Abstract
Objective
Brain arteriovenous malformations (AVMs) are an important cause of neurological morbidity in young adults. The pathophysiology of these lesions is poorly understood. A soluble form of endoglin (sEng) has been shown to cause endothelial dysfunction and induce preeclampsia. We tested if sEng would be elevated in brain AVM tissues relative to epilepsy brain tissues, and also investigated whether sEng overexpression via gene transfer in the mouse brain would induce vascular dysplasia and associated changes in downstream signaling pathways.
Methods
Expression levels of sEng in surgical specimens were determined by Western blot assay and ELISA. Vascular dysplasia, levels of MMP and oxidative stress were determined by immunohistochemistry and gelatin zymography.
Results
Brain AVMs (n=33) had higher mean sEng levels (245 ± 175 vs 100 ± 60, % of control, P=0.04) compared with controls (n=8), as determined by Western blot. In contrast, membrane-bound Eng was not significantly different (108 ± 79 vs 100 ± 63, % of control, P=0.95). sEng gene transduction in the mouse brain induced abnormal vascular structures. It also increased matrix metalloproteinase (MMP) activity by 490 ± 30% (MMP-9), 220 ± 30% (MMP-2), and oxidants by 260 ± 20% (4-hydroxy-2-nonenal) at 2 weeks after injection, suggesting that MMPs and oxidative radicals may mediate sEng-induced pathological vascular remodeling.
Interpretation
The results suggest that elevated sEng may play a role in the generation of sporadic brain AVMs. Our findings may provide new targets for therapeutic intervention for patients with brain AVMs.
Introduction
Brain arteriovenous malformations (AVMs) are complexes of tortuous, tangled vessels representing fistulous connections between arteries and veins lacking a capillary bed.1, 2 The pathophysiology underlying brain AVMs is not clearly understood. Most AVMs are sporadic, with probably less than 5% attributable to hereditary hemorrhagic telangiectasia (HHT) with mutations in the endoglin (ENG) gene.3
Endoglin (CD105) is a 180kDa homodimeric transmembrane glycoprotein mainly expressed by endothelial cells.4 In the human brain, endoglin is not only expressed in the endothelium of all vessels but also in the adventitia of arteries and arterioles.5 Endoglin modulates TGF-β superfamily signaling by serving as a co-receptor.6 TGF-β is required for proper vessel development, and defective signaling has been implicated in the pathogenesis of several vascular diseases. Mutations in the ENG gene are the underlying cause of HHT type 1, an autosomal dominant disease characterized by focal loss of capillaries and development of AVMs in multiple organs, including the brain.7 Furthermore, Eng null mice are embryonic lethal, but Eng-heterozygous mice have been shown to develop abnormal brain vascular structures reminiscent of AVMs.8 Recently, a circulating soluble form of endoglin (sEng) was observed at elevated levels in the sera of women with preeclampsia, where it acts as a decoy, sequestering TGF-β from its endothelial cell surface receptors and inhibiting signaling.9 Eng was also shown to be required for proper eNOS activation by facilitating eNOS association with Hsp90.10 Eng-heterozygous mice displayed impaired eNOS activity accompanied by elevated oxidants and vasomotor abnormalities.
We hypothesized that sEng is elevated in sporadic brain AVMs, where it contributes to disease progression. Increasing levels of a decoy receptor such as sEng would lead to inhibition of TGF-β signaling and create a situation analogous to that observed in HHT1 patients, where haploinsufficiency prevails and leads to reduced levels of functional membrane endoglin and impaired TGF-β signaling. We measured sEng protein levels in frozen brain AVM tissues and investigated whether sEng overexpression via gene transfer would lead to enlarged and dysmorphic vessels in the mouse brain and associated impaired signaling pathways.
Methods
All studies involving patients were approved by the Institutional Review Board (IRB) of the University of California, San Francisco (UCSF), and patients gave informed consent. Procedures for the use of laboratory animals were approved by the University’s Institutional Animal Care and Use Committee.
Study Subjects
Patients with AVMs evaluated at UCSF were entered into an ongoing prospective registry.11 We analyzed a subset of this group who underwent AVM surgery and had frozen tissue specimens or plasma samples available for analysis. Clinical parameters for the study cohort included in the Western blot analysis (n=33) are listed in Table 1. None of the patients had a history of HHT. A subset of these patients was included in the tissue ELISA assay (n=20). However, the majority of patients included in the plasma sEng assays (n=29) were different because tissue and plasma samples from the same patient were not available. Characteristics were similar between cohorts except for a higher percentage of patients presenting with hemorrhage (48% vs 39%) and embolization (73% vs 25%), and a lower percentage with brain AVM of size <3 cm (58% vs 75%) in the tissue vs. plasma cohort, respectively.
