Abstract
The tremendous need for bone tissue in numerous clinical situations and the limited availability of suitable bone grafts are driving the development of tissue engineering approaches to bone repair. In order to engineer viable bone grafts, one needs to understand the mechanisms of native bone development and fracture healing, as these processes should ideally guide the selection of optimal conditions for tissue culture and implantation. Engineered bone grafts have been shown to have capacity for osteogenesis, osteoconduction, osteoinduction and osteointegration - functional connection between the host bone and the graft. Cells from various anatomical sources in conjunction with scaffolds and osteogenic factors have been shown to form bone tissue in vitro. The use of bioreactor systems to culture cells on scaffolds before implantation further improved the quality of the resulting bone grafts. Animal studies confirmed the capability of engineered grafts to form bone and integrate with the host tissues. However, the vascularization of bone remains one of the hurdles that need to be overcome if clinically sized, fully viable bone grafts are to be engineered and implanted. We discuss here the biological guidelines for tissue engineering of bone, the bioreactor cultivation of human mesenchymal stem cells on three-dimensional scaffolds, and the need for vascularization and functional integration of bone grafts following implantation.
Keywords: Bone grafts, tissue engineering, mesenchymal cells, bone development, vascularization, bioreactor
INTRODUCTION
The field of tissue engineering has developed rapidly over the last 15 years. There are numerous reports on various tissues grown in vitro including bone, cartilage, ligament, muscle and blood vessels. These achievements have been facilitated by laudable accomplishments in multiple related disciplines, but particularly by the rapid advancements in the area of stem cell biology, along with our increasing understanding of how these cells respond to environmental cues. One of the key objectives of bone tissue engineering is the enhancement and guidance of osteogenic differentiation of stem cells within three-dimensional (3D) scaffolds, in a way that would enable to engineer in vitro clinically applicable bone constructs.
Tissue engineered bone constructs have the potential to alleviate the demand arising from the shortage of suitable autograft and allograft materials for augmenting healing of fracture critical-sized defects. Advancements in stem cell, biomaterial and bioreactor technologies have enabled tremendous progress in the quality of the grafts that can be generated in vitro. However, there are still no widely accepted guidelines for determining the minimal requirements (structural and functional) for engineered bone grafts, or standard clinical models for evaluating graft performance. This may be in part due to the wide variability in the types of clinical defects seen, which may in turn influence the choice of scaffold material, cell source, delivery methods, and therapeutic agents. Another challenge is that the size of bone grafts that can be grown in vitro is considerably smaller than the size of critical-sized defects.
Thus, this review attempts to discuss the biological and clinical contexts in which bone tissue engineering should be considered if it is to become a widely used therapeutic tool. Firstly, the basic development and structure of bone are described, as a basis for various tissue engineering approaches. Then we examine the current approaches (autograft and allograft technologies) used to address critical-sized defects in clinical situations. Against this backdrop, the need for engineered bone grafts and their minimum structural and biological requirements that can induce bone regeneration will be discussed. Various aspects of tissue engineered bone constructs are reviewed including clinically relevant cell sources, scaffold properties, and bioreactor platforms used to derive tissue engineered constructs, as well as studies in animal models. We then review approaches for vascularizing tissue-engineered bone constructs and provide perspective on the major challenges that need to be overcome.
1. BONE REPAIR
Bone Structure and Mechanical Properties
Bone provides mechanical support for anchoring muscles and facilitating movement, while protecting vital organs. The primary functions of bone are based on its structural characteristics. Flat bones and the outer part of long bones are comprised of compact (or cortical) bone which contains ~ 80 – 90 % mineralized tissue providing the mechanical strength. The ends of long bones are made up primarily of trabecular (or cancellous) bone. In contrast, only 15 – 25 % of the trabecular bone is mineralized. Thus, while trabecular bone contributes to the mechanical strength, its primary role is metabolic, as this bone functions as a reservoir of calcium and phosphate ions [1]. The structure and geometry of trabecular bone is described by a number of parameters including the trabecular thickness, average spacing, number of trabeculae per unit length and connectivity density, which characterizes the interconnectedness of the trabeculae [2].
The mechanical properties of bone generally depend on its structure and orientation. Due to different structural features, macroscopically the cortical bone has much higher compressive stiffness (12-20 GPa vs 0.2-0.8 GPa) and strength (100-230 MPa vs 2-12 MPa) than the cancellous bone [3]. The specific organization of tissue microstructure results in strong anisotropy in mechanical properties. For instance, the Young's modulus measured along the long axis of cortical bone is 17 GPa, much larger than that in the transverse direction (9.6 GPa) [4].
Development, Fracture Healing and Remodeling
Bone is an incredibly complex organ with huge variations of the skeletal shape in the different regions of the body [5]. The formation of skeletal elements is initiated with the process of cellular condensation, where dispersed mesenchymal cells migrate and proliferate as they become bound together by the expression of adhesion molecules [6]. Subsequent bone development occurs via one of two mechanisms: endochondral ossification (the formation of a cartilage template and its replacement by bone) or intramembranous ossification (direct differentiation of mesenchymal stem cells into osteoblasts).
Most of the bones in the body, including all long bones, form via endochondral ossification (Fig. 1). In this process, mesenchymal condensation is followed by directed differentiation of the precursor cells to pre-chondrocytes and chondrocytes, to create a cartilaginous anlage with a perichondrium at the border. At the center of this model, where primary ossification begins, chondrocytes become hypertrophic, mineralize their matrix and signal the migration of chondroclasts and blood vessels through vascular endothelial growth factor (VEGF). Blood vessels facilitate the influx of hematopoietic cells which interact with the stroma, and form the future bone marrow. Cells in the perichondrium are signaled to become osteoblasts and to secrete collagen I-rich matrix resulting in the formation of a bone collar [7]. Hypertrophic chondrocytes undergo apoptosis and are replaced by osteoblasts that form the bone matrix. Secondary ossification centers develop at the ends of the cartilage model, where again, chondrocytes stop proliferating, hypertrophy and signal the influx of blood vessels and osteoblasts. In between the primary and secondary ossification centers, zones of proliferating chondrocytes (known as the growth-plate) enable bone lengthening. Bone widening occurs via the proliferation and subsequent intramembranous ossification of mesenchymal cells at the surface (appositional growth).
