, Eichenbaum, & Tanila, 2006), including an increase in place field firing rate and rigidity (Wilson, S, Gallagher, Eichenbaum, & Tanila, 2005). While the place fields of young CA3 pyramidal neurons do exhibit expansion (Lee et al., 2004), this phenomenon has not been investigated in aged animals. Thus, it is possible that memantine affects the firing patterns of aged CA3 neurons, and this may lead to restoration of plasticity. Alternatively, memantine may affect both cell types, resulting in restored behaviorally-induced plasticity.
An additional mechanism for the observed improvement in place field expansion plasticity involves the affinity of memantine for the α7* nicotinic acetylcholine receptor (nAChR). The α7* nAChR is present on hippocampal neurons and is also a Ca2+ conducting receptor. In cultured hippocampal neurons, memantine has been shown to reduce Ca2+ conductance through the α7* nAChR (Aracava, Pereira, Maelicke, & Albuquerque, 2005). Thus, memantine could also act to restore normal plasticity mechanisms in aged neurons by reducing the amount of Ca2+ that enters the neurons through both the NMDA and the α7* nACh receptors.
Memantine also led to an increase in running speed but only at the highest dose (15mg/kg), and both the medium and high doses lead to an increase in firing rate. It is unlikely that memantine's reinstatement of behaviorally-induced place field expansion is due to its effect on running speed or firing rate. First, measures of experience-dependent place field expansion were used that are robust against changes in running speed, such as the number of spikes within a place field and the center of mass shift. Moreover, there was evidence of place field expansion reinstatement at both the low and medium doses, and these doses had no effect on the animal's running speed. Additionally, the low dose of memantine also lead to a reinstatement of place expansion but did not affect overall firing rate. Finally, the effects of the medium and high doses of memantine on firing rate only occurred at running speeds faster than 35 cm/s (see Fig 6B) and the aged rats did not often run at speeds this fast.
Dynamic place field expansion: comparison of reinstatement in old rats to young animals
In young rats repeated route following behavior leads to a 20-40% increase in place size between the first pass through a place field and the twentieth pass (estimated from Ekstrom et al., 2001; Shen et al., 1997). In the dorsal CA1 subregion of the hippocampus this translates to approximately a 6-7 cm increase in place field size (estimated from Ekstrom et al., 2001; Shen et al., 1997). In the current study, between lap one and lap twenty, the low, medium and high doses of memantine resulted in a 6.6 cm (26%), 6.2 cm (24%) and 5.7 cm (22%) increase in place field size, respectively. Thus, the magnitude of experience-dependent place field expansion plasticity is at least comparable between young rats and aged rats that have been administered the noncompetitive NMDA receptor antagonist memantine.
The current findings suggest a means to therapeutically treat normal age-associated neurobiological changes in plasticity mechanisms. By adjusting calcium influx via the NMDA receptor, memantine may alter the probability that synaptic weights of aged rats are adjusted over a dynamic range more similar to that of young animals.
Acknowledgments
We would like to thank Stephen Cowen and Peter Lipa for assistance with the analysis, Bruce L. McNaughton for helpful discussions, Gary L. Wenk for lending his pharmacology expertise, and Jie Wang, Jeri Meltzer, Frank Houston and Kim Bohne for their technical support. Additionally, we extend our deep thanks to Michelle Carroll and Luann Snyder for their administrative assistance.
Supported by: McKnight Brain Research Foundation, AG012609, AG010546, and the University of Arizona Undergraduate Biology Research Program.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Abbott LF, Blum KI. Functional significance of long-term potentiation for sequence learning and prediction. Cereb Cortex. 1996;6(3):406–16. doi: 10.1093/cercor/6.3.406. [DOI] [PubMed] [Google Scholar]
- Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312(3):1195–205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- Arai A, Kessler M, Ambros-Ingerson J, Quan A, Yigiter E, Rogers G, Lynch G. Effects of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience. 1996a;75(2):573–85. doi: 10.1016/0306-4522(96)00263-1. [DOI] [PubMed] [Google Scholar]
- Arai A, Kessler M, Rogers G, Lynch G. Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther. 1996b;278(2):627–38. [PubMed] [Google Scholar]
- Arai AC, Xia YF, Rogers G, Lynch G, Kessler M. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J Pharmacol Exp Ther. 2002;303(3):1075–85. doi: 10.1124/jpet.102.040360. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96(9):5280–5. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci. 1996;8(3):565–71. doi: 10.1111/j.1460-9568.1996.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, O'Keefe J. Loss of place specificity in hippocampal complex spike cells of senescent rat. Neurobiol Aging. 1983;4(2):113–9. doi: 10.1016/0197-4580(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Foster TC, McNaughton BL. Region-specific age effects on AMPA sensitivity: electrophysiological evidence for loss of synaptic contacts in hippocampal field CA1. Hippocampus. 1992;2(4):457–68. doi: 10.1002/hipo.450020413. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388(6639):272–5. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnar E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69(4):1031–55. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Blum KI, Abbott LF. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 1996;8(1):85–93. doi: 10.1162/neco.1996.8.1.85. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296(5576):2243–6. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Bubser M, Keseberg U, Notz PK, Schmidt WJ. Differential behavioural and neurochemical effects of competitive and non-competitive NMDA receptor antagonists in rats. Eur J Pharmacol. 1992;229(1):75–82. doi: 10.1016/0014-2999(92)90288-f. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52(3):445–59. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czurko A, Hirase H, Csicsvari J, Buzsaki G. Sustained activation of hippocampal pyramidal cells by ‘space clamping’ in a running wheel. Eur J Neurosci. 1999;11(1):344–52. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- Davis CM, Moskovitz B, Nguyen MA, Tran BB, Arai A, Lynch G, Granger R. A profile of the behavioral changes produced by facilitation of AMPA-type glutamate receptors. Psychopharmacology (Berl) 1997;133:161–7. doi: 10.1007/s002130050386. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Weinberg RJ. Enhanced expression of AMPA receptor protein at perforated axospinous synapses. Neuroreport. 1998;9(5):857–60. doi: 10.1097/00001756-199803300-00017. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14(3):249–58. