Abstract
AIM: To explore the influence of portal vein hemo-dynamic changes after portal venous arterialization (PVA) on peribiliary vascular plexus (PVP) morphological structure and hepatic pathology, and to establish a theoretical basis for the clinical application of PVA.
METHODS: Sprague-Dawley rats were randomly divided into control and PVA groups. After PVA, hemodynamic changes of the portal vein and morphological structure of hepatohilar PVP were observed using Doppler ultrasound, liver function tests, ink perfusion transparency management and three-dimensional reconstruction of computer microvisualization, and pathological examination was performed on tissue from the bile duct wall and the liver.
RESULTS: After PVA, the cross-sectional area and blood flow of the portal vein were increased, and the increase became more significant over time, in a certain range. If the measure to limit the flow in PVA was not adopted, the high blood flow would lead to dilatation of intrahepatic portal vein and its branches, increase in collagen and fiber degeneration in tunica intima. Except glutamic pyruvic transaminase (GPT), other liver function tests were normal.
CONCLUSION: Blood with a certain flow and oxygen content is important for filling the PVP and meeting the oxygen requirement of the bile duct wall. After PVA, It is the anatomic basis to maintain normal morphology of hepatohilar bile duct wall that the blood with high oxygen content and high flow in arterialized portal vein may fill PVP by collateral vessel reflux. A adequate measure to limit blood flow is necessary in PVA.
Keywords: Peribiliary vascular plexus, Portal venous arterialization, Liver transplantation, Bile duct neoplasms, Three-dimensional reconstruction, Hemodynamics
INTRODUCTION
In the clinical practice of enlarged radical operation and liver transplantation, revascularization of the liver is often needed. For this reason, portal venous arterialization (PVA) has been extensively studied and carried out in clinical practice[1-5]. PVA has been used in more than 10 cases during liver transplantation and some transplant centers have achieved good outcomes[6,7]. Blood flow and oxygen content in the peribiliary vascular plexus (PVP) play an important role in ensuring the normal physiological function of bile-duct epithelial cells[8]. After PVA, the influence of portal vein hemodynamic changes on hepatohilar PVP is important for the incidence of bile duct complications and is directly associated with clinical application of PVA. Therefore, the purpose of our study was to explore the influence of portal vein hemodynamic changes after PVA on PVP morphological structure, and liver function and pathology, and to establish a theoretical basis for the clinical application of PVA.
MATERIALS AND METHODS
All study methods were approved by the ethics committee of Xiamen First Hospital.
Materials
Healthy, adult, male, clean Sprague-Dawley rats weighing 250-300 g were provided from the Laboratory Animal Center, General Hospital of PLA, Beijing. All rats were maintained under specific pathogen-free conditions. The CK40 inverted phase contrast microscope and BX51TF system microscope were provided by Olympus. Perfusion ink was from China.
Establishment of PVA model
Rats were randomly divided into control and PVA groups (each group with 17 rats). Rats were fasted 12 h prior to operation, then anesthetized with 3% chloral hydrate (1 mL/100 g). Models were made under BX51TF system microscope. In the control group, after opening the abdomen, the porta hepatis was isolated, and then the abdomen was closed. In the PVA group, after opening the abdomen, the inferior vena cava, portal vein and common hepatic artery were isolated, and then double ligations were performed with 0 silk thread on the common hepatic artery and it was cut in the middle, the proximal broken end with silk thread and small round pin for further use. The portal vein and the celiac artery were blocked with vascular clamps, the stump of the common hepatic artery was connected to the portal vein, and then the celiac artery clamp was removed, and tremors from the portal vein were felt (Figure 1A). The portal vein clamp was removed and the blood flow was temporarily restored (cross-clamp for 5-7 min). Subsequently, the side-to-side anastomosis (cross-clamp for 10-12 min) of the portal vein and the inferior vena cava was performed, with the portal vein diameter the same as the anastomotic stoma diameter (Figure 1B). The anastomotic stoma was confirmed to be unobstructed. Finally, ligation was performed between the anastomotic stoma of the hepatic artery and the portal vein, and the right gastric vein branch of the portal vein. Therefore, only arterial blood flowed into the intrahepatic portal vein. All rats in the PVA and control groups were observed for 6 mo.
Figure 1.