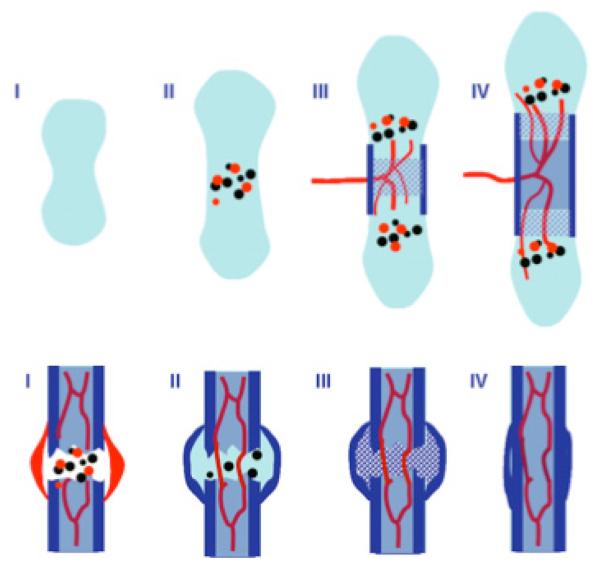
Fig. (1). Bone formation and fracture healing.
Many of the processes occurring during long bone formation are recapitulated during fracture healing. During bone formation, many of these processes occur concurrently but with distinct spatial distributions, while they occur as a temporal sequence during fracture healing. Upper Panel: Initial Stages of Bone Formation Via Endochondral Ossification. Stage I indicates formation of cartilaginous anlage via mesenchymal condensation and differentiation of progenitor cells into chondrocytes. During Stage II, cells at the center undergo hypertrophy and express both angiogenic (red circles) and osteogenic (black circles) growth factors. This stimulates vascular invasion (stage III) with accompanying chondroclastas and osteoblasts. The perichondrium is stimulated to form a bone collar (dark blue rectangle) and cartilage is replaced with trabecular bone. Subsequent bone lengthening (stage IV) results in the formation of a marrow cavity which extends outwards to the ends of the long bone. Lower Panel: Stages of Fracture Healing. (Stage I) Damaged blood vessels in the fracture region result in the formation of a hematoma (red). Growth factors associated with the hematoma are believed to recruit osteo-progenitor cells and induce new-angiogenesis. Progenitor cell migrate to the fracture-site and form a chondrified (internal) callus to stabilize the region (stage II). Meanwhile the periosteum and cortex form new bone via intramembranous ossification (external callus). Stage III: The internal callus then ossifies via endochondral ossification. In stage IV, the spongy bone is resorbed and mechanical continuity is established via remodeling of cortical bone.
Intramembranous (also called, ‘dermal’) bone occurs primarily with flat bones including the skull, scapula and mandible, and it involves the direct differentiation of mesenchymal cells into pre-osteoblasts and osteoblasts [1]. The process is not well characterized and it is still considered the developmental ‘exception’ since most bone forms via endochondral ossification [7]. There are distinct differences in the composition and structure of the bone matrix formed via endochondral and intramembranous ossification [8] but recent analysis have identified several shared molecular regulators of the process [9]. Endochondral ossification is tightly regulated by coordinated expression and interaction of several molecules including Indian Hedgehog (Ihh), parathyroid hormone related peptide (PTHrP), bone morphogenetic proteins (BMPs), VEGF and fibroblastic growth factors (FGFs). BMPs are part of a large family of proteins and have several roles in skeletal development including the initiation of mesenchymal condensations [7]. In the endochondral ossification process Ihh and PTHrP form a critical feedback loop that mediate the balance between chondrocyte proliferation and hypertrophy and regulate the thickness of the growth plate [7, 10]. During intramembranous bone formation, BMPs as well as Ihh and PTHrP are similarly required to induce uncommitted mensenchymal progenitor cells along the osteogenic pathway via a novel phenotype – a preosteoblast which co-expressed chondrocytic and osteoblastic markers simultaneously [9].
The remodeling process is required for the maintenance of normal healthy bone. Initial bone formation results in an irregular distribution of disorganized fiber bundles known as woven bone. This is subsequently remodeled via the coordinated interaction of osteocytes, osteoclasts and osteoblasts into lamellar (layered) structures [1]. Osteoclasts are of hematopoietic origin and are the main cells responsible for bone resorption. They are critical for remodeling processes that occur in response to mechanical stimulation during bone development as well as fracture healing. Upon activation, osteoclasts resorb bone at the endosteal surface. This is followed by bone formation by osteoblasts. The process between resorption and formation is tightly coordinated and balanced in healthy bone.
Bone, when damaged, is unique in its ability to heal without the formation of scar tissue. Fracture healing of long bones occurs via several stages (Fig. 1) and involves coordinated responses of the bone marrow, bone cortex, periosteum and the surrounding soft tissues, including regulation of cellular proliferation, migration and differentiation [11, 12]. The process combines elements of endochondral and intramembranous ossification recapitulating many of the developmental steps. Initially, a damage of blood vessels result in the formation of a hematoma accompanied by an inflammatory response. Many of the signaling molecules involved in the regulation of new bone formation (FGFs, BMPs, PDGF (platelet-derived growth-factor), VEGF etc.), are associated with this inflammation [11]. Primary bone formation occurs immediately at the cortex and periosteum, via intramembranous ossification. The external soft tissues stabilize the fracture by the formation of a callus, which subsequently undergoes chondrogenesis. The callus is comprised of two components: the hard callus formed by intramembranous ossification and the soft (cartilaginous) callus. After the callus forms, cell proliferation decreases, chondrocytes hypertrophy and begin to calcify the matrix. The calcified cartilage is targeted by in-growing blood vessels in a process that is highly similar to endochondral ossification. Chondroclasts resorb the calcified cartilage and osteoblastic progenitors begin the process of new bone formation, in which the mechanical continuity of the cortex is regained by subsequent remodeling.
Current Clinical Treatments of Bone Defects
In general, bone expresses excellent ability for healing, therefore the restoration of alignment and stable fixation suffice for the reconstruction after trauma or disease in most cases. Even relatively large bone defects may be bridged by natural mechanisms of bone repair over callus and woven bone. The bridging may be done acutely (typically in the metaphyseal bone segment such as filling up the gap in the open wedge osteotomy) [13], or continuously (over the callus distraction, by bone lengthening of the long bone diaphysis) [14]. Unfortunately, such bridging is limited to bone segments that allow rigid fixation and encounter adequate vascular supply. The major disadvantages of the callus bridging are technical limitations and the long time needed for structural bone formation to allow full weight-bearing.
However, in certain clinical situations the natural bone repair may be too slow (e.g., following deformity corrections with osteotomies or joint fusions) or inadequate (e.g., large bone defects after comminutive fractures, resections of bone tumors or tumor-like conditions, and endoprosthetic loosening), therefore some form of grafting is required. Cancellous bone is typically used when the requirement is solely to fill the defect to enhance bone formation. The cortico-cancellous block is used in cases where the support of a structure is required (e.g. cervical fusion and articular surface reconstructions). The autologous cancellous bone has the highest value for the routine usage since, besides being osteoproliferative and angiogenic, it is also safe, cheap and available to every surgeon. Smaller quantities of cancellous bone can be harvested from the metaphysis adjacent to the reconstruction site, but larger supplies are available from the pelvic girdle. Yet, the harvest of autologous tissue can result in prolonged pain and there is not enough material for extensive or multiple reconstructions. The structural cortico-cancellous grafts are typically harvested from the anterior or posterior iliac crest, which may result in serious cosmetic problems. A special type of structural graft is the vascularized fibular graft, which requires a microsurgical approach to connect the nutrient fibular vessel to a vascular bundle adjacent to the site of the defect. The operation causes a high degree of morbidity at the donor site, and its usage is generally limited to the tumor reconstructions [15-17].