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields”. Neuron. 2001;31(4):631–8. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7(6):602–12. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–70. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol. 2004;468(1):86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–16. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16(2):823–35. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger R, Staubli U, Davis M, Perez Y, Nilsson L, Rogers GA, Lynch G. A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse. 1993;15(4):326–9. doi: 10.1002/syn.890150409. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci. 1998;18(7):2740–7. doi: 10.1523/JNEUROSCI.18-07-02740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. The Organization of Behavior: A Neurophysiological Theory 1949 [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7(3):244–53. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–82. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16(6):947–54. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiol Aging. 1988;9(56):571–9. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J Neurophysiol. 1986;56(3):797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- Larson J, Lieu T, Petchpradub V, LeDuc B, Ngo H, Rogers GA, Lynch G. Facilitation of olfactory learning by a modulator of AMPA receptors. J Neurosci. 1995;15(12):8023–30. doi: 10.1523/JNEUROSCI.15-12-08023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004;42(5):803–15. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Lynch G, Granger R, Ambros-Ingerson J, Davis CM, Kessler M, Schehr R. Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol. 1997;145(1):89–92. doi: 10.1006/exnr.1997.6447. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10(1):31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus. 1994;4(4):410–21. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus. 2006;16(9):785–94. doi: 10.1002/hipo.20202. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Vanrhoads SR, Sutherland GR, Lipa P, McNaughton BL. Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus. 2005;15(7):841–52. doi: 10.1002/hipo.20114. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983a;52(1):41–9. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983b;8(4):391–7. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci U S A. 1997;94(16):8918–21. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Quirk MC, Wilson MA. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25(3):707–15. doi: 10.1016/s0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2004;311(2):677–82. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Lavoie AM, Kalyani A. Redistribution of spatial representation in the hippocampus of aged rats performing a spatial memory task. Behav Neurosci. 1996;110(5):1006–16. doi: 10.1037//0735-7044.110.5.1006. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–68. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M, Kasznaik A. Spatial memory and aging: performance on a human analog of the Morris water maze. Aging, Neuropsychology and Cognition. 2000;7:86–93. [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24(35):7648–53. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16(17):5382–92. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3(3):317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits in the ability to encode contextual change: a place cell analysis. Hippocampus. 2000;10(3):338–50. doi: 10.1002/1098-1063(2000)10:3<338::AID-HIPO14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305(5692):1972–5. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34(10):1239–58. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system - too little activation is bad, too much is even worse. Neuropharmacology. 2007;53(6):699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, McDonald S. Memantine Treatment in Mild to Moderate Alzheimer Disease: A 24-Week Randomized, Controlled Trial. Am J Geriatr Psychiatry. 2006 doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- Pieta Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, Siciliani Scalco F, Rewsaat Guimaraes M, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146(4):1719–25. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Sakellaridis N. Memantine and recognition memory: Possible facilitation of its behavioral effects by the nitric oxide (NO) donor molsidomine. Eur J Pharmacol. 2007;571(23):174–9. doi: 10.1016/j.ejphar.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41(2):461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8(8):1923–8. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Recce ML, O'Keefe J. The tetrode: an improved technique for multiunit extracellular recording. Soc Neurosci Abstr. 1989;15:1250. [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003;9(3):275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–79. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA. Age-related decrease in cholinergic synaptic transmission in three hippocampal subfields. Neurobiol Aging. 1996;17(3):439–51. doi: 10.1016/0197-4580(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, Skaggs WE, Weaver KL. The effect of aging on experience-dependent plasticity of hippocampal place cells. J Neurosci. 1997;17(17):6769–82. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6(2):149–72. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31(3):271–6. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Tanila H, Shapiro M, Gallagher M, Eichenbaum H. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci. 1997;17(13):5155–66. doi: 10.1523/JNEUROSCI.17-13-05155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272(5264):1017–20. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27(10):614–20. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–18. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Research. 2000:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- Wilkniss SM, Jones MG, Korol DL, Gold PE, Manning CA. Age-related differences in an ecologically based study of route learning. Psychol Aging. 1997;12(2):372–5. doi: 10.1037//0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- Wilson I, I S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterarions in place cells are subregion specific. Journal of Neuroscience. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29(12):662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261(5124):1055–8. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yang z, Houston FP, Barnes CA. Effects of ampakines on spatial learning and memory in aged rats. Paper presented at the Society for Neuroscience, New Orleans.2003. [Google Scholar]
- Zajaczkowski W, Quack G, Danysz W. Infusion of (+) -MK-801 and memantine -- contrasting effects on radial maze learning in rats with entorhinal cortex lesion. Eur J Pharmacol. 1996;296(3):239–46. doi: 10.1016/0014-2999(95)00716-4. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav. 2006 doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]