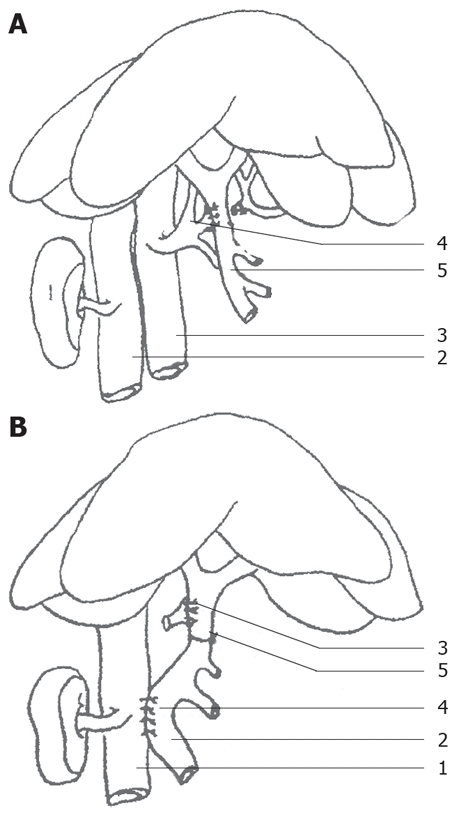
Animal PVA model establishment. A: Side-to-side anastomotic stoma of the hepatic artery and the portal vein. (1) kidney; (2) inferior vena cava; (3) abdominal aorta; (4) hepatic artery; (5) portal vein. B: Side-to-side anastomotic stoma of the portal vein and the venae cavae. (1) inferior vena cava; (2) portal vein; (3) arteriovenous anastomotic stoma; (4) anastomotic stoma of the portal vein and the venae cavae; (5) ligation of the portal vein.
Hemodynamic changes of arterialized portal vein
Color Doppler ultrasound was used to monitor the cross-sectional area and blood flow of the portal vein at the mid-point of the main portal vein in seven rats from the two groups, at 1 and 6 mo after PVA, which was carried out in the Department of Ultrasound, PLA General Hospital. The included angle between the ultrasound probe and the main portal vein was < 60° and the frequency of the probe was 5-13 MHz.
Liver functions in rats 6 mo after PVA
Two milliliters of blood were taken from the abdominal aorta in randomly selected 8 rats from the two groups respectively for determining liver functions. Then ink perfusion was performed in the 8 rats respectively (as follow).
Capillary morphology in hepatohilar bile duct wall after PVA
Ink perfusion: Six months after PVA, the portal vein and abdominal aorta were intubated in 10 rats (remained 2 rats and 8 rats above) in the control group, and only the abdominal aorta was intubated in 10 rats (remained 2 rats and 8 rats above) from the PVA group. In the 20 rats, the thoracic aorta was ligated, then a cut was made on the vena cava of the midriff, 1% heparin physiological saline was infused till clear efflux from thoracic cavity. Ink had not been equably and slowly infused under pressure of 16.0 kPa into the abdominal aorta until ink exuded from inferior vena cava of thoracic cavity, The perfusion state of the bile duct wall capillaries was closely observed with a dissecting microscope. The liver and extrahepatic bile ducts were removed, and fixed with 10% formaldehyde solution over 72 h.
Tissue dehydration, transparency and embedding: Five hepatohilar bile ducts from both groups were washed with lotic water, dehydrated in graded alcohol, and placed in dimethylbenzene for transparence over 24 h. The distribution of the hepatohilar bile duct wall capillaries was observed using an inverted phase contrast microscope. Another five hepatohilar bile ducts from the two groups were dehydrated in graded alcohol, placed in dimethylbenzene for transparence, embedded in paraffin, and continuously sectioned at a thickness of 20 μm. After adhering on, sections were slightly raised and heated until the paraffin disappeared, then they were incubated at 60°C for 24 h in a thermostat. The sections were again put in dimethylbenzene for transparence over 24 h, during which the transparence liquid was changed three times. After transparence, the sections were mounted in gum, and pictures were collected with Motic 0.65 times light microscope for three-dimensional reconstruction of computer microvisualization.
Three-dimensional reconstruction of computer microvisualization: Firstly, primitive three dimensional data field was constructed by registering the sequence of two-dimensional section images according to feature points of ink perfusion blood vessel on bile duct thick sections, then the wave filter of the primitive three dimensional data was performed with median filter to inhibit noises and to enhance image characteristics, improving the ratio of signal to noise. Lastly the isosurfaces of cube voxel were extracted from three dimensional data field with Marching Cubes algorithm to construct the three-dimensional structure of sequence sections. Three-dimensional reconstruction was completed with PIV 2.53 GHz, 512M RAM microcomputer and algorithm was finished with language C++. The resolution of each section was 686 × 548 × 8 bits.
Pathological changes in hepatohilar bile duct and liver 6 mo after PVA using hematoxylin and eosin (HE) and Masson staining
The rats that underwent ultrasonography were killed 6 mo after PVA. Samples of hepatohilar bile ducts and liver were removed, fixed with 10% neutral formalin for 48-72 h, embedded in paraffin, continuously sectioned at a thickness of 5 μm, and stained with HE and Masson stain.
The steps for Masson staining were as follows. Samples were fixed with neutral formalin, sectioned, deparaffinized in water, stained with Masson composite staining solution for 5 min, washed with 0.2% acetic acid solution, washed with 5% phosphotungstic acid for 5-10 min, washed twice with 0.2% acetic acid solution, stained with bright green staining solution for 5 min, washed twice with 0.2% acetic acid solution, dehydrated in absolute alcohol, put in xylene for transparency, and mounted with neutral gum. Collagen fibers were bluish green in color and red blood cells were orange.