The alternative solution for grafting is homologous bone from human donors. All the living cells are destroyed during the graft processing and storage in the tissue banks. This reduces the risk of disease transmissions and immunogenic reactions. Therefore, the decellularized, homologous cancellous bone has only osteoconductive and osteoinductive characteristics, and it takes longer for a defect to be filled by native bone tissue. The homologous cancellous bone is often mixed with autologous bone to improve the healing capacity. To diminish the possibilities of disease transmission the homologous bone can be used as a demineralized bone matrix, which has the same limitations for usage. The osteointegration and revascularization of large cortical grafts is limited, and often they remain as non-vital sequesters [16].
2. TISSUE-ENGINEERED BONE GRAFTS
Functional Requirements
Surgical interventions utilizing autografts and allografts have been shown to improve repair of bone defects in various degrees. However, none of currently used grafts has all the ideal characteristics: high osteoinductive and angiogenic potentials, biological safety, low patient morbidity, no size restrictions, ready access to surgeons, long shelf life, and reasonable cost [16, 18]. The promise of tissue engineering is to combine the advances in the fields of biomaterials and cell biology towards bone grafts matching most or all of these characteristics.
Because the primary function of orthopaedic tissues is biomechanical in nature, the restoration of normal biomechanical function becomes the major goal of orthopaedic tissue engineering [19]. Engineered bone constructs would provide physical and biological signals to simulate the natural remodeling mechanism, leading to the complete integration of bone grafts with the surrounding biological tissues and possibly the eventual replacement of the constructs with native bone tissue. Tissue engineered bone constructs, therefore, should ideally have mechanical properties similar to native bone during the entire process of tissue repair and regeneration, especially when constructs are to be implanted in load-bearing sites. Alternatively, scaffolds with inferior mechanical properties can potentially be used if they should allow for fast and strong bone formation. As the whole process usually takes long time (~1-2 years), it is important that the degradation rate of constructs match with the cellular rate of bone formation so that constructs can be mechanically stable with relatively constant stiffness and strength.
Bone Tissue Engineering
To date, the field of bone tissue engineering has been focused on creating tissue grafts that have capacity to enhance osteogenesis in the site of the bone defect. Constructs have been assembled in vitro by seeding cells with osteogenic potential into biodegradable scaffolds, and either directly transplanted in vivo to assess their bone forming potential, or cultured in vitro to enable development of new tissue and the formation of “mature” bone-like grafts. A variety of culture protocols have been used, employing biochemical osteoinductive signals (growth factors and/or cytokines) and specialized dynamic culture systems – bioreactors. These systems facilitate the homogenous tissue growth by improving nutrient transport (in the absence of a vascular network) and mechanical stimulation (shear-stress arising from the flow of culture medium). Only recently steps have been taken toward recapturing the complex, non-uniform tissue architecture found in native bone, and developing inherent vascular networks that would support long term survival and development of larger constructs upon in vivo im-plantation.
Cells
Large numbers of cells capable of producing bone extracellular matrix are needed for the production of clinically-sized engineered tissues. Mesenchymal stem cells, which differentiate and form bone during normal development, have long been the primary cell source for engineering bone grafts. It has long been recognized that adult bone marrow stem cells (BMSC) form multiple mesenchymal tissues in vivo including bone [20, 21], and have utility for engineering skeletal tissues. BMSC can be easily isolated from the marrow aspirate based on their ability to adhere and grow on tissue culture plastics, and can reach up to 50 population doublings in culture [22]. The quantity of stem cells initially isolated varies between different patients and aspirate preparations, and reportedly declines with the patient age [23]. It is most likely that the mesenchymal stem cells from bone marrow aspirates, which drive the normal bone remodeling and regeneration, are an excellent source of cells for bone repair. Studies have shown that the cell culture substrate [24, 25], and the growth factors supplemented to cell culture medium [26-28] help maintain the differentiation potential of these cells during expansion. The need to utilize the right cell phenotype for engineering of human tissues is widely recognized, but the exact phenotypic characteristics are not always well defined. For engineering and regeneration of bone, the properties of choice include high biosynthetic activity (critical for the further development and integration with the host), expression of osteogenic markers (critical for the development of “bona fide” bone tissue), and phenotypic stability (critical for avoiding nonspecific tissue development).
Another easily accessible, abundant source of autologous osteogenic cells is the adipose tissue [29, 30]. Isolation protocols of adipose stem cells (ASC) include density gradient centrifugation of the collagenase-digested lipoaspirate/minced adipose tissue, and culture expansion of the adherent cell population. ASC have been reported to undergo differentiation into various lineages, including osteogenic, chondrogenic and endothelial [29, 30]. Comparative studies of ASC and BMSC cells have indicated both similarities and differences [31]. BMSC populations obtained from the bone marrow of different donors share a common surface antigen expression pattern, including CD44, CD71, CD90, and CD105, whereas the expression of hematopoetic and endothelial lineage markers is low or absent [32-34]. While expression of CD34 is negative for BMSC, there are different reports for ADSC [29, 31, 35]. More work is needed to evaluate the comparative differentiation potential of the two cell types, and the optimal culture conditions required to achieve the functional properties of terminally-differentiated cells are still under investigation [29]. Other connective tissues are being investigated as sources of multipotent cells that could also be employed in bone repair. These include periosteum, umbilical cord, cord blood and fetal tissues [23, 36].
Scaffolds
The scaffold is crucial for the successful engineering of bone tissues as it provides a suitable environment for osteogenic cells to migrate, proliferate, differentiate, and promote new bone formation, and it also provides mechanical competence during the bone regeneration [19]. There are a few requirements to be considered in the design and construction of 3D bone scaffolds. First, the scaffold must be biocompatible and degrade with time into non-toxic products. It should also be highly porous and permeable for cell seeding (in vitro) and infiltration (in vivo), nutrient transport, tissue ingrowth, and vascularization. The scaffold should be mechanically stable, having properties similar to those of the native bone. Finally, an ideal bone scaffold should also be osteoconductive (to recruit bone cells from the recipient), osteoinductive (to differentiate stem cells into bone-forming cells), and osseointegrative (to provide permanent and functional attachment to native bone).
