Abstract
AIM: To evaluate the association between X-ray cross-complementing gene 1 (XRCC1) genetic polymorphism Arg399Gln and gastric cancer risk by means of meta-analysis.
METHODS: We searched PubMed and NCBI up to June 1, 2008. A total of 16 clinical trials and reports were identified, but only 8 trials qualified under our selection criteria. Statistical analysis was performed with the software program Review Manage, version 4.2.8.
RESULTS: Of the 8 case-control studies selected for this meta-analysis, a total of 1334 gastric cancer cases and 2194 controls were included. For Arg399Gln, the Gln/Gln genotype carriers did not have a decreased cancer risk compared with those individuals with the Arg/Arg genotype (OR = 0.92, 95% CI, 0.71-1.19; P = 0.51). Similarly, no associations were found in the recessive and dominant modeling (Gln/Gln vs Arg/Gln + Arg/Arg: OR = 0.96; 95% CI, 0.77-1.19; P = 0.70 and Gln/Gln + Arg/Gln vs Arg/Arg: OR = 0.90, 95% CI, 0.77-1.05; P = 0.18).
CONCLUSION: No association is found between the XRCC1 polymorphism Arg399Gln and gastric cancer risk.
Keywords: Gastric cancer, Gene polymorphism, X-ray cross-complementing gene 1, Meta-analysis
INTRODUCTION
Gastric cancer is a leading cause of death worldwide, with nearly a million new cases diagnosed each year. It is the fourth most common cancer and the second leading cause of cancer death worldwide[1]. Multiple environmental and lifestyle factors may increase the risk of gastric cancer (GC), including tobacco use[2], a diet poor in fresh fruits and vegetables or rich in salt[3], and Helicobacter pylori (H pylori) infection[4]. However, not all of those who have been exposed to the risk factors will develop gastric cancer, suggesting the inter-individual differences in susceptibility. These differences may in part be caused by genetic variation, such as single nucleotide polymorphism (SNPs)[5] in DNA repair gene that increase susceptibility to the DNA damage resulting from carcinogens, particularly when these SNPs are located within the coding or regulating regions causing altered protein expression.
One of the important DNA repair protein is X-ray cross-complementing gene 1 (XRCC1)[6], acting as a scaffolding protein for the base excision repair (BER) and single-strand break repair (SSBR). These overlapping pathways participate in the constitutive response to endogenous mutagens and exogenous exposures, including tobacco smoke. Specifically, XRCC1-mediated pathways repair damage to DNA bases, from oxidation or covalent binding of nonbulky electrophiles, and to the deoxyribose phosphate backbone. Quick resolution of this genetic damage is imperative because repair intermediates, such as abasic sites and SSB, are generally more genotoxic and cytotoxic than the initial lesion. Three common polymorphisms within the XRCC1 gene have been identified at codon 194, 280 and 399 (Arg194Trp, Arg280His, and Arg399Gln)[7]. These nonconservative amino acid changes may alter XRCC1 function. This change in protein biochemistry leads to the supposition that variant alleles may diminish repair kinetics, thereby influencing susceptibility to adverse health effects, including cancer.
Shen et al[8] first reported an association between XRCC1 codon 399 polymorphisms and gastric cancer. Since the publication of this report, many studies have appeared in the literature either supporting or negating the association. To clarify the effect of XRCC1 codon 399 polymorphisms on the risk of gastric cancer, we have undertaken a systematic review and meta-analysis.
MATERIALS AND METHODS
Literature search strategy
We searched in the Medline and Chinese National Knowledge Infrastructure (CNKI), covering all papers published up to June 1, 2008, with a combination of the following keywords: gastric cancer and XRCC1. We evaluated potentially relevant publications by examining their titles and abstracts and procured the most relevant publications for a closer examination. Besides the database search, the reference lists of the selected papers were also screened for other potential articles that may have been missed in the initial search. The search and evaluation were conducted from January to June 2008.