Statistical treatment
Data were expressed as mean ± SD. The t test was used for comparison between the two groups. Statistical analysis was performed with Stata software.
RESULTS
General status of experimental animals
In all rats, normal diet was restored, and fur was shiny. Body weight showed an increasing trend 3 d after operation, and then returned to normal 1 mo after PVA. There was no significant difference in body weight between the two groups at 6 mo after PVA.
Hemodynamic changes of the portal vein after PVA
Spectral changes of the portal vein and the status of vascular anastomosis 6 mo after PVA: In the portal vein, the continuous venous frequency spectrum was changed into a pulsatory arterial frequency spectrum 6 mo after PVA (Figure 2). Six months after PVA, the status of the vascular anastomosis of the portal vein with hepatic artery and vena cava is shown in Figure 3. The hepatic artery and the portal vein were obviously enlarged 6 mo after PVA.
Figure 2.
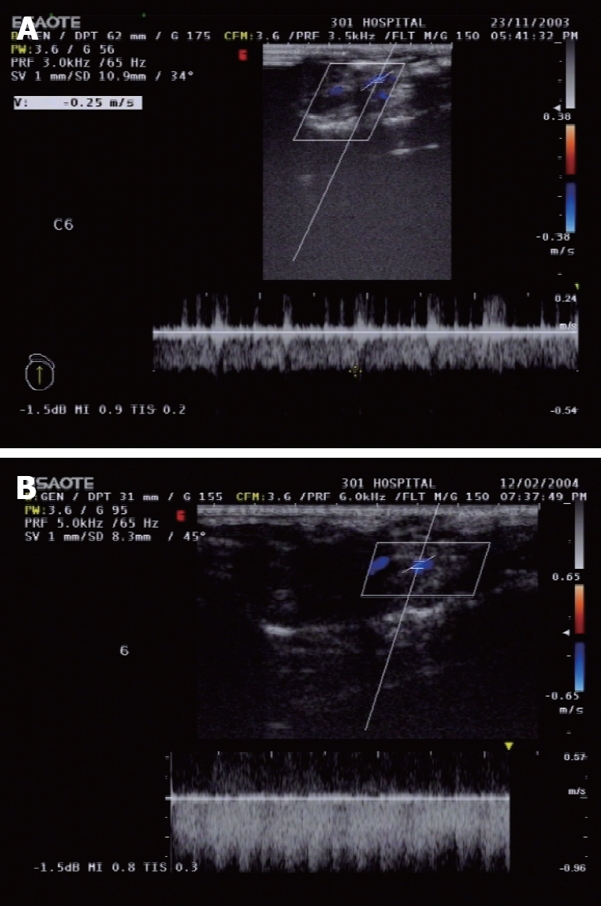
Frequency spectrums in the portal vein. A: Venous frequency spectrums in the portal vein of control group; B: Arterial frequency spectrums in the portal vein 6 mo after PVA.
Figure 3.
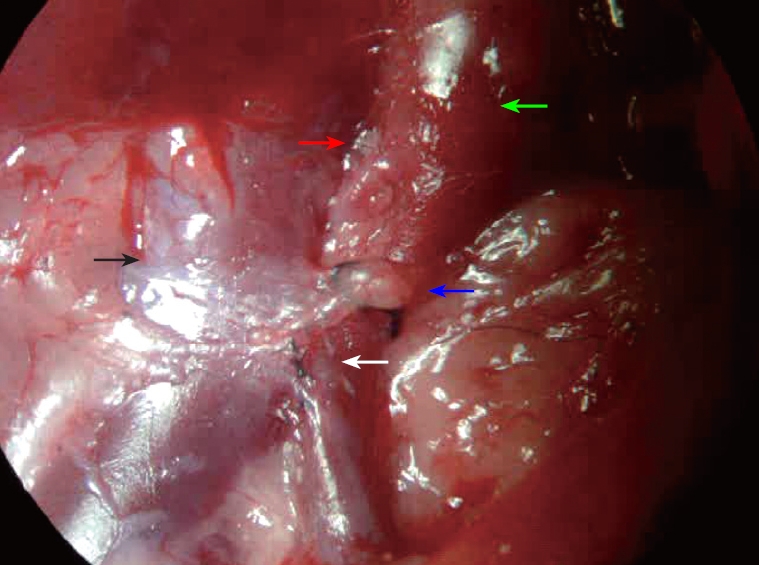
The status of the vascular anastomosis of the portal vein with hepatic artery and vena cava. The red arrow indicates the anastomotic site of the hepatic artery and portal vein. The white arrow indicates the side-to-side anastomotic stoma of the portal vein and the venae cavae. The green arrow indicates thickening of the portal vein. The black arrow indicates the venae cavae. The blue arrow indicates a thread blocking the portal vein.