A wide range of natural or synthetic materials has been investigated for bone tissue engineering as well as bone repair in clinic settings. These materials can be mostly categorized into three tiers: polymers, ceramics, and composites. The natural polymer matrix consisting of type I collagen can provide an excellent environment for osteoinduction and osteogenesis, but it has low mechanical modulus and therefore cannot provide sufficient structural support for cells inside [37]. In contrast, synthetic materials can, in principle, be tailored to satisfy all the requirements. The copolymers (PLGA) of poly(lactic acid) (PLA) and poly(glycolic acid) (PGA) have been widely used synthetic polymeric materials, because of their controllable degradation rate and mechanical properties. Other polymers for bone tissue engineering include polyanhydrides, polycarbonates, polyphosphazenes, polycaprolactone and polyfumarates [38]. Naturally produced ceramics such as corals have also been used for the repair of bones such as the distal phalanx of a thumb [39]. Corals have good biocompatibility, well-interconnected porous structure, and appropriate mechanical properties, but the high dissolution rate has limited coralline calcium carbonate in clinical applications, especially when the high load-bearing capacity of bone grafts is required. Synthetic calcium-based ceramics such as hydroxyapatite (HA) and hydroxyapatite-tricalciumphosphate are also osteoconductive materials, but they are usually fragile when high porosity is needed [40]. But when the bioactive calcium-based ceramics are combined with polymers, scaffold mechanical properties as well as the osteoconductivity can be improved as demonstrated by many composite-based scaffolds such as collagen-HA-PLGA, chitosan-hydroxyapatite, PLA-polyethyleneglycol (PEG), collagen-PLA-HA, and polycaprolactone (PCL)-HA [41].
When necessary, bone scaffolds may be surface-modified to enhance cellular attachment, migration, and osseointegration or be used to deliver cytokines, growth factors, and genes for osteogenic induction and bone formation [42-46]. Several excellent reviews can be consulted for more detail on these topics [38, 47-49].
In Vitro Cultivation
The potential for growing bone-like constructs from BMSC was first explored in static culture [50, 51], and the effects of various parameters (cell seeding density, scaffold properties, culture medium composition) on tissue development have been evaluated. Various osteogenic cell sources and biodegradable scaffolds, including synthetic and natural polymers [32, 52-55], ceramics [56] and composites [54, 57-59] have also been tested in static culture. These studies helped identify the importance of three-dimensional culture environments for proper signaling (cell condensation, cell-cell interactions and cell-matrix interactions) that can stimulate osteogenesis, but they also showed clearly that static culture limits the development of bone constructs due to the diffusional exchange of nutrients, oxygen and metabolites.
In order to achieve homogenous cellular growth and tissue development inside large (millimeter to centimeter sized) constructs, bioreactor cultivation systems with enhanced mass transport capabilities have been investigated. In stirred flasks and rotating bioreactors, medium convection in bulk medium enhances mass transport at the surfaces of cultured tissue constructs, whereas the transport in the construct interior remains by diffusion only. In stirred flasks, the scaffolds are fixed in place, whereas in the rotating bioreactors, they are cultured freely suspended in the culture medium [60-62]. In perfusion bioreactors, culture medium flows through the interstitial spaces (pores) of the construct, which enables local supply of oxygen and nutrients and removal of metabolites, thus providing much better control of the cell microenvironment [63].
Goldstein and colleagues [64] compared rat BMSC growth in four culture systems, and showed that perfusion and rotating bioreactor cultures result in constructs with more uniform cell distribution than stirred flasks and static dishes. In a longer follow-up study [65], the culture in stirred flasks was compared to rotating bioreactors and static culture, and exhibited the highest cell proliferation and osteogenesis. However other studies have indicated more favorable osteogenic outcomes when using rotating bioreactors, with significantly increased osteogenesis of human osteoblastic cell line [66], primary rat calvarial osteoblasts [67] and human BMSC [68] compared to static culture. The positive effects have been attributed to a combination of improved mass transport (depending on scaffold geometry) and mechanical conditioning of the constructs [69].
Several studies have shown positive effects of perfusion culture on bone development in vitro [63, 70-76]. In these cases, improved transport through the scaffold interior and fluid shear stresses to osteogenic cells are believed to better mimic the native bone environment. It has been shown that mechanical conditioning alone in the absence of dexamethasone, the standard osteogenic supplement, can induce osteogenic differentiation of BMSC in perfusion culture [72]. Recent results from our group indicate that employment of high perfusion rates in concurrence with adjustment of perfusion chamber designs could potentially support in vitro development of large custom-shaped bone grafts [77]. Additionally, bioreactor designs accommodating cell seeding, in vitro expansion and perfusion culture have been proposed [74, 76, 78].
In Vivo Models and Clinical Studies
One of the crucial aspects of bone tissue engineering is the evaluation/prediction of the obtained constructs capacity for bone healing. In order to assess osteogenesis, the constructs are often implanted into ectopic sites (e.g., subcutaneously), where bone formation does not occur naturally, and the osteoinductive signals arise from the implant itself. The outcome is a combination of the influences from systemic factors, the osteogenic (bone forming), osteoinductive (bone inducing) and osteoconductive (bone supporting) properties of the implant, and the surrounding tissue potential for ingrowth, vascularization and osteogenesis. For the evaluation of human cell-based constructs, studies have been performed in immuno-compromised rodent models [79-82]. These have shown the ability of cultured osteogenic cells to form bone tissue in vivo. Importantly, interspecies differences in bone formation requirements have been noted [83].
Implantation into orthotopic non-load bearing (e.g., rat calvaria) and load bearing (e.g., femour, tibia) sites, where the surrounding tissue itself has osteoinductive and osteogenic capacity, has also been used [84-86]. Studies have indicated that maturation of the grafts by in vitro cultivation enhances bone healing after implantation [32, 53], as compared to implantation of the unseeded scaffolds and scaffolds seeded with cells immediately prior to surgery,. It has been shown that the number of clonogenic BMSCs (and not the total number of cells per scaffold) is positively correlated to the bone forming potential [87]. Such parameters could be used as a predictive measure for evaluation of the constructs in the clinical setting.
The advantage of rodent models (besides the possibility of testing human cells) is the miniaturization of critical-size (non-healing) defects, which are in the range of < 1 cm, and roughly correspond to the sizes of constructs that can currently be prepared in vitro. On the other hand, studies performed in orthotopic sites of large animals, including skull and mandibular defects [88-91], and large segmental defects of long bones [92-95] have been limited to the use of autologous cells of the species under investigation. This work provided a proof of principle for rodent studies, as in many cases the improvement of bone healing was observed following the implantation of tissue engineered constructs.