Table 1.
Demographic information for the study cohort
| AVM (%) | Control (%) | ||
|---|---|---|---|
| Sample size | N | 33 | 8 |
| Age (Year) | Mean ± SD | 39 ±17 | 28 ± 9 |
| Embolization | Yes | 24 (73%) | -- |
| No | 9 (27%) | -- | |
| Initial Presentation | Unruptured | 17 (52%) | -- |
| Ruptured | 16 (48%) | -- | |
| Sex | Male | 20 (61%) | 1 (13%) |
| Female | 13 (39%) | 7 (87%) | |
| Race/Ethnicity | White | 19 (58%) | 5 (63%) |
| Hispanic | 13 (39%) | 2 (25%) | |
| Asian | 1 (3%) | 1 (12%) | |
| AVM Size | <3cm | 19 (58%) | -- |
| 3–6cm | 14 (42%) | -- | |
| >6cm | 0 (0%) | -- | |
| AVM venous drainage | Superficial | 17 (52%) | -- |
| Deep | 5 (15%) | -- | |
| Both | 11 (33%) | -- |
Control cerebral cortex was obtained from patients undergoing surgical treatment of epilepsy as described.12, 13 Middle cerebral artery was harvested from autopsy subjects.
Western Blot Analysis
Frozen tissues were homogenized in standard RIPA buffer (Santa Cruz Biotechnology). Equal amounts of proteins were fractionated by gel electrophoresis, and were electroblotted onto a PVDF membrane. The membranes were then probed with mouse anti-human endoglin antibody, 1:250 (BD Biosciences, Cat # DNDG00) followed by horseradish-peroxidase conjugated sheep anti-mouse IgG. β-actin was used as a loading control. CD31 is a specific endothelial cell marker and its expression levels are used to measure endothelial cell mass.14 Reactive bands were quantified by scanning densitometry and analyzed using NIH Image 1.63 software. The optical densities (OD) of sEng or mEng (transmembrane) were normalized to that of β-actin and CD31.
ELISA
Levels of soluble endoglin in tissue and plasma were measured using specific ELISA kits (R&D systems). We used a tissue lysis buffer (Tris buffed saline, proteases inhibitors) without strong detergents to selectively exclude membrane-bound proteins from the extract.
Immunohistochemistry
Immunostaining was performed as described.15 Briefly, brain sections (10µm) were incubated with mouse anti-endoglin antibody, washed, incubated with biotinylated goat anti-mouse IgG, and treated with the ABC streptavidin detection system. The resulting horseradish peroxidase signal was detected using 3,3’-diaminobenzidene. Negative controls were performed by omitting the primary antibodies during the immunostaining.
Animal Treatment
Adult C57BL/6 male mice underwent adeno-associated viral-mediated VEGF (AAV-VEGF) gene transduction (2 µl, 2×109 genome copies) into the right parenchyma as described.16 One week after AAV-VEGF gene transfer, mice were injected with adenoviral sEng (Ad-sEng), 1×107 pfu, or Ad-lacZ (lacZ is a reporter gene that codes for beta galactosidase) into the same region. Ad-sEng was prepared as described.9 Mice were sacrificed and perfused with PBS at 1 and 2 weeks after injection. A series of 20 µm brain coronal sections were collected.
Capillary Morphology
Capillary density and dysplastic capillaries were analyzed using lectin/α-SMA-staining imaging as described.17 Briefly, brain sections were incubated with lectin (fluorescein labeled Griffonia Simplicifolia lectin I), and then incubated with anti-α-smooth muscle actin antibody and Alexa 594 anti-mouse IgG. α-SMA was used to identify mural cells to distinguish capillaries from venules or arterioles. We used two operational criteria to code a vessel as “dysplastic”: (1) a diameter >15 µm, while α-SMA negative; (2) abnormal morphology defined as irregular vascular wall or contour, and aneurysmal dilation. Quantitation of dysplastic capillaries has been described in our previous study.17
Measurement of MMPs and 4-hydroxy-2-nonenal (HNE) Levels
The mouse brain caudate putamen portion on the viral transduction side was collected and homogenized on ice. After centrifugation, the supernatant was collected and analyzed by gelatin zymography as described.16 Aliquots of the supernatants were used for HNE Western blotting. Rabbit anti-HNE (Alpha Dignostic International) was used at 1:1000 dilution.