Cross-sectional area of the portal vein in the two groups after operation: Compared with the control group, the cross-sectional area of the portal vein in the PVA group was significantly increased (P < 0.01) (Table 1).
Table 1.
Comparison of cross section area and volume of blood flow of the portal vein between the two groups (mean ± SD, n = 7)
| Group | 1 mo | 6 mo |
| Cross section area of the portal vein (mm2) | ||
| Control group | 0.0317 ± 0.0098 | 0.0333 ± 0.0103 |
| PVA group | 0.0433 ± 0.0242 | 0.0657 ± 0.015112 |
| Volume of blood flow of the portal vein (mL/min) | ||
| Control group | 37.6 ± 11.36 | 37.72 ± 9.09 |
| PVA group | 74.70 ± 42.76 | 132.01 ± 78.723 |
Compared with control P < 0.01;
Compared with cross section area of the portal vein one month after PVA P < 0.05;
Compared with control group P < 0.01.
Volume of blood flow of the portal vein in the two groups after operation: Compared with the control group, the volume of blood flow in the portal vein 6 mo after PVA was obviously increased (P < 0.01) (Table 1).
Liver functions in rats 6 mo after PVA
Total bilirubin (TBIL), direct bilirubin (DBIL) and total bile acid (TBA) in the PVA group were greater than those in the control group, but there was no significant difference between the two groups (P > 0.05). Serum albumin (ALB) and alkaline phosphatase (AKP) in the PVA group were lower than those in the control group, but there was no significant significance between the two groups (P > 0.05). Glutamic pyruvic transaminase (GPT) in the PVA group was significantly greater than that in control group (P < 0.01) (Table 2).
Table 2.
Liver Functions in the two groups (mean ± SD, n = 8)
| Group | GPT | ALB | TBIL | DBIL | AKP | TBA |
| Control | 62.81 ± 10.98 | 31.46 ± 3.97 | 2.53 ± 1.95 | 1.56 ± 1.49 | 144.43 ± 73.86 | 46.97 ± 27.89 |
| PVA | 178.96 ± 62.801 | 29.71 ± 3.98 | 3.11 ± 0.95 | 2.19 ± 0.45 | 117.76 ± 53.41 | 72.68 ± 35.03 |
Compared with control group P < 0.01.
Morphological changes of hepatohilar capillaries after PVA
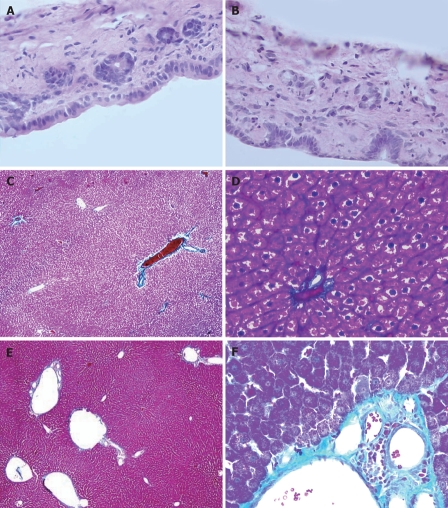
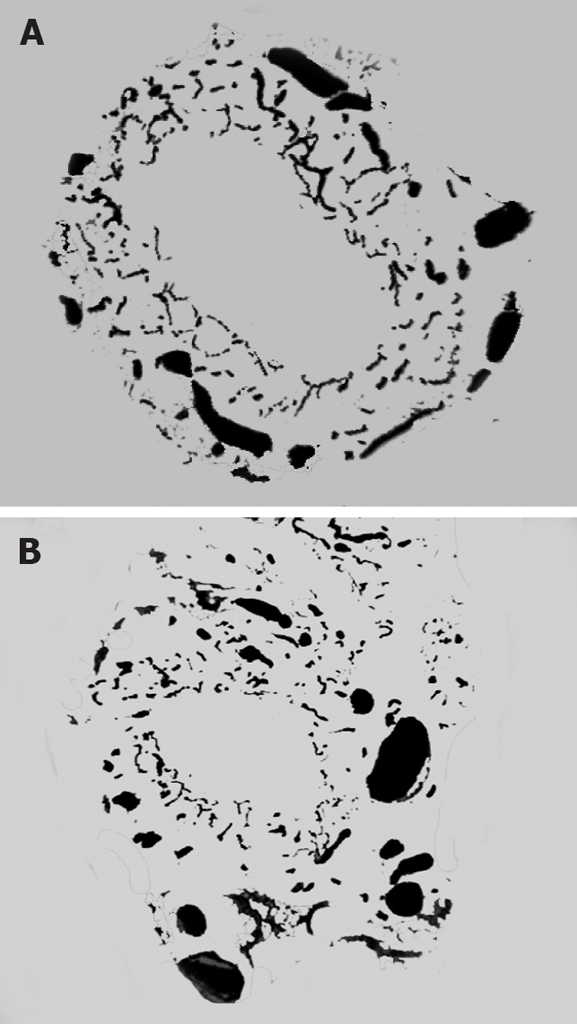
Ink perfusion sections showed that, compared with the control group, the capillaries of the outside layer of the PVP were obviously thickened and dilated, and capillary density of the inside layer was increased, and capillary diameter of the inside layer was enlarged in the PVA group (Figure 4). Ink perfusion gross transparent specimens clearly showed the capillary structure in the forked site of the hepatohilar bile duct in the two groups. Capillaries in the forked site of the bile duct were obviously thickened in the PVA group (Figure 5). Three-dimensional reconstruction clearly showed the PVP stereochemical structure of the hepatohilar bile ducts in the two groups. Peripheral vessels of the PVP were obviously thickened in the PVA group (Figure 6).
