Abstract
AIM: To compare the liver stiffness (LS) measurement by transient elastography (TE) to the liver biopsy (LB)-considered the “gold standard” in the evaluation of patients with chronic hepatitis C.
METHODS: During a period of 12 mo, we evaluated 199 consecutive patients with chronic hepatitis due to hepatitis C virus (HCV), in which LB and LS assessments (by means of TE) were performed during the same session.
RESULTS: Out of 199 patients, a valid measurement of the LS could not be obtained in 8. The mean value of LS in the cohort of 191 valid measurements was 8.45 ± 4.96 kPa, ranging from 2.3 to 38 kPa.The mean value of LS in patients with significant fibrosis at biopsy (161 patients with F ≥ 2 according to Metavir) was 9.02 ± 5.15 kPa, significantly higher than in patients with no or mild fibrosis (30 patients with F < 2 Metavir): 5.39 ± 1.81 kPa (P < 0.0001). For a cut-off value of 6.8 kPa, the LS had a PPV of 98%, a NPV of 30.1%, a sensitivity of 59.6% and a specificity of 93.3% for the presence of significant fibrosis (at least F2 Metavir), with a diagnostic performance of 77.3% (AUROC 0.773). Using this cut-off value, we reached the best discrimination between absence of fibrosis/mild fibrosis (F < 2 Metavir) and the presence of moderate to severe fibrosis (F ≥ 2 Metavir).
CONCLUSION: In patients with chronic hepatitis due to HCV, a cut-off value of 6.8 kPa measured by TE can differentiate between significant fibrosis and absent or mild fibrosis, with a PPV of 98%, a NPV of 30.1%, a sensitivity of 59.6%, a specificity of 93.3%, and a diagnostic performance of 77.3%.
Keywords: Liver stiffness, Transient elastography, Liver biopsy, Chronic C hepatitis, FibroScan
INTRODUCTION
In the evolution of chronic viral and non-viral hepatitis, liver fibrosis is a very important factor associated with prognosis. Hence, it is necessary to evaluate precisely the severity of fibrosis in those patients, in order to perform a correct staging and, eventually, to take a decision regarding the treatment.
Currently, the biopsy examination of the liver is considered the optimal method to evaluate changes in fibrosis over time[1]. Nevertheless, the liver biopsy (LB) has its shortcomings: the intra- and interobserver variability[2,3], the sampling variability (as proven in a study by Ratziu et al[4]), and, last but not least, the fact that LB is an invasive method, with morbidity and mortality greater than zero.
Based on these premises, noninvasive methods for the evaluation of liver fibrosis have been developed during the last few years in order to replace the LB. The most promising noninvasive methods are, currently, the FibroTest-ActiTest[5] and the transient elastography (TE) measured with the FibroScan device[6,7].
TE is an ultrasound-based method. By using an ultrasound transducer probe mounted on the axis of a vibrator, the transmission of low-frequency vibrations from the right intercostal space creates an elastic shear wave that propagates into the liver. A pulse-echo ultrasound acquisition is then used to detect the velocity of wave propagation. This velocity is proportional to the liver stiffness, with faster wave progression occurring through stiffer tissues. Measurement of liver stiffness (LS) is then performed and measured in kPa[6].
The FibroScan assessment of LS was validated as method of evaluation in chronic hepatitis C. Also, there are some articles that proved the value of this method in other chronic hepatopathies, like chronic hepatitis due to the hepatitis B virus (HBV) infection, hemochromatosis, primary billiary cirrhosis or non-alcoholic steato-hepatitis[8-11].
MATERIALS AND METHODS
During a 12 mo period (June, 2007 to June, 2008), all patients with chronic hepatitis C from the Departments of Gastroenterology and Hepatology and Infectious Diseases of Timisoara were investigated in the same session by means of two methods: LS measurement by means of TE and LB, in order to compare the value of that noninvasive method for the assessment of liver fibrosis. Patients with proven liver cirrhosis (ascites, jaundice, esophageal varices) were excluded from the study, as were patients who had another cause of chronic hepatitis, apart from hepatitis C virus (HCV) (HBV, alcoholic hepatitis, etc).