The following criteria were used for the literature selection for the meta-analysis: (1) The articles should be published in either English or Chinese between January 1989 and March 2008; (2) Only the case-control studies were considered; (3) The paper should clearly describe gastric cancer diagnoses and the sources of cases and controls; (4) The authors must offer the size of the sample, odds ratios (ORs) and their 95% confidence intervals (CIs) or the information that can help infer the results in the papers; (5) The definition of the exposure/risk genotypes was similar in all papers; (6) The methods of data collection and analysis should be statistically acceptable; (7) Those publications that presented data allowing such outcomes to be derived were also included.
Accordingly, the following exclusion criteria were also used: (1) The design and the definition of the exposure were obviously different from those of the selected papers; (2) Not offering the source of cases and controls and other essential information; (3) Reviews and repeated literatures were also excluded.
Data extraction
To minimize the bias and to improve the reliability, two reviewers checked all potentially relevant studies independently. Data on the following characteristics were also extracted: the first author, year of publication, journal, study population, number of genotyped cases and controls, and odds ratios and their confidence intervals.
Meta analysis
The strength of the associations between gastric cancer and the XRCC1 polymorphism was estimated by ORs and 95% CI. For the Arg399Gln polymorphism, we first estimated the risk of the variant genotype Gln/Gln and compared with the wild-type Arg/Arg homozygote, and then evaluated the risks of Gln/Gln vs (Arg/Gln + Arg /Arg) and (Arg/Gln + Gln/Gln) vs Arg/Arg, which assumed the recessive and dominant effect of the variant Gln399 allele.
We assessed the departure from the Hardy-Weinberg equilibrium for the control group in each study using the HWE program (http://linkage.rockefeller.edu/ott/linkutil.htm) for goodness of fit. The Peto Mantel-Haenszel fixed effect model or DerSimonian Laird random effect model was selected to summarize the combined OR dependent on the results of heterogeneity test among individual studies. And the heterogeneity was considered significant if P < 0.05. If there was no heterogeneity, fixed effects model was used; otherwise, a random effect model based on the DerSimonian and Laird estimator was used[9]. Inverted funnel plots were used to provide diagnosis of publication biases.
All of the statistical analyses were performed with Review Manager (version 4.2.8, The Cochrane Collaboration).
RESULTS
Literature search and meta-analysis databases
We selected 16 published papers dealing with case-control studies of the polymorphisms .We reviewed all papers in accordance with the criteria defined above, and excluded 8 papers because their study designs were different from others or they did not list data clearly enough for further analysis or repeated literatures. Hence, data were available from 8 case-control studies, including 1334 gastric cancer cases and 2194 controls (Table 1)[8,10-16]. We established a database according to the extracted information from each article. Given in Table 1 are the lists of the publication year, first author, and the number of cases and controls for each XRCC1 codon 399 genotype. Other necessary information is also listed in the forest plots in our meta-analysis. No qualified researches were acquired before 2000, which suggested that the researches between XRCC1 polymorphism and gastric cancer were started very late.
Table 1.
Distribution of XRCC1 genotype among gastric cancer cases and controls included in the meta-analysis
|
Distribution |
XRCC1 condon 399 |
||||||||
| First author | Area | Yr |
Prostate cancer |
Control |
HWE | ||||
| Arg/Gln | Arg/Gln | Gln/Gln | Arg/Gln | Arg/Gln | Gln/Gln | ||||
| Shen et al[8] | China | 2000 | 13 | 83 | 92 | 13 | 59 | 64 | Yes |
| Lee et al[10] | South Korea | 2002 | 110 | 71 | 9 | 94 | 69 | 9 | Yes |
| Ratnasinghe et al[11] | China | 2004 | 49 | 34 | 3 | 192 | 193 | 33 | Yes |
| Duarte et al[12] | Brazil | 2005 | 73 | 67 | 20 | 70 | 57 | 23 | Yes |
| Huang et al[13] | Poland | 2005 | 124 | 121 | 36 | 166 | 179 | 45 | Yes |
| Song et al[14] | China | 2006 | 46 | 48 | 8 | 54 | 44 | 3 | Yes |
| Zhang et al[15] | China | 2006 | 136 | 82 | 18 | 369 | 275 | 64 | Yes |
| Ruzzo et al[16] | Italy | 2007 | 10 | 45 | 36 | 10 | 59 | 50 | Yes |
HWE: Hardy-Weinberg equilibrium.