Figure 4.
PVP plane structure of hepatohilar bile duct. A: Control group (20 μm, × 100); B: PVA group (20 μm, × 100).
Figure 5.
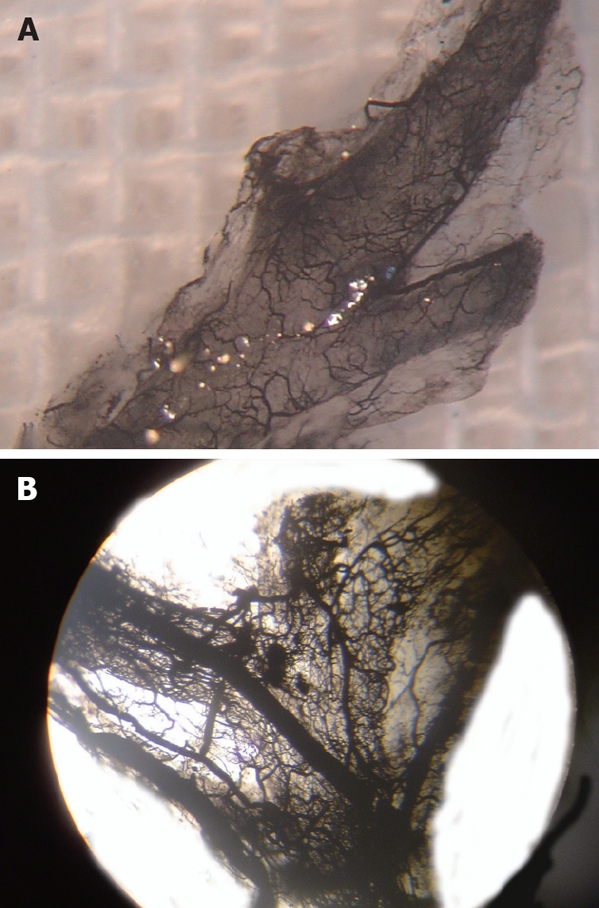
Microvascular distribution in forked site of hepatohilar bile ducts. A: Control group (× 100); B: PVA group (× 100).
Figure 6.
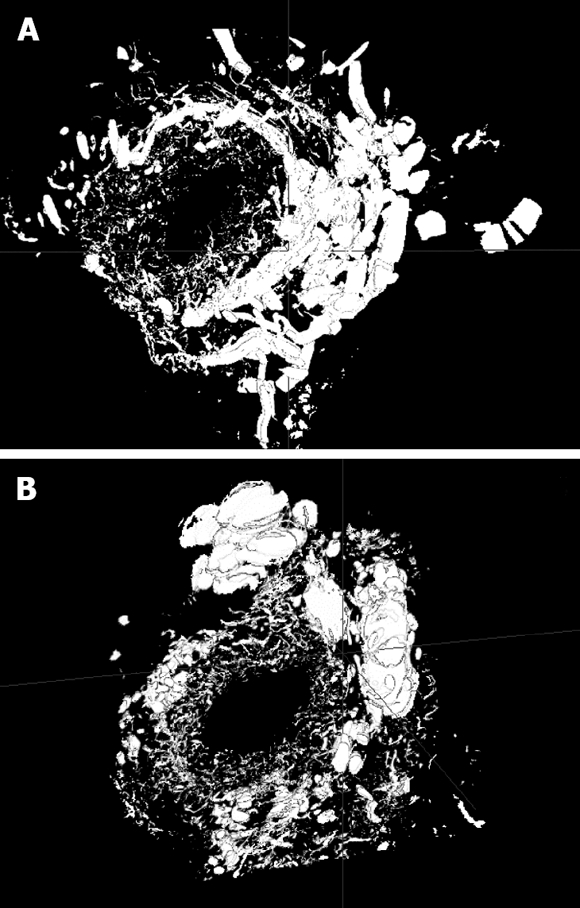
PVP three-dimensional reconstruction of hepatohilar bile ducts. A: Control group (12 × 20 μm); B: PVA group (12 × 20 μm).