The LS was evaluated by means of TE with a FibroScan device (Echosens-Paris, France) by three experienced physicians. Measurements were performed in the right lobe of the liver through the intercostal spaces while the patients were lying in dorsal decubitus position with the right arm in maximal abduction. The tip of the transducer was covered with coupling gel and placed on the skin, between the ribs, aiming at the right lobe of the liver. The operator, assisted by ultrasound A-mode images provided by the system, located a portion of the liver that was at least 6 cm thick, free of large vascular structures. Once the area of measurement had been located, the operator pressed the probe button to begin an acquisition. Acquisitions that did not have a correct vibration shape or a correct follow-up of the vibration propagation were automatically rejected by the software.
Ten successful acquisitions were performed on each patient. The success rate was calculated as the ratio of the number of successful acquisitions over the total number of acquisitions. The median value of the 10 valid measurements was then calculated by the device and considered to be the value of LS in that patient.
Only patients in which LS measurements had a success rate of at least 60%, with interquartile range (IQR) of all validated measurements less than 30% of the median values (IQR < 30%), were considered for further analysis.
We performed echoguided LB, with Menghini type modified needles, 1.4 and 1.6 mm in diameter. Only LB fragments of at least 2 cm, including at least 8 portal tracts were considered adequate for pathological interpretation. The LB was assessed according to the Metavir score by a senior pathologist. Fibrosis was staged on a 0-4 scale: F0-no fibrosis; F1-portal fibrosis without septa; F2-portal fibrosis and few septa extending into lobules; F3-numerous septa extending to adjacent portal tracts or terminal hepatic venules and F4-cirrhosis
The statistical analysis was performed using Microsoft Excel and GraphPad Prism programs. For the statistical study of quantitative variables, the mean and standard variation were calculated. The diagnostic performance of LS measurements was assessed by using receiver operating characteristics (ROCs) curves. Connected with any cutoff value is the probability of a true positive (sensitivity, Se) and the probability of a true negative (specificity, Sp). The ROC curve is a plot of Se vs 1-Sp for all possible cutoff values. The most commonly used accuracy index is the area under the ROC curve, when values close to 1.0 indicate high diagnostic accuracy. ROC curves were thus built for the detection of significant fibrosis (F ≥ 2 Metavir) and severe fibrosis (F ≥ 3 Metavir). Optimal cutoff values for LS measurements were chosen to maximize the sum of Se and Sp.
RESULTS
The study group included 199 patients with chronic hepatitis C, 138 women and 61 men, with a mean age of 49.79 ± 10.98 years. In 8 cases we could not obtain valid measurements, as we did in the remaining 191 patients (96%).
The mean value of LS in the subgroup of 191 patients with valid measurements was 8.45 ± 4.96 kPa, ranging from 2.3 to 38 kPa.
We divided patients according to the degree of fibrosis, i.e. into a subgroup with significant fibrosis (F ≥ 2 Metavir, patients who should receive antiviral therapy) and another one with no or mild fibrosis (F < 2 Metavir, in which antiviral treatment is currently not recommended).
The mean value of LS in patients with significant fibrosis (161 patients with F ≥ 2 Metavir) was 9.02 ± 5.15 kPa, significantly higher than in patients with no or mild fibrosis (30 patients with F < 2 Metavir): 5.39 ± 1.81 kPa (P < 0.0001) (Figure 1A).
Figure 1.