Meta analysis
The Gln/Gln genotype carriers did not have an increased cancer risk compared with those individuals with the Arg/Arg genotype (OR, 0.92; 95% CI, 0.71-1.19; P = 0.51). Similarly, no associations were found in the recessive and dominant modeling (Gln/Gln vs Arg/Gln + Arg/Arg: OR, 0.96; 95% CI, 0.77-1.19; P = 0.70 and Gln/Gln + Arg/Gln vs Arg/Arg: OR, 0.90; 95% CI, 0.77-1.05; P = 0.18). We did not find any associations between Arg399Gln polymorphism and gastric cancer risk in our study (Figure 1).
Figure 1.
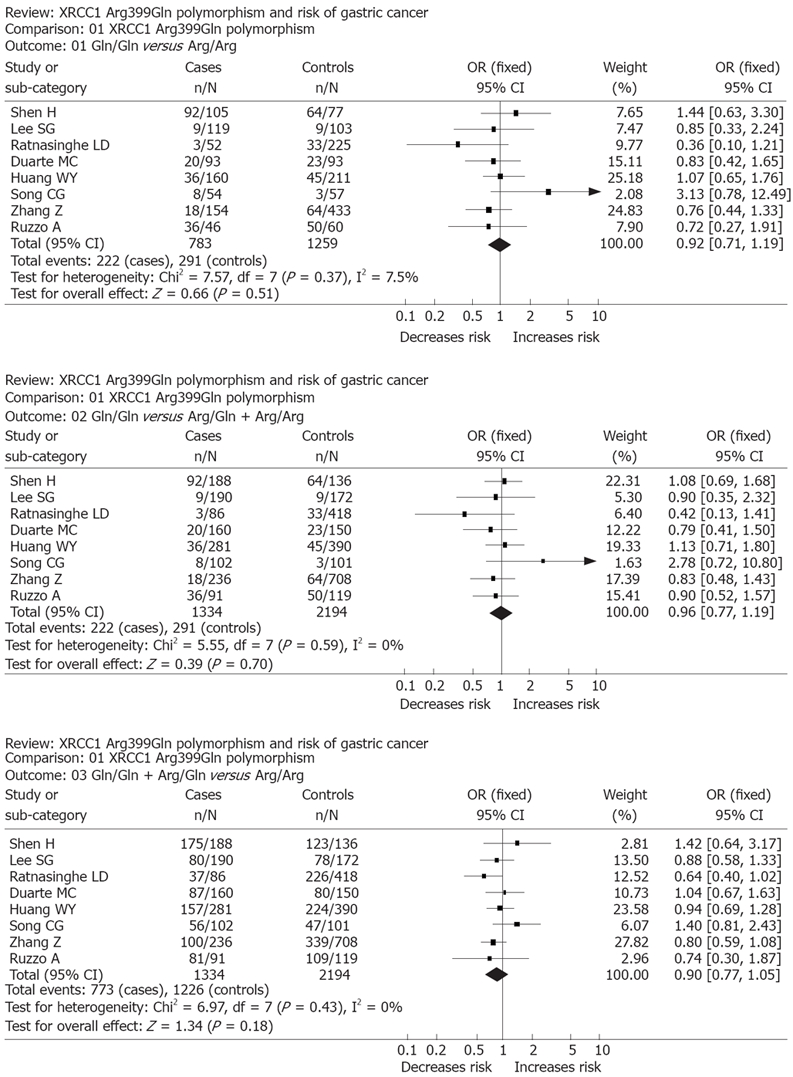
Forest plot of a meta-analysis of the association between XRCC1 polymorphism Arg399Gln and gastric cancer risk (fixed-effects mode).