Human clinical trials addressing tissue engineered grafts in bone repair are limited only to case reports [96-98]. The autologous bone marrow-derived mesenchymal stem cells were loaded on ceramic, slowly absorbable scaffolds. The results confirmed safety of the products and demonstrated integration of the constructs into the bone. More information are awaited from the randomized trials, but there is no general agreement on the best clinical model for the tissue engineered bone.
3. VASCULARIZATION OF BONE GRAFTS
Vascularization following implantation is of critical importance for the survival, integration and functionality of engineered bone tissue, as it is for the development of native bone [99]. In both cases, vascular supply is necessary to assure efficient gas and nutrition exchange with all cells within the tissue. At short distances (≤ 200 – 300 μm), the mass transport requirements (for oxygen in particular) can be met by molecular diffusion, while limitations of diffusional transport over greater distances results in acellular regions [64, 100]. Enhanced cell survival can be supported within larger scaffolds (≤ 5 mm) by culturing bone constructs in bioreactors [63, 68]. But for successful outcome of the implantation of the construct in vivo, vascularization of the graft needs to be considered. There are several approaches being utilized in order to vascularize bone grafts, and generally one or combination of three major principles can be followed (Fig. 2).
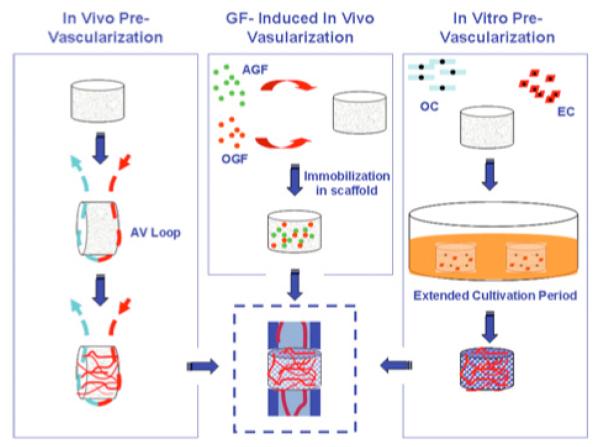
Fig. (2). Approaches to Vascularizing Engineered Bone Scaffolds.
Left: The arterio-venous (AV) loop is shown as an example of an in vivo approach for pre-vascularizing scaffolds. Other methods include intramuscular or cutaneous implantation. The AV-loop method is advantageous in that it results in blood vessels with a generally consistent orientation and is less restricted by anatomical location. Center: One cell-free approach is to immobilize angiogenic growth factors (AGF) and osteogenic growth factors (OGF) unto scaffolds and directly implant into the site of interest. In this method, the growth-factors induce migration of angiogenic and osteo-progenitor cells and provide them with the stimuli for neo-vessel formation and osteogenic differentiation. Right: Cell-based, tissue-engineering approach utilizes osteogenic cells (OC) and endothelial cells (EC) in three-dimensional co-culture. This method includes an extended cultivation period to facilitate functional organization of cells and differentiation into appropriate cell types.
In Vivo Pre-Vascularization
Bone grafts can be implanted into environments rich in vascular supply (subcutaneous, intramuscular, or intraperito-neal sites), where the constructs can be invaded with new vascular networks at their surfaces. However, formation of new vessels within the implanted bone proceeds in random patterns, and the transplantation to the site of interest is impossible without damaging the initial vascular network. Alternative approaches have been described, including the ‘intrinsic’ vascularization which is discussed in several good reviews [101, 102]. Here, angiogenesis is induced via a vessel located centrally in the graft: for example, the use of the carotid artery, jugular vein or saphenous bundle in osteoconductive hydroxyapatite scaffolds seeded with BMSC resulted in de novo bone deposition and neo-vascularization within viable bone grafts which can be potentially transplanted to orthotopic sites of interest [103]. Another vessel configuration, the arteriovenous loop, has been employed to prevascularize scaffolds derived from bovine cancellous bone. Subsequently injected osteoblasts showed better survival rates but they failed to produce new bone tissue [104]. On the other hand, the combination of a vascular element and osteogenic cells in coralline implants enhanced neovascularization as well as osteogenesis when implanted in ectopic intramuscular sites [105].
Utilization of Angiogenic Factors
Vascularization of an implanted graft can also be accelerated by the local delivery of angiogenic growth factors. Growth factors, such as VEGF, PDGF and FGF play crucial roles in angiogenesis [106]. Incorporation of these factors into scaffolds and control of their local release rate and delivery regime present one possibility for accelerating the vascular in-growth in vivo. The growth factors can be incorporated in two methods: by mixing with the polymer particles of the scaffold itself (which later results in fast release), or they can be encapsulated in microspheres to facilitate their controlled release over longer periods of time [107]. Since the development of tissues is orchestrated by the coordinated interactions of multiple growth factors along spatial and temporal gradients, allowing for the delivery of multiple growth factors with distinct release kinetics are of special importance. Utilizing this approach, delivery of (VEGF)-165 and (PDGF)-BB resulted in rapid formation of a mature vascular network [108]. VEGF-releasing, biomineralized PLGA scaffolds implanted in rat cranium defects showed an increase in vascularization as well as in the production of mineralized tissue, when compared with scaffolds without VEGF [109]. Vascularization can also be enhanced by the combination of angiogenic factors with cells: VEGF-releasing PLG scaffolds in combination with microvascular endothelial cells resulted in the formation of functional vessels one week after implantation in SCID mice [110]. The combination of osteogenic (BMP-4) and angiogenic (VEGF) factors together with bone marrow stromal cells promoted bone formation at an ectopic site [111]. The combined delivery of cells, osteogenic and angiogenic factors resulted in a significant increase in the quantity of regenerated bone compared with any factor alone or any two factors combined.
In Vitro Prevascularization of TE Grafts
Another promising approach to achieve vascularization of tissue engineered bone grafts is seeding and co-culturing endothelial and osteogenic cells into the bone constructs engineered in vitro. As a source of endothelial cells, adult endothelial cells can be used, but recently, adult mesenchymal stem cells have also been shown to have the potential to differentiate toward endothelial lineage [112-114]. Endothelial cells have the potential to form new vessels within the scaffolds with the potential to anastomose with host vasculature when implanted in vivo. It is important, however, that besides endothelial cells, also the presence of other cell types (smooth muscle cells, pericytes) is considered, since interaction of different cell types is needed for functional vasculature. Using this co-culture principle, human skin was engineered in vitro using keratinocytes, fibroblasts and endothelial cells and then transplanted into nude mice. The network of in vitro engineered capillary-like structures successfully anastamosed to the host's vasculature [115]. Similarly, survival and vascularization of an in vitro engineered, prevascularized muscle implant was improved after the transplantation [116]. Prevascularized constructs for use in bone tissue engineering have also been generated in vitro using various biomaterials (porous hydroxyapatite, porous calcium phosphate, porous nickel-titanium and fibroin nets), seeded with human dermal microvascular endothelial cells and primary osteoblasts or cell lines MG-63 [117]. The formation of 3D prevascular networks was also reported for human umbilical vein endothelial cells (HUVEC) grown in pellet co-culture with human osteoprogenitor cells. However, upon in vivo implantation anastomosis with host vasculature was limited [118]. On the other hand significant increase in bone formation was observed when endothelial cells was transplanted at orthotopic site together with BMSC [119].