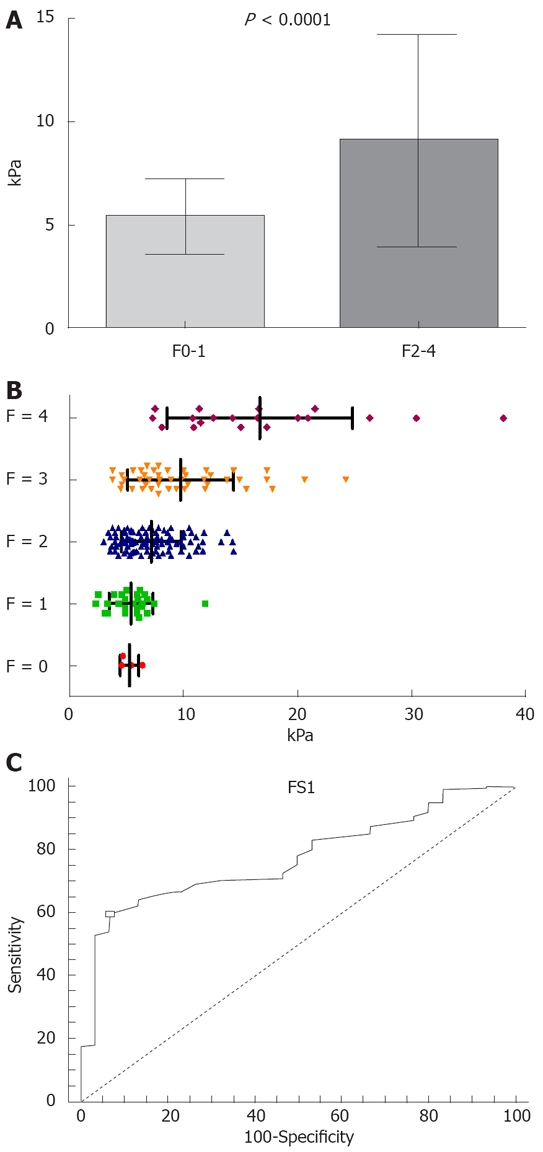
The mean value of LS and AUROC for LS predictive value. A: Mean value of LS in patients with significant fibrosis (F ≥ 2 Metavir) as compared to those with no or mild fibrosis (F < 2 Metavir); B: Mean value of LS according to fibrosis; C: The AUROC for LS predictive value of the presence of significant fibrosis (at least F2 Metavir).
The values of LS in various subgroups of patients, divided according to fibrosis stage, were (Figure 1B): 5.27 ± 0.83 kPa in 4 patients with F0; 5.41 ± 1.92 kPa in 26 cases with F1; 7.18 ± 2.62 kPa in 97 patients with F2; 9.75 ± 4.63 kPa in 45 cases with F3; and 16.68 ± 8.10 kPa in 19 cases with F4.
The statistical significance of the differences between the LS in these subgroups was: F0 vs F1 P = 0.8081; F0 vs F2 P = 0.1117; F0 vs F3 P < 0.0001; F0 vs F4 P < 0.0001; F1 vs F2 P = 0.0048; F1 vs F3 P < 0.0001; F1 vs F4 P < 0.0001; F2 vs F3 P = 0.0011; F2 vs F4 P < 0.0001; F3 vs F4 P = 0.0002.
The statistical analysis of these subgroups showed that there were no significant differences between the mean values of LS in the F0 vs F1 subgroup (so that the cases with mild and no fibrosis cannot be differentiated by means of FibroScan evaluation of LS). The comparison of the mean values of LS between the other subgroups showed significant (S) or very significant (ES) differences.
The mean value of LS in patients with severe fibrosis (64 patients with F ≥ 3 Metavir) was 11.81 ± 6.62 kPa, significantly higher than in patients with at most moderate fibrosis (127 patients with F < 3 Metavir) 6.76 ± 2.56 kPa (P < 0.0001).
We tried to establish the value of LS measured by means of FibroScan, which best predicts the presence of significant fibrosis (F ≥ 2 Metavir) in patients with chronic hepatitis C.
For a cut-off value of 6.8 kPa, the LS had a PPV of 98%, a NPV of 30.1%, a sensitivity of 59.6%, a specificity of 93.3% for the presence of significant fibrosis (at least F2 Metavir), and a diagnostic performance of 77.3% (AUROC 0.773) (Figure 1C).
For a cut-off value of 7.2 kPa, the PPV was 97.8%, the NPV was 28.6%, the sensitivity was 56.5% and the specificity was 93.3% for the presence of significant fibrosis (at least F2 Metavir).