In all of the studies, the genotype frequencies were consistent with Hardy-Weinberg equilibrium (Table 1). No significant between-study heterogeneities were present in the heterogeneity tests. In the funnel plot analysis of publication biases, the shape of the funnel plot (Figure 2) appeared to be approximately symmetrical, and the magnitude of the main ORs was in dispersion on the left side of 1. Therefore, the funnel plot analysis suggested that publication biases might not have a significant influence on the results of the XRCC1 gene.
Figure 2.
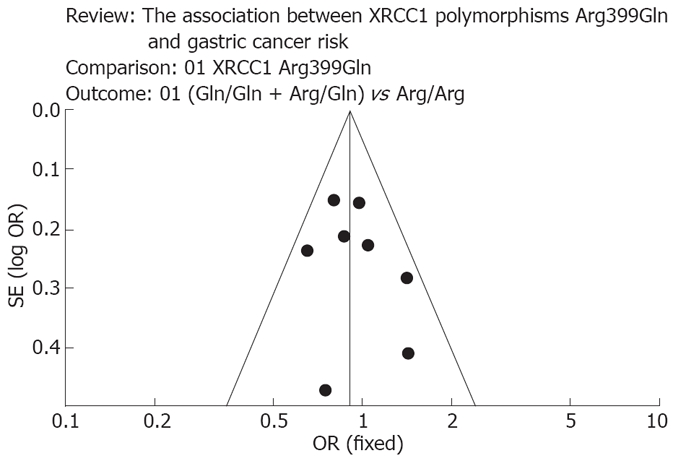
Funnel plot to explore publication bias. The graphical funnel plot of the 8 published studies appears to be asymmetrical.
DISCUSSION
XRCC1 protein is an important component of the BER pathway, which fixes base damage and DNA single strand breaks caused by ionizing radiation and alkylating agents. The XRCC1 protein has no known catalytic activity but serves to orchestrate BER through its role as a central scaffolding protein for DNA ligase III, DNA polymerase β, and PARP, and also through its function in recognizing and binding to single-strand breaks[17]. The Arg399Gln polymorphism[18] is located in the region of the BRCT-I interaction domain of XRCC1 with ADPribosepolymerase, the presence of the variant 399Gln allele has been shown to be associated with measurable reduced DRC as assessed by the persistence of DNA adducts, tumor-suppressor gene P53 mutations, increased red blood cell glycophorin A, elevated levels of sister chromatid exchanges and prolonged cell-cycle delay.
To the best of our knowledge, it is the first systematic review that has investigated the association of XRCC1 codon 399 polymorphisms and gastric cancer, and no evidence has shown any associations between Arg399Gln polymorphism and gastric cancer susceptibility. One possible explanation is that human cells have five different DNA repair systems, characterized by the involvement of more than 150 proteins coded by genes, the majority of which are polymorphic. Thus, given that inter-individual variation in DNA repair capacity is in part explained by SNPs in repair genes, it is possible that the analyzed variants do not influence the basal level of damage. Nevertheless, based on our study, it is worth noting that, even if a common variant in the functional region of a definitively meaningful gene had an effect on human disease, such as cancer, it may play only a minor role in the disease causation.
Although the relationship is inconsistent in bladder cancer[19], other previous studies have found that the XRCC1 399Gln/Gln genotype is associated with an increased risk of lung cancer among Asians[20] and breast cancer in African Americans[21]. Our present and previous studies were similar in most aspects, and the reasons for this apparent difference in risk with different tumors are as yet unknown, possibly due to the overall heterogeneity in the studies that find strong positive or inverse associations. Many meta-analyses of several gene-disease associations have shown that initially promising associations often gravitate toward null over time[22].