Statistical Analysis
Data are presented as mean ± SD. For Western blot quantification of sEng, we determined that the distribution of values was moderately skewed and used the non-parametric Mann-Whitney test to compare AVMs to control tissues. Multivariable models were used to assess possible confounding factors such as hemorrhage and AVM size on sEng levels. Results from animal studies were analyzed using one-way analysis of variance (ANOVA), followed by Fisher’s protected least significant difference test. A probability value of less than 5% was considered statistically significant.
Results
Clinical Characteristics
Table 1 shows the demographic and clinical characteristics for AVM patients and epilepsy controls included in the Western blot analysis. The mean age (years) for AVM patients was 39 ± 17 and 28 ± 9 for control patients (P=0.08). Race/ethnicity was similar between brain AVM and control groups (P=0.40). A greater percentage of AVM patients were male (61%) compared to the control group (13%, P=0.02).
Higher sEng Levels in Human Brain AVMs
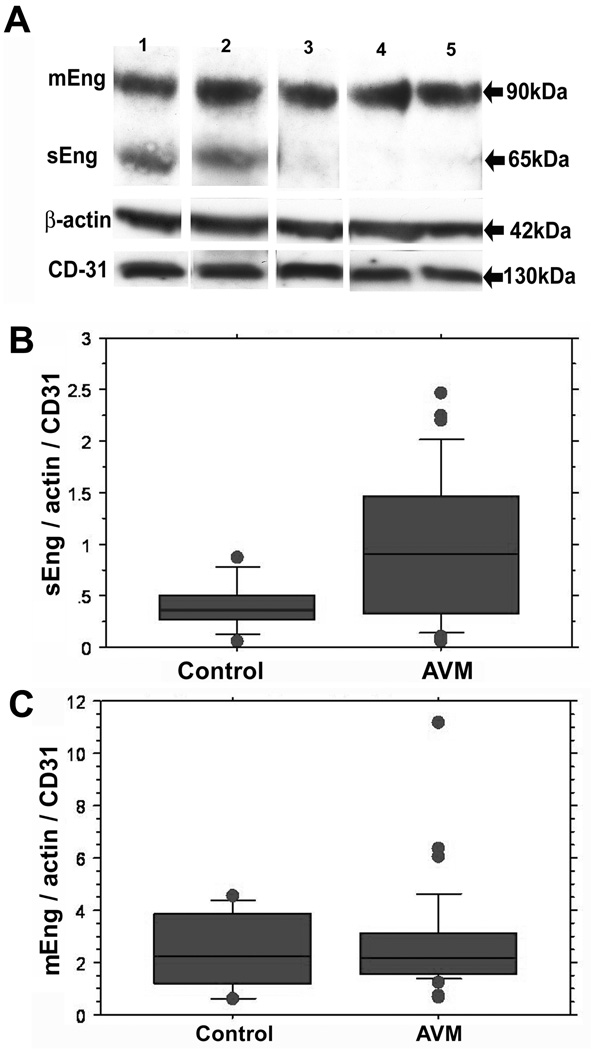
We first determined sEng levels in AVM and control tissues using Western blots. As shown in Fig 1A, the transmembrane Eng (mEng) was seen at 90kDa, corresponding to monomeric mEng, while the band at 65kDa was considered sEng, based on previous reports. Results from the Mann-Whitney test showed that brain AVMs (n=33), compared to controls (n=8), had higher sEng levels (245% ± 175 vs 100% ± 60, P=0.04, Fig 1B). Membrane Eng was not significantly different (108 ± 79 vs 100 ± 63, % of control, P=0.95, Fig 1C). After adjusting for age, race, and gender, sEng between brain AVMs and controls was still higher but no longer statistically significant (P=0.08). Of note, AVMs had higher vascular density as shown by higher CD31/actin ratio.
Figure 1. Western blot analysis of endoglin in brain AVM and control tissues.
A. Representative Western blot shows the membrane form of Endoglin (mEng) as a 90kDa monomer in all samples, while the 65kDa fragment corresponding to the soluble form (sEng) is seen only in AVMs. β-actin blot was used to control for protein loading and CD31 for measurement of endothelial cell mass. Lanes 1 and 2: AVMs; Lane 3: middle cerebral artery from autopsy; Lanes 4 and 5: epilepsy cortex samples. B. Quantification of sEng level. The sEng, actin and CD31 bands were quantified by densitometry and sEng levels normalized to those of β-actin and CD31. Brain AVMs (n=33) had higher sEng levels (0.98 ± 0.7, vs 0.4 ± 0.24, P< 0.05) compared with controls (n=8). We normalized sENG to CD31 expression to control for artifactual estimates of increased sENG caused by higher vascular density in AVM tissues. C. Quantification of mEng level. The bands corresponding to the plasma membrane form of Eng were quantified and expressed relative to β-actin and CD31. The values were not significantly different (2.6 ± 1.9, in brain AVMs vs 2.4 ± 1.5 in control samples, P=0.95).