For a cut-off of value of 7.5 kPa, the PPV was 98.8%, the NPV was 27.4%, the sensitivity was 52.2% and the specificity was 96.7%.
DISCUSSION
We compared our results with data from the literature (Table 1). In a multicentric study coordinated by Beaugrand[12] on 494 patients chronically infected with HCV, who were evaluated in the same session by means of percutaneous LB and valid FibroScan, a significant correlation was found (r = 0.57, P < 0.0001) between the LS and histological fibrosis, with AUROC [confidence interval (CI) 95%] 0.84, 0.93 and 0.96 for F ≥ 2, F ≥ 3 and F = 4, respectively. This study tried to establish cut-off values in order to differentiate among various histological stages. Thus, a cut-off value of 7.5 kPa differentiates F01/F234 with a sensitivity of 67%, a specificity of 87%, a PPV of 86% and a NPV of 68%, with 76% diagnostic accuracy.
Table 1.
Cut-off values for significant fibrosis (at least F2 Metavir) in various studies
In a study performed by Foucher et al[13] that compared LB with LS evaluation by means of FibroScan in 354 patients with chronic hepatitis C, the cut-off value was 7.2 kPa, for moderate and severe fibrosis (F234), 12.5 kPa for F34 and 17.6 kPa for cirrhosis.
In a Korean study[14], the cut-off values were 7.3 kPa for F ≥ 2, 8.8 kPa for F ≥ 3 and 15.1 kPa for F = 4. In a study performed in the Netherlands[15], using a cut-off value of 7.1 kPa, 88% of the patients who did not have significant fibrosis (F < 2 Metavir) were correctly identified.
In a study performed in Romania[16], on 324 consecutive patients chronically infected with HCV, evaluated both by TE and LB in the same session, the LS values were strongly correlated with fibrosis (r = 0.759, P < 0.0005), but also with steatosis (r = 0.255, P < 0.005), necroinflammatory activity (r = 0.378, P < 0.0005) and hepatic iron deposition (r = 0.143, P = 0.03). The univariable regression analysis of this study demonstrated that fibrosis (R2 = 0.610, P < 0.0005), activity (R2 = 0.145, P < 0.0005) and steatosis (R2 = 0.037, P < 0.002) were correlated with LS. In multiple regression analysis, all three variable independently influenced LS: fibrosis (P < 0.0005), activity (P = 0.039) and steatosis (P = 0.025). The conclusions of this study showed that fibrosis is the main predictor of LS, but also that the latter bis influenced by activity and steatosis.
In a study performed by Coco and co-workers[17], the value of LS was evaluated considering the level of aminotransferases. The LS dynamics profiles paralleled those of ALT, increasing 1.3 to 3 fold during ALT flares. This study showed that the LS remained unchanged in patients with stable biochemical activity.
All these studies, coming from different regions of the world, completed previous studies from France[18-22], demonstrating the value of non-invasive, FibroScan evaluation of LS for the fibrosis assessment in chronic hepatitis C. This method is not accurate enough to differentiate among contiguous stages of fibrosis (especially 0, 1 and 2), but is sensitive enough to differentiate between the absence and/or mild fibrosis from significant fibrosis, an essential step for the decision regarding treatment. In the future, we shall still find exactly whether histological activity, steatosis or biological activity (ALT) have an important role in the assessment of LS by means of FibroScan.
Considering the data presented from our study, the cut-off value of 6.8 kPa is the most accurate for the discrimination between absence or mild fibrosis (F < 2 Metavir) and the presence of moderate and severe fibrosis (F ≥ 2 Metavir), thus allowing us to select the patients with chronic hepatitis C who should be treated (knowing that patients with F < 2 Metavir are not candidates for treatment, since they have mild disease). In our study, using a cut-off value of 6.8 kPa, the sum of sensitivity and specificity was the highest.