In our study, no significant between-study hetero-geneities were present in the heterogeneity tests, indicating that our present combined analyses were unbiased, and no obvious publication bias existed in our meta-analysis, since the ‘‘funnel plot’’ was symmetrically plotted. However, serious limitations were still inherited from the published studies. First, our assessment was based on relatively few studies, and because the papers included in our meta-analysis were limited to those published in either English or Chinese only in the periods between 1989 and 2008, it is possible that some relevant published studies and unpublished studies that are likely to have null results were not included, which may have biased the results. Therefore, although the test for publication bias was not statistically significant, possible bias, especially the outcome-reporting bias, still could not be ruled out. Second, most studies selected case subjects from Asian populations; therefore, we did not conduct a subgroup analysis according to different ethnic groups. The other studies were from Brazil, Poland and Italy, which have different patterns of lifestyle risk factors, genetic backgrounds, and gastric cancer risks. Third, we did not test for gene x environment interactions because of the issue of multiple testing and the lack of sufficient studies. Nevertheless, it is possible that these polymorphisms alter risk in subgroups of the population that have been exposed to specific environmental and lifestyle factors. Therefore, larger and well-designed studies are needed to further evaluate the association between XRCC1 polymorphism and gastric cancer risk.
In summary, our meta-analysis evaluated the relationship between genetic polymorphisms and gastric cancer risk and revealed that XRCC1 polymorphism Arg399Gln could not alter susceptibility to gastric cancer.
COMMENTS
Background
A G to A transition of the X-ray cross-complementing gene 1 (XRCC1) gene at codon 399 has been implicated as a risk factor for gastric cancer, but individual studies have been inconclusive or controversial. The aim of this meta-analysis was to clarify the effect of XRCC1 Arg399Gln polymorphism on the risk of gastric cancer.
Research frontiers
To date, there have been many studies on the association between XRCC1 genetic polymorphism Arg399Gln and gastric cancer risk, but no meta-analyses.
Innovations and breakthroughs
XRCC1 polymorphism Arg399Gln could not alter susceptibility to gastric cancer. Further studies are needed to prove it.
Applications
It can be seen from this paper that XRCC1 polymorphism Arg399Gln could not alter susceptibility to gastric cancer. It suggests that, even if a common variant in the functional region of a definitively meaningful gene had an effect on human disease, such as cancer, it may play only a minor role in the disease causation.
Peer review
The study is nicely designed and the analytical data appear to be scientifically sound. The outcome clearly shows that XRCC1 polymorphism Arg399Gln could not alter susceptibility to gastric cancer.
Footnotes
Peer reviewers: Dr. Mark S Pearce, Pediatric and Lifecourse Epidemiology Research Group, School of Clinical Medical Sciences, University of Newcastle, Sir James Spence Institute, Royal Victoria Infirmary, Newcastle upon Tyne, NE1 4LP, United Kingdom; Yoshiharu Motoo, MD, PhD, FACP, FACG, Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Zhong XY L- Editor Ma JY E- Editor Yin DH
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281; quiz 313-314. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 4.Masuda G, Tokunaga A, Shirakawa T, Togashi A, Kiyama T, Kato S, Matsukura N, Bou H, Watanabe M, Tajiri T. Helicobacter pylori infection, but not genetic polymorphism of CYP2E1, is highly prevalent in gastric cancer patients younger than 40 years. Gastric Cancer. 2007;10:98–103. doi: 10.1007/s10120-007-0414-y. [DOI] [PubMed] [Google Scholar]
