Abstract
Coffee drinking has been reported to have beneficial effects on insulin resistance which has been directly associated with endometrial cancer. Although a relationship between coffee consumption and endometrial cancer risk is biologically plausible, this hypothesis has been previously explored in only two prospective studies, with a small number of cases.
We used data from the Swedish Mammography Cohort, a population-based prospective cohort study of 60 634 women. During 17.6 years of follow-up, 677 participants were diagnosed with incident endometrial cancer (adenocarcinoma). We examined the association between self-reported coffee consumption (at baseline 1987–90 and in 1997) and endometrial cancer risk using Cox proportional hazards models.
Each additional cup (200g) of coffee per day was associated with a RR of 0.90 (95% CI 0.83–0.97). In women drinking four or more cups of coffee a day the risk reduction of endometrial cancer was RR 0.75 (95% CI 0.58–0.97) as compared to those who drank one cup or less. The association seemed largely confined to overweight and obese women, who showed a respective risk reduction of 12% (95% CI 0–23%) and 20% (95% CI 7–31%) for every cup of coffee, but was not observed among normal weight women. There was a statistically significant interaction between coffee consumption and body mass index (pinteraction <0.001).
These data indicate that coffee consumption may be associated with decreased risk of endometrial cancer, especially among women with excessive body weight. If confirmed by other prospective studies, these results are of major public health significance.
Keywords: Diet, Prospective study, Coffee, Endometrial cancer, Epidemiology
Background
Coffee consumption has been reported to improve insulin sensitivity 1 and to decrease the risk of developing diabetes 2. Endometrial cancer risk has been directly associated with obesity, insulin resistance, and the resultant hyperinsulinemia 3–7. Therefore, an association between coffee consumption and endometrial cancer risk, possibly modified by body weight, is biologically plausible. Endometrial cancer risk in relation to coffee consumption has been studied, to date, in only two prospective studies each with a small number of cases; one showed a statistically significant decrease in risk8 and the other a non –significant decrease in risk9. Eight case-control studies have evaluated the possible association between coffee drinking and endometrial cancer risk with five studies showing statistically significant decreased risk with increasing consumption of coffee 10–14, one showing a non-significant decreased risk 15 and two a statistically non-significant increase in risk 16, 17.
To address prospectively whether coffee consumption is associated with risk of endometrial cancer we used data from the Swedish Mammography Cohort, a population-based cohort study of over 60 000 women including 677 endometrial cancer cases. Thus, ours is the largest prospective study of endometrial cancer to date. Coffee consumption in the cohort was assessed by self-reports that were collected twice, once at baseline and the other 10 years later. Sweden has the second highest per capita coffee consumption in the world 18, and thus our cohort provides a wide range of coffee intake. Moreover, decaffeinated coffee is very uncommon in Sweden, which allows for the study of caffeinated coffee. In addition, we examined whether the association between coffee consumption and endometrial cancer risk may be modified by factors related to insulin resistance including overweight and obesity.
Methods
From 1987 to 1990, questionnaires and invitations to participate in a free mammography screening program were mailed to all women born 1914–1948 and living in the Uppsala County of central Sweden (n=48 517) and to all women that were born 1917–1948 and were living in the adjacent Västmanland County (n=41 786). A total of 66 651 women (74%) returned a completed questionnaire on diet including coffee consumption, as well as information about weight, height, parity and education.
In 1997, a second questionnaire was sent to all 56 030 cohort members who were still living in the study area; the second questionnaire was extended with information about medical history including diabetes and hypertension, age at menarche, history of oral contraceptive use, age at menopause, postmenopausal hormone use, and lifestyle factors, such as history of cigarette smoking, physical activity and use of dietary supplements; 39 227 (70%) women returned a completed questionnaire. Women who did not answer the second questionnaire were on average older, less educated, and had a slightly higher BMI. On the other hand their average coffee consumption did not differ.