Based on our results, we may use the FibroScan to evaluate LS in patients with chronic hepatitis C prior therapy. By using a cut-off value of 6.8 kPa, we can differentiate patients who needed to be treated (F ≥2 Metavir) from those who, according to most current guidelines, should not receive treatment (F < 2 Metavir).
The cut-off value of 6.8 kPa in our study had a PPV of 98%, meaning that we can identify quite accurately the patients who should be treated (F ≥ 2 Metavir). For patients with values of LS smaller than 6.8 kPa, we think appropriate to perform LB, because the NVP is low (30.1%), and otherwise we could miss patients with significant fibrosis using only TE. In our cohort of 191 patients with valid measurements of LS, using a cut-off value of 6.8 kPa, we could avoid LB in 160 patients who had a LS greater than 6.8 kPa and who could directly receive treatment, without LB. On the other hand, a LB should have been performed only in 31 patients (a 83.8% reduction in the number of LB performed in our Department during this same interval of time).
Considering the data from the literature and looking to our own, we could use the TE evaluation of LS in patients with chronic hepatitis C in order to decide about therapy. All studies show that, by using a cut-off value of 6.8-7.5 kPa, we could identify accurately enough the patients who need to be treated (F ≥ 2 Metavir) vs those who should not be treated (F < 2 Metavir), and this without performing a LB[13-15]. The PPV of 98% obtained in our study for a cut-off value of 6.8 kPa suggests that we can quite accurately identify patients who must be treated (F ≥ 2 Metavir). For patients with LS smaller than 6.8 kPa, we think appropriate to perform a LB, because the NPV is low (30.1%) and therefore we may miss patients with significant fibrosis if only based on LS measurement.
CONCLUSION
The FibroScan evaluation of LS is a method in which valid measurements can be obtained in the great majority of scanned patients (96% of the cases). In patients with chronic hepatitis C, when compared to the LB considered to be the “gold standard”, LS measurement by means of TE can differentiate between significant fibrosis and absent or mild fibrosis. We found that a cut-off value of 6.8 kPa is the one that best differentiates absence or mild fibrosis (F < 2 Metavir) from significant fibrosis (F ≥ 2 Metavir), with PPV of 98%, NPV of 30.1%, sensitivity of 59.6%, specificity of 93.3% and a diagnostic performance of 77.3%. In our cohort of patients, if the cut-off value of 6.8 kPa had been used for the presence of significant fibrosis, more than 80% of the LB would have been avoided.
Considering all these facts, the FibroScan evaluation of LS is a useful non-invasive method for the evaluation of patients with chronic hepatitis C in clinical practice, and can replace in many cases the LB for the decision of therapy.
COMMENTS
Background
In the evolution of chronic viral and non-viral hepatitis, liver fibrosis is a very important factor associated with prognosis. Hence, it is necessary to evaluate precisely the severity of fibrosis in those patients, in order to perform a correct staging and, eventually, to take a decision regarding the treatment. Fibrosis can be assessed through invasive [liver biopsy (LB)] and non-invasive methods such as transient elastography (TE) assessment of liver stiffness (LS), developed in the last few years in order to replace LB.
Research frontiers
The aim of the study was to assess the value of LS measurement by TE as compared to the LB-considered to be the “gold standard”, in the evaluation of patients with chronic hepatitis C.
Innovations and breakthroughs
This study concluded that LS measurement by TE can predict the presence of significant fibrosis (at least F2 Metavir, that requires treatment) in patients with chronic hepatitis C, confirming previously published studies.
Applications
Transient evaluation of LS could replace in many cases the LB for the decision of therapy in patients with chronic hepatitis C, if the values of LS are higher than the established cut-off values for significant fibrosis (6.8-7.5 kPa according to various studies). The LB should be performed only if the LS is lower than the cut-off, in order to avoid “missing” patients with significant fibrosis.
Terminology
TE is an ultrasound-based method that assesses the LS as a marker of fibrosis. By using an ultrasound transducer probe mounted on the axis of a vibrator, the transmission of low-frequency vibrations from the right intercostal space creates an elastic shear wave that propagates into the liver. A pulse-echo ultrasound acquisition then is used to detect the velocity of wave propagation. This velocity is proportional to the tissue stiffness, with faster wave progression occurring through stiffer material. The stiffer the liver, the higher the degree of fibrosis.