We also measured sEng levels in AVM and control tissues using ELISA. We used a tissue lysis buffer that did not contain strong detergents in order not to solubilize membrane-bound proteins. The absence of integral membrane proteins such as mEng in the lysates was confirmed by Western blotting (Fig 2B). As shown in Fig 2A, AVM tissue (n=20) had higher average levels of sEng (4.0 ± 3.6 vs 0.9 ± 0.5 ng/mg, P=0.002) compared to control tissue (n=14). This difference remained significant (P=0.002) in multivariable analysis adjusting for age, gender and race. In addition, sEng analysis by ELISA and Western blotting was correlated (R2= 0.84, P<0.001, n=20).
Figure 2. Measurement of Eng protein levels.
A. ELISA assay showing that brain AVM tissue (n=20) had higher average sEng protein levels (4.0 ± 3.6, vs 0.9 ± 0.5, ng/mg, P< 0.05) compared to control tissue (n=14). B. Western blotting confirmed absence of membrane Eng (90kDa) and presence of sEng (65kDa) in tissue lysates used for ELISA. C Immmunohistochemical staining indicates that Eng was present in endothelial cells (arrows) and adventitia (arrowheads) in both brain AVMs (a, b) and controls (c, d), and no obvious differences in staining intensity were noted. Images b and d are higher magnification of the boxed areas from images a and c, respectively. Size bar= 50µm.
Immunohistochemical staining revealed that Eng was present in endothelial cells as well as adventitia in both brain AVM and control tissues (Fig 2C), and no obvious differences in staining intensities were noted. However, the antibody that was used recognized both sEng and mEng forms.
Plasma levels of sEng in patients with AVMs (2.69 ± 0.82 ng/ml; n= 29) were not different from those of controls (3.28 ± 0.9 ng/ml; n=7), and were within the normal range.
Analysis among AVM patients by multivariable models showed that gender, race, age, AVM size/location, and embolization did not significantly impact sEng levels. However, initial presentation with hemorrhage was significantly associated with sEng levels, as shown by Western blot (P=0.04), but not by ELISA (P=0.73).
sEng Induces Dysplastic Capillaries in the Mouse Brain
The success and degree of sEng gene transduction was confirmed by immunohistochemistry and Western blot. As shown in Fig 3A, numerous sEng positive cells were detected in sEng-transduced mouse brain. No sEng positive cells were detected in the lacZ-transduced brain, as expected from the minimal cross-reactivity of the antibody to human endoglin with mouse endoglin. Strong human sEng expression was detected 1 week after Ad-sEng injection and was reduced slightly at 2 weeks post-injection, whereas no reactivity was observed in the brain injected with Ad-LacZ at either time point (Fig 3B).
Figure 3. Expression of human sEng in the adult mouse brain after AdsEng transduction.
A Immunostaining of human sEng (brown) is shown for Ad-sEng-transduced mouse brain; Ad-lacZ-transduced mouse brain is used for control. Staining was performed 1 week after viral injection. Upper Bar = 1000 µm, lower bar=100µm. B Representative Western blots showing human sEng expression (65kDa) in the mouse brain after Ad-sEng transduction. Samples were collected at 1 and 2 weeks after adenoviral injection. Lanes 1 and 2: lacZ, 1 week; Lanes 3 and 4: lacZ, 2 weeks; Lanes 5 and 6: sEng, 1 week; Lanes 7 and 8: sEng, 2 weeks.
The number and morphology of capillaries were examined to determine if sEng overexpression stimulated capillary dysplasia in the adult mouse brain in the presence of VEGF hyperstimulation, as sEng alone would likely have no effect. Control mice with Ad-lacZ treatment were injected with the same amount of VEGF. There was no significant difference in the total number of capillaries between the groups with or without sEng treatment (data not shown). However, dysplastic vessels were detected at 2 weeks after Ad-sEng injection (Fig 4A). There were more dysplastic capillaries in the Ad-sEng-transduced mouse brains than those treated with Ad-lacZ (Fig 4B, 2.1± 0.5 vs. 0.2±0.2, P<0.01). These dysplastic capillaries usually developed adjacent to the needle track. Dysplastic vessels were not observed in the brains of mice injected with Ad-LacZ.
Figure 4. sEng induces dysplastic capillaries in the mouse brain.