Data on coffee consumption was collected at baseline 1987–90 by use of a self-administered food-frequency questionnaire that included 67 food items commonly consumed in the study population. Women were asked to report how often on average they consumed coffee during the last six months; they could choose one from 8 pre-specified frequencies ranging from “never or seldom” to “4 times per day or more”. We used age-specific (<53, 53–65, >65 years) coffee servings that were based on mean values obtained from 129 randomly chosen women from the SMC who weighed and recorded food intake for four 1-week periods (Wolk A: unpublished data). The Spearman correlation coefficient between food frequency questionnaires and weighted records for coffee consumption was 0.6. In the second questionnaire of 1997 there was an open ended question on how many coffee cups per day or per week the women consumed during the last year. Information about tea consumption was collected in the same way as that for coffee.
Body mass index was calculated as weight in kg divided with the square of the height in meters (BMI, kg/m2). The validity for self-reported weight and height as compared to measurements in Swedish women has been studied and the Pearson correlation coefficients for these were r = 0.9 and 1.0, respectively 19. Physical inactivity was assessed in the second questionnaire using five predefined categories for time spent per day watching TV/sitting (inactive leisure time, less than 1h daily to more than 6 h daily). Education was assessed with six questions ranging from 6 years of basic education to university studies. Diabetes was self-reported on the second questionnaire and assessed with the question “have you ever been diagnosed with diabetes”; in case of women with diabetes who were hospitalized we also obtained information by linkage of the cohort to the Swedish In-patient Register.
Follow-up of the Cohort
We performed linkage of the cohort with the National Swedish Cancer Register through December 31, 2005 and with the Regional Cancer Register in the study area through December 31, 2007. The National Swedish Cancer Register and the Regional Cancer Register have been estimated to be almost 100% complete 20. Furthermore, by linkage with the nationwide Swedish In-patient Register, we identified women who had a hysterectomy for reasons other than endometrial cancer. Dates of death or migration from the study area were ascertained by linkage with the Swedish Death Register and the Swedish Population Register, respectively. Of the 66 651 women who responded to the first questionnaire in 1987–90, we excluded those with a missing identification number, with age outside the range 40–76 years, those with a cancer diagnosis (other than non-melanoma skin cancer) before the study baseline, with a history of hysterectomy before entry to the cohort and with extreme values of reported energy intake. Furthermore, we also excluded women with missing information on coffee consumption (n=592). After these exclusions, 60 634 women aged 40 to 76 years at baseline remained for this analysis, including 677 incident endometrial cancer (endometroid adenocarcinoma) cases. This study was approved by the Ethics Committees at the Uppsala University Hospital (Uppsala, Sweden) and the Karolinska Institutet (Stockholm, Sweden). Completion of the self-administered questionnaire was considered to imply informed consent to participate in this study.
Statistical Analysis
To estimate the risk of endometrial cancer we used the Cox proportional hazards models. We calculated person-years of follow-up for each woman from the date of mammography to the date of endometrial cancer diagnosis, the date of a hysterectomy, the date of death from any cause, the date of migration out of the study area during December 31, 2005 – December 31, 2007 (since for this time period we only have regional information on cancer incidence), or the end of follow-up on December 31, 2007, whichever came first. We computed incidence rate ratios by dividing the number of incident cancer cases by the number of person-years of follow-up in each category, stratified on age in months. The rate ratios (RRs) of endometrial cancer (with 95% confidence intervals [CIs]) were calculated by dividing the incidence rates among women in the two upper categories of coffee consumption with women in the lowest category of coffee consumption. The data conformed to the proportional hazards assumption21. We performed age-adjusted and multivariable analyses. In the main analysis we included coffee consumption from the baseline questionnaire, BMI and smoking. We categorized coffee in three groups according to the distribution in the cohort. Since majority of the women drank 2–3 cups of coffee a day the middle group was the largest and the third group included 20% of the population. We also performed analysis further adjusting for known risk factors and potential confounders such as years of education (<10, 10–12, >12, other), age at menopause (<48, 48–49, 50–52, >53), age at menarche (<12, 12, 13, >14), oral contraceptive use (yes, no), postmenopausal hormone therapy (yes, no), parity (yes, no), history of diabetes (yes, no) and total energy intake (continuously), as well as such foods correlated with coffee drinking as tea (continuously) and consumption of buns, cookies and cakes (continuously). Missing values for any potential confounder were treated as a separate “missing category” in the model.