Endothelial cells not only contribute to form the vasculature to provide nutrients to the bone but are also important in terms of interaction with and differentiation of osteoprogenitor cells. It was seen that the lifespan of endothelial cells was prolonged when cells were cultured together with osteoblast cells [117] and the presence of endothelial cells accelerated the expression of an osteogenic phenotype in osteoprogenitor cells, [118, 120-122]. However, it has also been reported that endothelial cells can inhibit the differentiation of human mesenchymal stem cells into mature osteoblasts [123] and that the ability of HUVEC to form tubular structures was decreased when co-cultured with osteoblasts [124]. Different effects of co-culturing observed might be due to different stages of cells' differentiation or different culture conditions used, what implies the need for further research in order to understand and achieve good co-culture systems and conditions. Also, different environmental cues can have important effect on cell differentiation: for example, hypoxic conditions stimulate angiogenesis in vitro [125], but have at the same time neutral to negative effects on osteogenic differentiation of mesenchymal stem cells [126-129]. The use of perfusion culture conditions showed improved vasculogenic properties after ASC were implanted in vivo [130].
Summary and Future Perspectives
In summary, bone is one of the tissues with excellent capability for regeneration following injury. However, this is the case only if the defect is below a certain critical size. The needs of bone repair in many patients suffering from large bone resections or significant trauma motivates the creation of bone grafts using tissue-engineering approaches. Because of the intrinsic capability of the bone tissue to re-establish its complex hierarchical structure during regeneration, the tissue engineering approaches are in many cases “biomimetic”. To take advantage of the intrinsic ability of the cells to form bone, osteogenic cells can be cultured on a scaffold serving as a structural and logistic template for bone formation, in a bioreactor providing the necessary molecular and physical signals. Several areas of ongoing active research are directly relevant to the translation of research results into the clinical practice, including: development of bioreactor systems for automated, standardized and scalable production of TE bone grafts; scale up to large, clinically sized bone grafts with capacity for further development and integration; evaluation of engineered grafts in large animal models; rapid establishment of functional blood flow through the implanted graft; accommodation for patient to patient and site to site variability.
ACKNOWLEDGMENTS
The tissue engineering work described in this review has been generously supported by the NIH (R01 DE16525 and P41-EB002520 to G V-N), the New York Stem Cell Foundation (Stanley and Fiona Druckenmiller Fellowship to DM), Ministry of Higher Education, Science and Technology, Republic of Slovenia (3311-04-831828) and Ministry of Defense, Republic of Slovenia (TP MIR 31; TP MIR 06/ RR/ 12).
REFERENCES
- 1.Baron R. In: Diseases of Bone and Mineral Metabolism. Arnold A, editor. Darmouth, MA: 2008. Endotext.com. [Google Scholar]
- 2.Huiskes R, Van Reitbergen B. In: Basic Orthopaedic Biomechanics and Mechano-Biology. Mow VC, Huiskes R, editors. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 123–79. [Google Scholar]
- 3.Hench LL, Wilson J, editors. Introduction to Bioceramics. World Scientific; Singapore: 1993. [Google Scholar]
- 4.Guo XE. In: Bone Mechanics Handbook. Cowin SC, editor. CRC press; Boca Raton, FL: 2001. pp. 10–1. [Google Scholar]
- 5.Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316–8. doi: 10.1038/nature01654. [DOI] [PubMed] [Google Scholar]
- 6.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–47. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 8.Scott CK, Hightower JA. The Matrix of Endochondral Bone Differs from the Matrix of Intramembranous Bone. Calcif Tissue Int. 1991;49:349–54. doi: 10.1007/BF02556258. [DOI] [PubMed] [Google Scholar]
- 9.Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–44. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- 10.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 11.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury, Int J Care Injured. 2005;36:1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 13.Staubli AE, De Simoni C, Babst R, Lobenhoffer P. TomoFix: a new LCP-concept for open wedge osteotomy of the medial proximal tibia--early results in 92 cases. Injury. 2003;34(Suppl 2):B55–62. doi: 10.1016/j.injury.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Paley D, Herzenberg JE, Paremain G, Bhave A. Femoral lengthening over an intramedullary nail. A matched-case comparison with Ilizarov femoral lengthening. J Bone Joint Surg Am. 1997;79:1464–80. doi: 10.2106/00004623-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Grenshaw AH. In: Campbell's Operative Orthopaedics. 11th editon Canale TS, Beaty JH, editors. Mosby Elsevier; Philadelphia: 2008. pp. 14–22. [Google Scholar]
- 16.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454–64. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to Auto-genous Bone Graft: Efficacy and Indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Vunjak-Novakovic G, Goldstein SA. In: Basic Orthopaedic Biomechanics and Mechano-biology. 3rd edition Mow VC, Huiskes R, editors. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 343–408. [Google Scholar]
- 20.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone-marrow osteogenic stem-cells - in vitro cultivation and transplantation in diffusion-chambers. Cell Tissue Kinet. 1987;20:263–72. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 23.Barrilleaux B, Phinney DG, Prockop DJ, O'Connor KC. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–19. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 24.Mauney JR, Volloch V, Kaplan DL. Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials. 2005;26:6167–75. doi: 10.1016/j.biomaterials.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Mauney JR, Kaplan DL, Volloch V. Matrix-mediated retention of osteogenic differentiation potential by human adult bone marrow stromal cells during ex vivo expansion. Biomaterials. 2004;25:3233–43. doi: 10.1016/j.biomaterials.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138:4456–62. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- 27.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 28.Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Effect of different growth factors on human osteoblasts activities: A possible application in bone regeneration for tissue engineering. Biomol Eng. 2007;24:613–8. doi: 10.1016/j.bioeng.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 31.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 32.Meinel L, Karageorgiou V, Hofmann S, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71A:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 33.Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: Biological properties, characteristics, and applications in maxillofacial surgery. J Oral Maxillofac Surg. 2007;65:1640–7. doi: 10.1016/j.joms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 35.Noel D, Caton D, Roche S, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–84. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Sudo K, Kanno M, Miharada K, et al. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells. 2007;25:1610–7. doi: 10.1634/stemcells.2006-0504. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno M, Shindo M, Kobayashi D, Tsuruga E, Amemiya A, Kuboki Y. Osteogenesis by bone marrow stromal cells maintained on type I collagen matrix gels in vivo. Bone. 1997;20:101–7. doi: 10.1016/s8756-3282(96)00349-3. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–86. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 39.Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–4. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 40.Grundel RE, Chapman MW, Yee T, Moore DC. Autogeneic bone marrow and porous biphasic calcium phosphate ceramic for segmental bone defects in the canine ulna. Clin Orthop Relat Res. 1991:244–58. [PubMed] [Google Scholar]