A Photomicrographs show lectin-stained blood vessels in brains injected with Ad-lacZ (a), or Ad-sEng (b, c, and d) following AAV-VEGF transduction. Many dysplastic capillaries were detected in Ad-sEng-injected brains (arrow pointing to enlarged giant capillaries and arrowhead pointing to morphologically changed capillaries). Bar = 100 µm. B. Bar graph shows the dysplasia index in the treated groups of mice. Data are mean ± SD. *P<0.01, Ad-sEng-transduced mice vs. Ad-lacZ-transduced mice.
sEng Upregulates MMP-9 and MMP-2 Activities
As shown in Fig 5, MMP-9 activity greatly increased 2 weeks after sEng transduction compared to lacZ transduction (4.9±0.3 vs 1.0±0.9, p<0.05). MMP-2 activity also increased about 2-fold in the sEng-treated group compared to the lacZ control group (Fig 5C, 2.2±0.3 vs1.0±0.1, P<0.05).
Figure 5. Effects of sEng overexpression on MMP-9 and MMP-2 activity.
A. Representative photograph of a zymogram gel. St: MMP standards. Lanes 1 and 2: lacZ -treated mice, 2 weeks; Lanes 3 and 4: sEng -treated mice, 2 weeks. B. Bar graph demonstrates densitometric analysis of the band mean intensity of MMP-9 activity. C. Quantitation of MMP-2 activity. Data are mean ± SD, n= 6, * P<0.05, sEng vs. lacZ
sEng Stimulates Superoxide Production
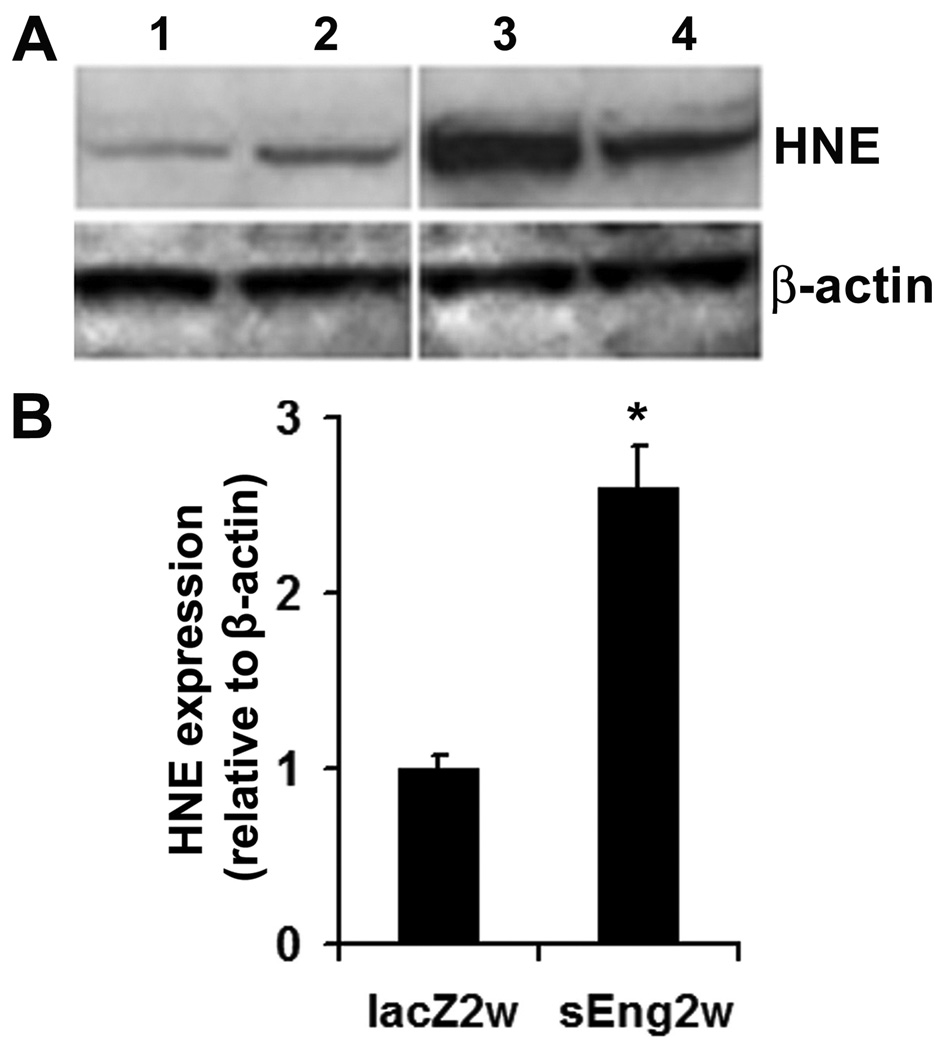
HNE, a major product of polyunsaturated fatty acid peroxidation, was used as an oxidative marker. There was no significant difference in the level of HNE protein adducts 1 week after sEng or lacZ injection (data not shown). However, a 2.6-fold increase in HNE level was noted 2 weeks after sEng injection compared to that of lacZ injection (Fig 6, P<0.05).