Histopathological changes in the hepatohilar bile duct wall
Figure 7A and B shows that, in both groups, the epithelial cell morphology of the bile ducts was normal, the thickness of bile duct was not increased, and there was no inflammatory cell infiltration.
Figure 7.
The pathological changes of hepatohilar bile duct and the liver in rats 6 mo after PVA using HE staining and Masson staining. A: Hepatohilar bile ducts in control group (HE, × 400); B: Hepatohilar bile ducts in PVA group (HE, × 400). C: Masson staining of the liver 6 mo after operation in control group (× 40); D: Masson staining of the liver 6 mo after operation in control group (× 400). E: Masson staining of the liver 6 mo after PVA in PVA group (× 40); F: Masson staining of the liver 6 mo after PVA in PVA group (× 400).
Masson staining of the liver
In the PVA group, Figure 7C-F shows the dilated portal vein and its branches, thickened blood vessel wall, increased collagen fiber and fiber degeneration in the tunica intima. Hepatic lobule structure was intact. Pseudolobule formation was not present. Hepatic sinusoids were widened. The morphology of the hepatic cells was not obviously abnormal.
DISCUSSION
Since the incidence of hepatic encephalopathy is increased because of the decrease in hepatic blood perfusion after portosystemic shunting in portal hypertension, Cohn & Herrod[9] and Fisher et al[10] originally thought that portal vein blood was replaced with arterial blood in hepatic perfusion. Subsequently, many researchers have carried out a large number of animal experiments and confirmed that when the portal vein is perfused with arterial blood at an appropriate flow and pressure, animals are able to maintain normal liver blood flow and levels of blood ammonia and blood colalin, to ensure normal liver detoxification and cell morphology. Since 1960, PVA has attracted extensive attention because of its various clinical applications[11-13], especially in the enlarged radical operation for hepatohilar cancer that invades the hepatic artery and liver transplantation. PVA has been used in reconstructing hepatic vascular access because of the presence of thrombosis of the portal and other veins, or the anatomical variation of the portal and mesenteric veins[14,15]. Morphological changes in PVP after PVA have attracted the attention of many workers. Hepatohilar bile ducts have become a concern because of their unique anatomical factors, including less collateral circulation and easily-damaged blood supply. The purpose of this study was to explore the influence of PVA on the morphology of hepatohilar bile ducts and PVP, epithelial cells of bile ducts, and to provide a theoretical basis for the clinical application of PVA.
Within 6 mo after PVA, rats were well and did not have jaundice. Their body weights showed an increasing trend. If the measure to limit blood flow was not taken within 1 mo after PVA, the cross-sectional area and blood flow of the portal vein were increased, and the increasing trend became more significant over time in a certain range. Hemodynamic changes after PVA will have an influence on hepatic structure. In our study, Masson staining 6 mo after PVA showed that, when measures to limit blood flow were not adopted, high blood flow in the portal vein led to dilation of the intrahepatic portal vein and its branches, increased collagen fibers in the tunica intima, widening of the hepatic sinusoids, and collagen fiber hyperplasia in the sinusoidal walls. However, the structure of hepatic lobule was not obviously disordered and pseudolobule formation was not present. Liver function tests showed that compared with the control group, except for GPT (significant increase P < 0.01), ALB in the PVA group was slightly lower, and bile acid and BIL were slightly increased, but there was no significant difference between the two groups. This demonstrated that PVA had no significant influence on ALB synthesis, bile acid and BIL metabolism and bile salt enterohepatic circulation. The increase in GPT may have been associated with high blood flow after PVA.
The PVP is common in mammals and its structure differs with various parts. It has been reported that the PVP of the large bile ducts consists of three layers of capillaries. The capillaries of the inside layer are arranged in an orderly manner and are open to capillaries beneath the epithelial lining. The middle and outside layer consist of a few capillaries, venules and arterioles in the bile duct wall or in peribiliary tissue[16]. It has been reported that different diseases will lead to different pathological features of the bile ducts, and PVP morphology will also show different pathological changes[17]. Blood flow and oxygen content in the PVP play important roles in ensuring normal physiological function of bile duct epithelial cells.
Ink perfusion thick sections and transparent specimens indicated that after PVA, capillaries of the outside layer of the PVP were obviously thickened, the number of blood vessels in the outside layer was not reduced, and capillary morphology in the hepatohilar bile duct walls was not disordered. This demonstrates that after the PVP loses its arterial blood supply, the blood in the arterialized portal vein can meet fully the requirement of the PVP, by portal vein collateral branch reflux, which may be the theoretical basis for clinical application of PVA. A semi-quantitative study of the PVP with normal portal pressure and portal hypertension has been performed by Terada et al[18] using immunohistochemistry. Results indicated that intrahepatic peribiliary vascular density was obviously increased in congenital portal hypertension and other forms of portal hypertension, because of extrahepatic portal vein thrombus and portal vein cancer embolus, but was only slightly increased in hepatic cirrhosis. PVP density is associated with portal vein pressure. It may be that the blood of the portal vein flows back into the PVP by internal routes, or flows directly into the PVP via arteries, which leads to PVP hyperplasia. This further indicates there are collateral vessels between the portal vein and PVP.