We calculated the RR of endometrial cancer (with 95% CI) using updated information on coffee for those answering the second questionnaire, for the first time period we used information from the first questionnaire only and for the second time period after 1 January 1998 we used the average coffee consumption from the two questionnaires. In test for linear trend, we used the median value in each category as a continuous variable in the model. To model and graph the multivariable adjusted rate ratio for coffee consumption and endometrial cancer incidence in a flexible way we used restricted cubic splines (three knot positions)22, 23. Both, BMI and physical inactivity are related to insulin resistance, therefore we conducted analyses stratifying on BMI (baseline) and physical inactivity (second time period only). We also separately analyzed a subgroup of women with diabetes. Furthermore we stratified the analysis postmenopausal hormone use. Statistical significance of interactions was tested by adding an interaction term to the Cox model, simultaneously containing the main variables and age in months.
Analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC) and Stata (version 9.2; Stata Corp, College Station, TX). All p-values are 2-sided.
Results
During a mean follow-up time of 17.6 years among 60 634 women in the cohort (1 066 348 person-years) 677 incident adenocarcinoma endometrial cancer cases were diagnosed. The mean age at diagnosis of endometrial cancer was 67.3 (± 9.2) years. Table 1 shows the distribution of known risk factors and potential confounders for endometrial cancer in the cohort by categories of coffee consumption. Women with a high coffee consumption were on average younger, less educated, drank less tea and were more likely to smoke. Other characteristics did not vary substantially with respect to coffee consumption.
Table 1.
Age-standardized baseline characteristics of women in the Swedish Mammography Cohort according to coffee consumption.
| Coffee consumption per day |
|||
|---|---|---|---|
| ≤1 cups* | 2–3 cups | ≥4 cups | |
| Median (gram/day) | 218 | 464 | 746 |
| Characteristics | n=19 338 | n=30 274 | n=11 022 |
| Age, y | 59.8 | 51.2 | 49.8 |
| Body mass index, kg/m2 | 24.7 | 23.2 | 24.9 |
| History of smoking, % | 22.3 | 28.3 | 37.2 |
| Age at menarche, y | 13.3 | 12.2 | 13.2 |
| Number of children | 2.1 | 2.0 | 2.2 |
| Oral contraceptive use, % | 42.3 | 44.2 | 42.1 |
| Age at menopause, y | 50.0 | 45.7 | 50.0 |
| Postmenopausal hormone therapy, % | 48.0 | 47.4 | 46.8 |
| Total energy intake, kcal | 1535 | 1505 | 1656 |
| Tea, gram/day | 221 | 116 | 74 |
| Buns, cookies and cakes, gram/day | 11.8 | 14.9 | 16.2 |
| Education ≥12years, % | 14.1 | 12.5 | 11.8 |
| History of diabetes, % | 4.8 | 3.2 | 4.7 |
Note: All values other than for age have been directly standardized according to the age distribution of the cohort.
Non-drinkers 3.7% and <1 cup/day 4.4% in the entire cohort.