Figure 6. sEng overexpression induces production of HNE.
A. Photograph represents HNE protein adduct expression (≈70 kDa) by Western blot analysis. B. Bar graph shows densitometric analysis of the major HNE band mean intensity. Lanes 1 and 2: lacZ treated, after 2 weeks; Lanes 3 and 4: sEng -treated, after 2weeks. * P<0.05, sEng vs. lacZ
Discussion
In this study, we demonstrated that: (1) sEng protein levels were elevated in AVM tissues compared to controls; (2) sEng overexpression promoted dysplastic capillary formation by VEGF in the normal mouse brain; and (3) sEng increased the activity of MMPs and oxidant levels.
Increased sEng in AVM tissues that we observed suggests that sEng-mediated changes in TGF-β signaling may play a role in AVM pathophysiology. mEng has been shown to be present in all vessels and in newborns and adults.5 Although sEng is present in small amounts under normal conditions,9, 18, 19 we experimentally increased sEng expression and this caused aberrant vascular remodeling and formation. Our data are consistent with a previous report that levels of mEng in AVM tissues are not different compared to normal tissues.5 Interestingly, mEng levels in AVM patients with HHT1 are reduced but to the same extent as in the non-affected vessels in these patients, due to haploinsufficiency associated with the disease.20 In our study, a few AVM patients showed little or very low levels of sEng, suggesting that elevated sEng levels alone are not sufficient to explain the brain AVM phenotype. Alternatively, it is also conceivable that a period of increased sEng expression occurs earlier in the course of the disease, and is not observed by the time the patient comes for surgical resection. Furthermore, it seems likely that sEng acts in concert with other angiogenic factors such as VEGF to promote AVM formation and progression.
Our data showing increased local sEng in AVMs are interesting in view of the recent report of elevated serum sEng derived from the placenta of patients with preeclampsia.9 In that study, a systemic effect was observed whereby circulating sEng acted as a decoy to inhibit the effects of TGF-β on the endothelium, leading to systemic hypertension. Soluble VEGF receptor 1 levels were also increased in patients with preeclampsia, and acted in concert with sEng to induce severe preeclampsia in rats.9 In our study, we observed an increase in brain sEng but not in systemic sEng, and the overexpression of sEng in the mouse brain treated with VEGF led to local vascular dysplasia. These findings suggest cross-talk between VEGF and TGF-β pathways. They are implicated in NO production and vasomotor regulation and may well be both deregulated in various vascular pathologies.
Cerebral sEng levels are associated with initial presentation of intracranial hemorrhage in AVM patients, suggesting that rupture of the endothelium may contribute to increased release of sEng. It is not clear how sEng is formed. A related Type III TGF-β receptor, betaglycan, appears to be shed by a process that is mediated by membrane-type matrix metalloprotease-1 (MT1-MMP).21 We have found that several different MMPs are increased in AVM nidal tissue and it would be of interest to assess MT1-MMP.22–24 Shedding from the plasma membrane is therefore a possible mechanism of sEng production. However, alternative splicing remains a possible mechanism for the generation of sEng. Multiple missense Eng mutations are present in HHT1 patients, but they are associated with non-functional proteins and do not give rise to stable secreted proteins.25 A more general mechanism of sEng production is likely amplified in different vascular pathologies such as preeclampsia, brain AVMs and tumor-associated angiogenesis. Brain AVM tissue seems to be characterized by a proinflammatory state.3, 23, 24, 26–28 We have shown that a promoter polymorphism in the TNF-α gene is associated with increased risk of new intracranial hemorrhage in the natural course of brain AVMs.29 Interestingly, TNF-α can induce release of sEng from normal placental villous explants.30
How sEng might contribute to AVM pathogenesis needs further study. Endoglin may modulate eNOS activation and thereby contribute to the local regulation of vascular tone and integrity.10 Eng-heterozygous mice show impaired arterial myogenic responses, and Eng-deficient endothelial cells produce less NO but generate more eNOS-derived superoxide (O2−). These studies suggest that oxidative stress contributes to vascular dysfunction. Consistent with this notion, we have found that sEng overexpression via gene transfer enhances the mouse brain levels of HNE adducts that are associated with oxidative stress. Our data suggest that free radicals may mediate sEng-induced aberrant vascular remodeling.