Peer review
This paper investigated the usefulness of elastography in assessing the stage of biopsy-proven fibrosis in patients with chronic hepatitis C. This is a straightforward study and the authors concluded that this new technique can predict the presence of severe fibrosis that require treatment.
Footnotes
Peer reviewer: Raymund Rabe Razonable, PhD, Division of Infectious Diseases, Mayo Clinic Institution, 200 First Street SW, Rochester, MN 55905, United States
S- Editor Li DL L- Editor Negro F E- Editor Yin DH
References
- 1.McHutchison J, Poynard T, Afdhal N. Fibrosis as an end point for clinical trials in liver disease: a report of the international fibrosis group. Clin Gastroenterol Hepatol. 2006;4:1214–1220. doi: 10.1016/j.cgh.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 3.Persico M, Palmentieri B, Vecchione R, Torella R, de SI. Diagnosis of chronic liver disease: reproducibility and validation of liver biopsy. Am J Gastroenterol. 2002;97:491–492. doi: 10.1111/j.1572-0241.2002.05507.x. [DOI] [PubMed] [Google Scholar]
- 4.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 5.Munteanu M. Non-invasive biomarkers FibroTest-ActiTest for replacing invasive liver biopsy: the need for change and action. J Gastrointestin Liver Dis. 2007;16:173–174. [PubMed] [Google Scholar]
- 6.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8–14. doi: 10.1053/j.gastro.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa E, Furusyo N, Toyoda K, Takeoka H, Otaguro S, Hamada M, Murata M, Sawayama Y, Hayashi J. Transient elastography for patients with chronic hepatitis B and C virus infection: Non-invasive, quantitative assessment of liver fibrosis. Hepatol Res. 2007;37:1002–1010. doi: 10.1111/j.1872-034X.2007.00160.x. [DOI] [PubMed] [Google Scholar]
- 9.Adhoute X, Foucher J, Laharie D, Terrebonne E, Vergniol J, Castéra L, Lovato B, Chanteloup E, Merrouche W, Couzigou P, et al. Diagnosis of liver fibrosis using FibroScan and other noninvasive methods in patients with hemochromatosis: a prospective study. Gastroenterol Clin Biol. 2008;32:180–187. doi: 10.1016/j.gcb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371–378. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 12.Ziol M, Marcellin P, Douvin C, de Ledinghen V, Poupon R, Beaugrand M. Liver stiffness cut off values in HCV patients: validation and comparison in an independent population. Hepatology. 2006;44:Suppl 1: 269A. [Google Scholar]
- 13.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KM, Choi WB, Park SH, Yu E, Lee SG, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Diagnosis of hepatic steatosis and fibrosis by transient elastography in asymptomatic healthy individuals: a prospective study of living related potential liver donors. J Gastroenterol. 2007;42:382–388. doi: 10.1007/s00535-007-2016-1. [DOI] [PubMed] [Google Scholar]
- 15.Berends MA, Snoek J, de Jong EM, Van Krieken JH, de Knegt RJ, van Oijen MG, van de Kerkhof PC, Drenth JP. Biochemical and biophysical assessment of MTX-induced liver fibrosis in psoriasis patients: Fibrotest predicts the presence and Fibroscan predicts the absence of significant liver fibrosis. Liver Int. 2007;27:639–645. doi: 10.1111/j.1478-3231.2007.01489.x. [DOI] [PubMed] [Google Scholar]
- 16.Lupşor M, Badea R, Stefănescu H, Grigorescu M, Sparchez Z, Serban A, Branda H, Iancu S, Maniu A. Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C. Results from a cohort of 324 patients. J Gastrointestin Liver Dis. 2008;17:155–163. [PubMed] [Google Scholar]
- 17.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Ledinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 22.Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Ledinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628–634. doi: 10.1016/j.jhep.2006.11.010. [DOI] [PubMed] [Google Scholar]