- 41.Hutmacher DW, Schantz JT, Lam CX, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245–60. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann S, Hagenmuller H, Koch AM, et al. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials. 2007;28:1152–62. doi: 10.1016/j.biomaterials.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Uebersax L, Hagenmuller H, Hofmann S, et al. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006;12:3417–29. doi: 10.1089/ten.2006.12.3417. [DOI] [PubMed] [Google Scholar]
- 44.Yang XB, Tare RS, Partridge KA, et al. Induction of human osteo-progenitor chemotaxis, proliferation, differentiation, and bone formation by osteoblast stimulating factor-1/pleiotrophin: osteoconductive biomimetic scaffolds for tissue engineering. J Bone Miner Res. 2003;18:47–57. doi: 10.1359/jbmr.2003.18.1.47. [DOI] [PubMed] [Google Scholar]
- 45.Yang XB, Bhatnagar RS, Li S, Oreffo RO. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10:1148–59. doi: 10.1089/ten.2004.10.1148. [DOI] [PubMed] [Google Scholar]
- 46.Kirker-Head C, Karageorgiou V, Hofmann S, et al. BMP-silk composite matrices heal critically sized femoral defects. Bone. 2007;41:247–55. doi: 10.1016/j.bone.2007.04.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–64. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 48.Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58:555–76. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Dimitriou R, Babis GC. Biomaterial osseointegration enhancement with biophysical stimulation. J Musculoskelet Neuronal Interact. 2007;7:253–65. [PubMed] [Google Scholar]
- 50.Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 51.Martin I, Shastri VP, Padera RF, et al. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res. 2001;55:229–35. doi: 10.1002/1097-4636(200105)55:2<229::aid-jbm1009>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Rhie JW, Oh DY, Ahn ST. Osteogenic differentiation of human adipose tissue-derived stromal cells (hASCs) in a porous three-dimensional scaffold. Biochem Biophys Res Commun. 2008;370:456–60. doi: 10.1016/j.bbrc.2008.03.123. [DOI] [PubMed] [Google Scholar]
- 53.Meinel L, Karageorgiou V, Fajardo R, et al. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–22. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Kim UJ, Vunjak-Novakovic G, Min BH, Kaplan DL. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005;26:4442–52. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res A. 2008;84A:191–7. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 56.Ng AMH, Tan KK, Phang MY, et al. Differential osteogenic activity of osteoprogenitor cells on HA and TCP/HA scaffold of tissue engineered bone. J Biomed Mater Res A. 2008;85A:301–12. doi: 10.1002/jbm.a.31324. [DOI] [PubMed] [Google Scholar]
- 57.Gravel M, Gross T, Vago R, Tabrizian M. Responses of mesenchymal stem cell to chitosan-coralline composites microstructured using coralline as gas forming agent. Biomaterials. 2006;27:1899–906. doi: 10.1016/j.biomaterials.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Zhao F, Grayson WL, Ma T, Bunnell B, Lu WW. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials. 2006;27:1859–67. doi: 10.1016/j.biomaterials.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 59.Xu C, Wang Y, Yu X, et al. Evaluation of human mesenchymal stem cells response to biomimetic bioglass-collagen-hyaluronic acid-phosphatidylserine composite scaffolds for bone tissue engineering. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31931. [DOI] [PubMed] [Google Scholar]
- 60.Chao PHG, Grayson W, Vunjak-Novakovic G. Engineering cartilage and bone using human mesenchymal stem cells. J Orthop Sci. 2007;12:398–404. doi: 10.1007/s00776-007-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freed LE, Vunjak-Novakovic G. In: Principles of Tissue Engineering. 2nd edition Lanza R, Langer R, Vacanti J, editors. Academic Press; San Diego: 2000. pp. 143–56. [Google Scholar]
- 62.Grayson WL, Chao PG, Marolt D, et al. In: Translational Approaches in Tissue Engineering and Regenerative Medicine. Mao JJ, Vunjak-Novakovic G, Mikos A, Atala A, editors. Artech House; Boston: 2007. pp. 353–74. [Google Scholar]
- 63.Grayson WL, Bhumiratana S, Cannizzaro C, et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng. doi: 10.1089/ten.tea.2007.0255. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279–88. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 65.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–48. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 66.Botchwey EA, Pollack SR, Levine EM, Laurencin CT. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res. 2001;55:242–53. doi: 10.1002/1097-4636(200105)55:2<242::aid-jbm1011>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu XJ, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: The influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proc Natl Acad Sci U S A. 2004;101:11203–8. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marolt D, Augst A, Freed LE, et al. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–49. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Botchwey EA, Dupree MA, Pollack SR, Levine EM, Laurencin CT. Tissue engineered bone: Measurement of nutrient transport in three-dimensional matrices. J Biomed Mater Res A. 2003;67A:357–67. doi: 10.1002/jbm.a.10111. [DOI] [PubMed] [Google Scholar]
- 70.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100:14683–8. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MAK, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33:63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- 72.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72A:326–34. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 73.Holtorf HL, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann Biomed Eng. 2005;33:1238–48. doi: 10.1007/s10439-005-5536-y. [DOI] [PubMed] [Google Scholar]
- 74.Braccini A, Wendt D, Jaquiery C, et al. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23:1066–72. doi: 10.1634/stemcells.2005-0002. [DOI] [PubMed] [Google Scholar]
- 75.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103:2488–93. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scaglione S, Braccini A, Wendt D, et al. Engineering of osteoinductive grafts by isolation and expansion of ovine bone marrow stromal cells directly on 3D ceramic scaffolds. Biotechnol Bioeng. 2006;93:181–7. doi: 10.1002/bit.20677. [DOI] [PubMed] [Google Scholar]
- 77.Grayson WL, Fröhlich M, Yeager K, Cannizzaro C, Vunjak-Novakovic G. Bioreactor Culture of Anatomically-Shaped Mandibular Condyles; Orthopeadic Research Society conference; San Francisco, USA. 2008. [Google Scholar]
- 78.Timmins NE, Scherberich A, Fruh JA, Heberer M, Martin I, Jakob M. Three-dimensional cell culture and tissue engineering in a TCUP (Tissue Culture Under Perfusion) Tissue Eng. 2007;13:2021–8. doi: 10.1089/ten.2006.0158. [DOI] [PubMed] [Google Scholar]
- 79.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–8. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 80.Dennis JE, Haynesworth SE, Young RG, Caplan AI. Osteogenesis in marrow-derived mesenchymal cell porous ceramic composites transplanted subcutaneously: effect of fibronectin and laminin on cell retention and rate of osteogenic expression. Cell Transplant. 1992;1:23–32. doi: 10.1177/096368979200100106. [DOI] [PubMed] [Google Scholar]