We used viral-mediated focal brain VEGF stimulation in combination with sEng overexpression. The rationale for this approach is derived from human observations. First, VEGF expression is a prominent aspect of sporadic AVM lesion phenotype.12 Second, Eng mutations lead to HHT1. These patients have a high incidence of AVMs, especially cerebral AVMs that are phenotypically similar to sporadic AVMs.31, 32 Our animal studies show that hyperstimulation with sEng and VEGF induces vascular dysplasia in the mouse brain, and increases MMP-9 and MMP-2 activities as well as oxidant levels. One caveat of our study is that we did not examine the ultrastructure of new vessels induced by sEng and VEGF treatment. Our animal study data have shown that both MMP-9 and MMP-2 are upregulated by combined sEng and VEGF treatment. MMP-2 has also been found to be upregulated in preeclampsia33 and diabetic vasculature.34 In brain AVMs, we could only observe increased levels of MMP-9.35 Failure to detect changes in MMP-2 levels in brain AVMs may be due to the small sample size of the population studied.
Our dysplasia phenotype is not to be taken as a direct model of the disease, and is only producing what may be a microvascular aberration that might be an early manifestation of the clinical phenotype. Even though the capillary dysplasia observed in our mouse model does not represent intranidal vessels of the human disease, it may also be mimicking the dysplastic capillaries that have been known for some time to inhabit the margins of the lesion.36 Interestingly, recent studies suggest that the dysmorphic dilated capillaries surrounding the clinical lesions are remarkably similar to those induced by sEng and VEGF in the current study.37, 38 The mouse models proposed here will provide us with an opportunity to discern causal relationships in the formation of an abnormal vascular phenotype, and eventually allow better characterization of the signaling pathways involved.
In our study, the choice of control tissue for the AVM nidus has inherent limitations. We have described previously the use of brain tissue taken from surgical epilepsy patients,12, 35 but others have proposed using superficial temporal arteries.39 Neither of these tissues is ideal, as neither contains intracranial blood vessels of the caliber found in the brain AVM nidus. An additional reason for using tissues from surgical epilepsy patients is that it serves as quality control assay to rule out the possible effects of acute surgical trauma on neural tissue. We have used autopsy-derived middle cerebral artery and observed that sEng was minimally expressed.
Together, our data provide the first evidence that elevated sEng may contribute to sporadic brain AVM pathophysiology. Mechanistically, sEng likely inhibits the normal regulation by TGF-β of endothelium function, and MMPs and oxidants may be downstream mediators of sEng-stimulated capillary dysplasia. Whether TGF-β expression and signaling are altered in brain AVMs warrants future investigation. The study provides important new insights into the generation/progression of brain AVMs.
Acknowledgments
This study was supported in part by PHS grants: R01 NS27713 (WLY), R01 NS34949 (WLY), P01 NS44155 (WLY, HS, GYY), R21 NS053943 (YC), and K23 NS058357 (HK). For their collaboration and support, we thank the members of the UCSF BAVM Study Project (http://avm.ucsf.edu), including Nancy J. Quinnine, RN; Nerissa U, Ko, MD, Christopher F. Dowd, MD, Van V. Halbach, MD; Randall T. Higashida, MD; Brad Dispensa, BS; and Philippe Jolivalt for their efforts with maintaining our AVM database. S. Ananth Karumanchi, MD, is an investigator of the Howard Hughes Medical Institute. The authors thank Voltaire Gungab for assistance with manuscript preparation.
References
- 1.Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812–1818. doi: 10.1056/NEJM199906103402307. [DOI] [PubMed] [Google Scholar]
- 2.Young WL. Intracranial arteriovenous malformations: pathophysiology and hemodynamics (Chapter 6) In: Jafar JJ, Awad IA, Rosenwasser RH, editors. Vascular Malformations of the Central Nervous System. New York: Lippincott Williams & Wilkins; 1999. pp. 95–126. [Google Scholar]
- 3.Kim H, Marchuk DA, Pawlikowska L, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. doi: 10.1007/978-3-211-09469-3_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol. 1988;141:1925–1933. [PubMed] [Google Scholar]
- 5.Matsubara S, Bourdeau A, terBrugge KG, et al. Analysis of endoglin expression in normal brain tissue and in cerebral arteriovenous malformations. Stroke. 2000;31:2653–2660. doi: 10.1161/01.str.31.11.2653. [DOI] [PubMed] [Google Scholar]
- 6.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 7.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 8.Satomi J, Mount RJ, Toporsian M, et al. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34:783–789. doi: 10.1161/01.STR.0000056170.47815.37. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 10.Toporsian M, Gros R, Kabir MG, et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–692. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 11.Halim AX, Singh V, Johnston SC, et al. Characteristics of brain arteriovenous malformations with coexisting aneurysms: a comparison of two referral centers. Stroke. 2002;33:675–679. doi: 10.1161/hs0302.104104. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Wu Y, Lawton MT, et al. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56:1058–1065. [PubMed] [Google Scholar]