The three-dimensional reconstruction of computer microvisualization realistically illustrated PVP three-dimensional structure. Peribiliary capillaries after PVA were obviously thickened, which may have been caused by the increases in blood flow and blood flow rate of the arterialized portal vein. MoticBuaa3DVol three-dimensional reconstruction software used in this study established a foundation for further research on capillary structure of hepatohilar bile ducts.
To maintain normal physical activities of bile duct tissue, adequate blood flow and a certain degree of blood oxygen content are required. Early study has shown that, since it possesses low blood flow and low oxygen content, the blood in the portal vein cannot completely fill the PVP, and local tissue is in a state of chronic hypoxia. Chronic inflammatory hyperplasia will occur in the hepatohilar bile duct administered by portal vein blood[19]. After PVA, blood flow and blood oxygen content were able to meet the requirements of the local tissues of the bile duct wall, and no ischemic lesion occurred in the bile ducts. HE staining results of this experiment disclosed that after PVA, there was no pathological change in bile duct tissue, which further verified this inference. At the same time, there were no significant differences in BIL and ALP between the two groups, which further confirmed the HE staining results.
In short, blood with a certain flow and oxygen content has important significance for filling the PVP and meeting the oxygen requirement of the bile duct wall. After PVA, it is the anatomic basis to maintain normal morphology of hepatohilar bile duct wall that the blood with high oxygen content and high flow in arterialized portal vein may fill PVP by collateral vessel reflux. The high blood flow in the portal vein after PVA is the main reason for the obvious dilation of the intrahepatic portal vein and collagen fibroplasia. Therefore, certain measures to limit blood flow in PVA are necessary.
COMMENTS
Background
During the enlarged radical operation for hepatohilar cancer that invades the hepatic artery and liver transplantation, the presence of portal vein and internal organ vein thrombosis or the congenital absence of the portal and mesenteric veins often require liver revascularization. For this reason, many researchers have carried out a large number of experiments and have applied portal venous arterialization (PVA) in clinical practice. Blood flow and oxygen content in the peribiliary vascular plexus (PVP) play an important role in ensuring normal physiological functions of bile duct epithelial cells. After PVA, the influence of portal vein hemodynamic changes on hepatohilar PVP has an important effect on the incidence of bile duct complications, and is directly associated with the clinical application of PVA. Therefore, to establish a theoretical basis for clinical application of PVA, we explored the influence of portal vein hemodynamic changes after PVA on PVP morphological structure and hepatic pathological structure.
Research frontiers
Liver revascularization is important in enlarged radical operation and liver transplantation. PVA is applied in clinical practice to resolve this problem, but some complications occur after PVA. In order to make clinical application of PVA better and reduce incidence of complications, it is necessary to study some subjects related to PVA.
Innovations and breakthroughs
In this study, we observed hemodynamic changes in the portal vein, morphological structure of hepatohilar PVP, and bile duct and liver pathology after PVA. We found that after PVA, the cross-sectional area and blood flow of the portal vein were increased and this trend became more significant over time in a certain range. If measures to limit the flow in PVA are not adopted, the high blood flow will lead to dilatation of the intrahepatic portal vein and its branches, and increased collagen and fiber degeneration in the tunica intima. Therefore, it is necessary to limit blood flow in PVA.
Applications
Measures to limit blood flow are likely to reduce the incidence of complications after PVA.
Terminology
PVA: portal venous arterialization; PVP: peribiliary vascular plexus.
Peer review
The paper addresses an important issue in hepatobiliary and liver transplantation practice, particularly in the cases of diffuse mesenteroportal venous thrombosis. In the current study, the authors used a model with a portacaval shunt in conjunction with complete arterialization of the portal inflow of the liver. The results are interesting.