- 81.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 82.Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12:1606–14. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 83.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RVB, Rowe DW, Robey PG. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–69. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 84.Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow-cells and porous ceramic - experiments in rats. Acta Orthop Scand. 1989;60:334–9. doi: 10.3109/17453678909149289. [DOI] [PubMed] [Google Scholar]
- 85.Puelacher WC, Vacanti JP, Ferraro NF, Schloo B, Vacanti CA. Femoral shaft reconstruction using tissue-engineered growth of bone. Int J Oral Maxillofac Surg. 1996;25:223–8. doi: 10.1016/s0901-5027(96)80035-x. [DOI] [PubMed] [Google Scholar]
- 86.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–62. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 87.Braccini A, Wendt D, Farhadi J, et al. The osteogenicity of implanted engineered bone constructs is related to the density of clonogenic bone marrow stromal cells. J Tissue Eng Regen Med. 2007;1:60–5. doi: 10.1002/term.11. [DOI] [PubMed] [Google Scholar]
- 88.Schliephake H, Knebel JW, Aufderheide M, Tauscher M. Use of cultivated osteoprogenitor cells to increase bone formation in segmental mandibular defects: an experimental pilot study in sheep. Int J Oral Maxillofac Surg. 2001;30:531–7. doi: 10.1054/ijom.2001.0164. [DOI] [PubMed] [Google Scholar]
- 89.Shang QX, Wang Z, Liu W, Shi YH, Cui L, Cao YL. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg. 2001;12:586–93. doi: 10.1097/00001665-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Wu W, Chen X, Mao T, Chen F, Feng X. Bone marrow-derived osteoblasts seeded into porous beta-tricalcium phosphate to repair segmental defect in canine's mandibula. Ulus Travma Acil Cerrahi Derg. 2006;12:268–76. [PubMed] [Google Scholar]
- 91.He Y, Zhang ZY, Zhu HG, Qiu WL, Jiang XQ, Guo W. Experimental study on reconstruction of segmental mandible defects using tissue engineered bone combined bone marrow stromal cells with three-dimensional tricalcium phosphate. J Craniofac Surg. 2007;18:800–5. doi: 10.1097/scs.0b013e31806901f5. [DOI] [PubMed] [Google Scholar]
- 92.Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–37. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 93.Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 94.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80A:985–96. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Viateau V, Guillemin G, Bousson V, et al. Long-bone critical-size defects treated with tissue-engineered grafts: A study on sheep. J Orthop Res. 2007;25:741–9. doi: 10.1002/jor.20352. [DOI] [PubMed] [Google Scholar]
- 96.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 97.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–55. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 98.Krec̆ic̆-Stres H, Krkovic̆ M, Koder J, et al. Mesenchymal Stem Cells: a Modern Approach to Treat Long Bones Defects. 11th Mediterranean Conference on Medical and Biomedical Engineering and Computing; Springer; Berlin Heidelberg. 2007. [Google Scholar]
- 99.Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–9. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 100.Gill DR, Ireland DC, Hurley JV, Morrison WA. The prefabrication of a bone graft in a rat model. J Hand Surg [Am] 1998;23:312–21. doi: 10.1016/S0363-5023(98)80133-0. [DOI] [PubMed] [Google Scholar]
- 101.Polykandriotis E, Arkudas A, Horch RE, Sturzl M, Kneser U. Autonomously vascularized cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med. 2007;11:6–20. doi: 10.1111/j.1582-4934.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon's point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawamura K, Yajima H, Ohgushi H, et al. Experimental study of vascularized tissue-engineered bone grafts. Plast Reconstr Surg. 2006;117:1471–9. doi: 10.1097/01.prs.0000197883.17428.22. [DOI] [PubMed] [Google Scholar]
- 104.Arkudas A, Beier JP, Heidner K, et al. Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng. 2007;13:1549–60. doi: 10.1089/ten.2006.0387. [DOI] [PubMed] [Google Scholar]
- 105.Pelissier P, Villars F, Mathoulin-Pelissier S, Bareille R, Lafage-Proust MH, Vilamitjana-Amedee J. Influences of vascularization and osteogenic cells on heterotopic bone formation within a madreporic ceramic in rats. Plast Reconstr Surg. 2003;111:1932–41. doi: 10.1097/01.PRS.0000055044.14093.EA. [DOI] [PubMed] [Google Scholar]
- 106.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 107.Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 108.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 109.Murphy WL, Simmons CA, Kaigler D, Mooney DJ. Bone regeneration via a mineral substrate and induced angiogenesis. J Dent Res. 2004;83:204–10. doi: 10.1177/154405910408300304. [DOI] [PubMed] [Google Scholar]
- 110.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–78. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 111.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 112.Valarmathi MT, Yost MJ, Goodwin RL, Potts JD. A three-dimensional tubular scaffold that modulates the osteogenic and vasculogenic differentiation of rat bone marrow stromal cells. Tissue Eng Part A. 2008;14:491–504. doi: 10.1089/tea.2007.0235. [DOI] [PubMed] [Google Scholar]
- 113.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–55. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 114.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 115.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–10. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 116.Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 117.Unger RE, Sartoris A, Peters K, et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965–76. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 118.Rouwkema J, De Boer J, Van Blitterswijk CA. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–93. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 119.Kaigler D, Krebsbach PH, Wang Z, West ER, Horger K, Mooney DJ. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633–7. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 120.Villars F, Guillotin B, Amedee T, et al. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775–85. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 121.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–7. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 122.Choong CSN, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolac-tone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521–31. doi: 10.1089/ten.2006.12.2521. [DOI] [PubMed] [Google Scholar]
- 123.Meury T, Verrier S, Alini M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem. 2006;98:992–1006. doi: 10.1002/jcb.20818. [DOI] [PubMed] [Google Scholar]
- 124.Wenger A, Stahl A, Weber H, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004;10:1536–47. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- 125.Phillips PG, Birnby LM, Narendran A. Hypoxia induces capillary network formation in cultured bovine pulmonary microvessel endothelial cells. Am J Physiol. 1995;268:L789–800. doi: 10.1152/ajplung.1995.268.5.L789. [DOI] [PubMed] [Google Scholar]
- 126.Xu Y, Malladi P, Chiou M, Bekerman E, Giaccia AJ, Longaker MT. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–93. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 127.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 128.Martin-Rendon E, Hale SJ, Ryan D, et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–12. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 129.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–57. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 130.Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823–9. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]