- 13.Hashimoto T, Lawton MT, Wen G, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410–423. doi: 10.1227/01.neu.0000103421.35266.71. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto T, Emala CW, Joshi S, et al. Abnormal pattern of Tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery. 2000;47:910–918. doi: 10.1097/00006123-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Xu B, Arderiu G, et al. Retroviral delivery of homeobox d3 gene induces cerebral angiogenesis in mice. J Cereb Blood Flow Metab. 2004;24:1280–1287. doi: 10.1097/01.WCB.0000141770.09022.AB. [DOI] [PubMed] [Google Scholar]
- 16.Hao Q, Chen Y, Zhu Y, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 17.Hao Q, Su H, Marchuk DA, et al. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol. 2008;295:H2250–H2256. doi: 10.1152/ajpheart.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabro L, Fonsatti E, Bellomo G, et al. Differential levels of soluble endoglin (CD105) in myeloid malignancies. J Cell Physiol. 2003;194:171–175. doi: 10.1002/jcp.10200. [DOI] [PubMed] [Google Scholar]
- 19.Blann AD, Wang JM, Wilson PB, Kumar S. Serum levels of the TGF-beta receptor are increased in atherosclerosis. Atherosclerosis. 1996;120:221–226. doi: 10.1016/0021-9150(95)05713-7. [DOI] [PubMed] [Google Scholar]
- 20.Bourdeau A, Cymerman U, Paquet ME, et al. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol. 2000;156:911–923. doi: 10.1016/S0002-9440(10)64960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasco-Loyden G, Arribas J, Lopez-Casillas F. The shedding of betaglycan is regulated by pervanadate and mediated by membrane type matrix metalloprotease-1. J Biol Chem. 2004;279:7721–7733. doi: 10.1074/jbc.M306499200. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto T, Matsumoto M, Li JF, et al. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol. 2005;5:1. doi: 10.1186/1471-2377-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Pawlikowska L, Yao JS, et al. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Fan Y, Poon KY, et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- 25.Paquet ME, Pece-Barbara N, Vera S, et al. Analysis of several endoglin mutants reveals no endogenous mature or secreted protein capable of interfering with normal endoglin function. Hum Mol Genet. 2001;10:1347–1357. doi: 10.1093/hmg/10.13.1347. [DOI] [PubMed] [Google Scholar]
- 26.Pawlikowska L, Tran MN, Achrol AS, et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 27.Achrol AS, Pawlikowska L, McCulloch CE, et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Hysi PG, Pawlikowska L, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27:176–182. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achrol AS, Pawlikowska L, McCulloch CE, et al. TNFa-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations (Abstract) Stroke. 2006;37:638–639. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 30.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 31.Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum Mol Genet. 2003;12:R97–R112. doi: 10.1093/hmg/ddg103. [DOI] [PubMed] [Google Scholar]
- 32.Letteboer TG, Mager JJ, Snijder RJ, et al. Genotype-phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet. 2006;43:371–377. doi: 10.1136/jmg.2005.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narumiya H, Zhang Y, Fernandez-Patron C, et al. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20:185–194. doi: 10.1081/PRG-100106968. [DOI] [PubMed] [Google Scholar]
- 34.Chung AW, Hsiang YN, Matzke LA, et al. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and −9 in human type 2 diabetic arterial vasculature. Circ Res. 2006;99:140–148. doi: 10.1161/01.RES.0000232352.90786.fa. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Wen G, Lawton MT, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- 36.McCormick WF. Pathology of vascular malformations of the brain. In: Wilson CB, Stein BM, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984. pp. 44–63. [Google Scholar]
- 37.Attia W, Tada T, Hongo K, et al. Microvascular pathological features of immediate perinidal parenchyma in cerebral arteriovenous malformations: giant bed capillaries. J Neurosurg. 2003;98:823–827. doi: 10.3171/jns.2003.98.4.0823. [DOI] [PubMed] [Google Scholar]
- 38.Sato S, Kodama N, Sasaki T, et al. Perinidal dilated capillary networks in cerebral arteriovenous malformations. Neurosurgery. 2004;54:163–168. doi: 10.1227/01.neu.0000097518.57741.be. [DOI] [PubMed] [Google Scholar]
- 39.Shenkar R, Elliott JP, Diener K, et al. Differential gene expression in human cerebrovascular malformations. Neurosurgery. 2003;52:465–478. doi: 10.1227/01.NEU.0000044131.03495.22. [DOI] [PMC free article] [PubMed] [Google Scholar]