- 5.Zienolddiny S, Campa D, Lind H, Ryberg D, Skaug V, Stangeland L, Phillips DH, Canzian F, Haugen A. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis. 2006;27:560–567. doi: 10.1093/carcin/bgi232. [DOI] [PubMed] [Google Scholar]
- 6.Caldecott KW, Aoufouchi S, Johnson P, Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular 'nick-sensor' in vitro. Nucleic Acids Res. 1996;24:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell. 2001;104:107–117. doi: 10.1016/s0092-8674(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 8.Shen H, Xu Y, Qian Y, Yu R, Qin Y, Zhou L, Wang X, Spitz MR, Wei Q. Polymorphisms of the DNA repair gene XRCC1 and risk of gastric cancer in a Chinese population. Int J Cancer. 2000;88:601–606. doi: 10.1002/1097-0215(20001115)88:4<601::aid-ijc13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee SG, Kim B, Choi J, Kim C, Lee I, Song K. Genetic polymorphisms of XRCC1 and risk of gastric cancer. Cancer Lett. 2002;187:53–60. doi: 10.1016/s0304-3835(02)00381-6. [DOI] [PubMed] [Google Scholar]
- 11.Ratnasinghe LD, Abnet C, Qiao YL, Modali R, Stolzenberg-Solomon R, Dong ZW, Dawsey SM, Mark SD, Taylor PR. Polymorphisms of XRCC1 and risk of esophageal and gastric cardia cancer. Cancer Lett. 2004;216:157–164. doi: 10.1016/j.canlet.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Duarte MC, Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, Silva AE. Polymorphisms of DNA repair genes XRCC1 and XRCC3, interaction with environmental exposure and risk of chronic gastritis and gastric cancer. World J Gastroenterol. 2005;11:6593–6600. doi: 10.3748/wjg.v11.i42.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang WY, Chow WH, Rothman N, Lissowska J, Llaca V, Yeager M, Zatonski W, Hayes RB. Selected DNA repair polymorphisms and gastric cancer in Poland. Carcinogenesis. 2005;26:1354–1359. doi: 10.1093/carcin/bgi084. [DOI] [PubMed] [Google Scholar]
- 14.Song CG, Lu HS, Huang CM, Liu X, Hou PF, Zhang XF. Relationship between gene polymorphism of XRCC1 Arg399Gln and the risk of gastric cancer patients in Fujian. Zhonghua Shiyan Waike Zazhi. 2006;23:1021. [Google Scholar]
- 15.Zhang Z, Miao XP, Tan W, Guo YL, Zhang XM, Lin DX. [Correlation of genetic polymorphisms in DNA repair genes ADPRT and XRCC1 to risk of gastric cancer] Ai Zheng. 2006;25:7–10. [PubMed] [Google Scholar]
- 16.Ruzzo A, Canestrari E, Maltese P, Pizzagalli F, Graziano F, Santini D, Catalano V, Ficarelli R, Mari D, Bisonni R, et al. Polymorphisms in genes involved in DNA repair and metabolism of xenobiotics in individual susceptibility to sporadic diffuse gastric cancer. Clin Chem Lab Med. 2007;45:822–828. doi: 10.1515/CCLM.2007.143. [DOI] [PubMed] [Google Scholar]
- 17.Marintchev A, Mullen MA, Maciejewski MW, Pan B, Gryk MR, Mullen GP. Solution structure of the single-strand break repair protein XRCC1 N-terminal domain. Nat Struct Biol. 1999;6:884–893. doi: 10.1038/12347. [DOI] [PubMed] [Google Scholar]
- 18.Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res. 1999;59:2557–2561. [PubMed] [Google Scholar]
- 19.Wang C, Sun Y, Han R. XRCC1 genetic polymorphisms and bladder cancer susceptibility: a meta-analysis. Urology. 2008;72:869–872. doi: 10.1016/j.urology.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 20.Kiyohara C, Takayama K, Nakanishi Y. Association of genetic polymorphisms in the base excision repair pathway with lung cancer risk: a meta-analysis. Lung Cancer. 2006;54:267–283. doi: 10.1016/j.lungcan.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Duell EJ, Millikan RC, Pittman GS, Winkel S, Lunn RM, Tse CK, Eaton A, Mohrenweiser HW, Newman B, Bell DA. Polymorphisms in the DNA repair gene XRCC1 and breast cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:217–222. [PubMed] [Google Scholar]
- 22.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]