Footnotes
Supported by Science and Technology Plan of Xiamen City, No. 3502Z20064005; and Health Bureau of Xiamen City, No. WSk0521
Peer reviewer: Justin H Nguyen, MD, Division of Transplant Sugery, Mayo Clinic, 4205 Belfort Road, Suite 1100, Jacksonville, Florida 32256, United States
S- Editor Xiao LL L- Editor Kerr C E- Editor Zheng XM
References
- 1.Sheil AG, Halliday JP, Drummond JM, Bookallil MJ, Gaudry PL, Yezerski SD. A modified technique for orthotopic liver transplantation. Arch Surg. 1972;104:720–724. doi: 10.1001/archsurg.1972.04180050094024. [DOI] [PubMed] [Google Scholar]
- 2.Blumhardt G, Ringe B, Lauchart W, Burdelski M, Bechstein WO, Pichlmayr R. Vascular problems in liver transplantation. Transplant Proc. 1987;19:2412. [PubMed] [Google Scholar]
- 3.Erhard J, Lange R, Giebler R, Rauen U, de Groot H, Eigler FW. Arterialization of the portal vein in orthotopic and auxiliary liver transplantation. A report of three cases. Transplantation. 1995;60:877–879. [PubMed] [Google Scholar]
- 4.Tsivian M, Neri F, Prezzi D, Puviani L, Pacile V, Bertelli R, Cavallari G, Mattioli B, Bianchi E, Piras GL, et al. Portal vein arterialization in hepatobiliary surgery and liver transplantation. Transplant Proc. 2007;39:1877–1878. doi: 10.1016/j.transproceed.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 5.Schleimer K, Stippel DL, Kasper HU, Tawadros S, Suer C, Schomäcker K, Hölscher A, Beckurts KT. Auxiliary liver transplantation with flow-regulated portal vein arterialization offers a successful therapeutic option in acute hepatic failure--investigations in heterotopic auxiliary rat liver transplantation. Transpl Int. 2006;19:581–588. doi: 10.1111/j.1432-2277.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Ott R, Böhner C, Müller S, Aigner T, Bussenius-Kammerer M, Yedibela S, Kissler H, Hohenberger W, Reck T, Müller V. Outcome of patients with pre-existing portal vein thrombosis undergoing arterialization of the portal vein during liver transplantation. Transpl Int. 2003;16:15–20. doi: 10.1007/s00147-002-0505-y. [DOI] [PubMed] [Google Scholar]
- 7.Nivatvongs S, Sirijindakul B, Nontasoot B. Portal vein arterialization for liver transplantation with extensive portomesenteric vein thrombosis: a case report. Transplant Proc. 2004;36:2267–2268. doi: 10.1016/j.transproceed.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 8.Takasaki S, Hano H. Three-dimensional observations of the human hepatic artery (Arterial system in the liver) J Hepatol. 2001;34:455–466. doi: 10.1016/s0168-8278(00)00058-1. [DOI] [PubMed] [Google Scholar]
- 9.Cohn R, Herrod C. Some effects upon the liver of complete arterialization of its blood supply. Surgery. 1952;32:214–218. [PubMed] [Google Scholar]
- 10.Fisher B, Russ C, Updegraff H. A suitable technique for total arterialization of the dog liver. Surgery. 1954;35:879–884. [PubMed] [Google Scholar]
- 11.Hulten O. Arterialization of the liver in cirrhosis. Scand J Clin Lab Invest Suppl. 1966;18:43–43. [PubMed] [Google Scholar]
- 12.Maillard JN, Benhamou JP, Rueff B. Arterialization of the liver wh portacaval shunt in the treatment of portal hypertension due to intrahepatic block. Surgery. 1970;67:883–890. [PubMed] [Google Scholar]
- 13.Otte JB, Reynaert M, De Hemptinne B, Geubel A, Carlier M, Jamart J, Lambotte L, Kestens PJ. Arterialization of the portal vein in conjunction with a therapeutic portacaval shunt. Hemodynamic investigations and results in 75 patients. Ann Surg. 1982;196:656–663. doi: 10.1097/00000658-198212001-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashfi A, Mehrabi A, Pahlavan PS, Schemmer P, Gutt CN, Friess H, Gebhard MM, Schmidt J, Büchler MW, Kraus TW. A review of various techniques of orthotopic liver transplantation in the rat. Transplant Proc. 2005;37:185–188. doi: 10.1016/j.transproceed.2004.12.257. [DOI] [PubMed] [Google Scholar]
- 15.Orlando G, De Luca L, Toti L, Zazza S, Angelico M, Casciani CU, Tisone G. Liver transplantation in the presence of portal vein thrombosis: report from a single center. Transplant Proc. 2004;36:199–202. doi: 10.1016/j.transproceed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Nakanuma Y, Matsui O. Intrahepatic peribiliary vascular plexus in various hepatobiliary diseases: a histological survey. Hum Pathol. 1994;25:940–946. doi: 10.1016/0046-8177(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 17.Qian YB, Liu CL, Lo CM, Fan ST. Risk factors for biliary complications after liver transplantation. Arch Surg. 2004;139:1101–1105. doi: 10.1001/archsurg.139.10.1101. [DOI] [PubMed] [Google Scholar]
- 18.Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic large bile ducts in normal livers and portal hypertension. J Gastroenterol Hepatol. 1989;4 Suppl 1:276–278. [PubMed] [Google Scholar]
- 19.Li WG, Hu SX, Xue BD, Jiang ZG, Huang ZQ. Observation of hepatohilar peribiliary vascular plexus with complete absence of hepatic artery blood supply in rats. Transplant Proc. 2007;39:3424–3428. doi: 10.1016/j.transproceed.2007.08.108. [DOI] [PubMed] [Google Scholar]