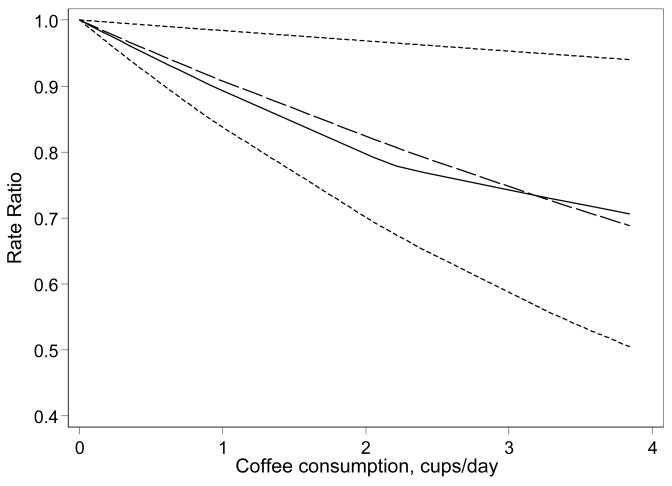
Overall, coffee consumption was statistically significantly and inversely associated with endometrial cancer risk in analyses adjusted for age and in analyses further adjusted for BMI and smoking (Table 2). Each additional cup of coffee (standardized to 200 gram) was associated with a RR of 0.90 (95%CI 0.83–0.97). This association was further investigated using updated information on coffee consumption from the second questionnaire in the cohort (Table 2). Spline analyses showed a clear dose-response between coffee consumption and decreased risk of endometrial cancer (Figure 1).
Table 2.
Rate Ratios (RRs) and 95% CIs of coffee consumption in relation to endometrial cancer for 60,634 women in the Swedish Mammography Cohort.
| Coffee consumption | ≤1 cups | 2–3 cups | ≥4 cups | Ptrend | Per 1 cup * |
|---|---|---|---|---|---|
| Baseline | |||||
| No of cases | 271 | 312 | 94 | ||
| Person-years | 320 532 | 548 404 | 197 412 | ||
| Age-adjusted RR (95% CI) † | 1.00 (ref) | 0.75 (0.62–0.92) | 0.72 (0.56–0.93) | 0.01 | 0.89 (0.82–0.95) |
| Multivariable adjusted RR (95% CI) ‡ | 1.00 (ref) | 0.78 (0.64–0.95) | 0.75 (0.58–0.97) | 0.02 | 0.90 (0.83–0.97) |
| Long-term | |||||
| No of cases | 224 | 304 | 149 | ||
| Age-adjusted RR (95% CI) † | 1.00 (ref) | 0.82 (0.68–0.98) | 0.82 (0.66–1.02) | 0.02 | 0.92 (0.85–0.99) |
| Multivariable adjusted RR (95% CI) ‡ | 1.00 (ref) | 0.82 (0.69–0.98) | 0.85 (0.69–1.05) | 0.03 | 0.93 (0.86–1.00) |
Cup size standardized to 200gram
Rate ratios from Cox proportional hazards models adjusted for age in months.
Rate ratios from Cox proportional hazards models adjusted for age in months, BMI (<20, 20–25, 26–30, >30) and smoking (never/ever/missing).
Figure 1.
Rate ratios (RRs) and 95% CIs of coffee consumption among women in the Swedish Mammography Cohort at baseline in relation to endometrial cancer risk using restricted cubic splines.
Multivariable adjusted rate ratios for coffee consumption in relation to endometrial cancer incidence adjusted for age, BMI and smoking. Plot of the restricted cubic splines (solid line), linear trend (long-dash line), and dotted lines 95% confidence limits of the linear trend.
In the analyses of baseline coffee consumption additionally adjusting for education, age at menopause, age at menarche, oral contraceptive use, postmenopausal hormone use, parity, history of diabetes, total energy intake, tea consumption and intake of foods correlated with coffee consumption such as buns, cookies and cakes, RRs for the second and third category of coffee consumption as compared to the lowest one were 0.77 (95% CI 0.63–0.94) and 0.75 (95% CI 0.57–0.98), respectively. To eliminate the possible residual confounding by smoking we performed an analysis confined to those women that had never smoked (including 20,348 women and 283 cases). Results from this analysis did not differ substantially from those for the entire cohort; e.g, RRs for the second and third category of coffee consumption as compared to the lowest one were 0.83 (95% CI 0.62–1.12) and 0.66 (95%CI 0.41–1.07), respectively. To eliminate potential effects of early undiagnosed endometrial cancer, we repeated our analysis after excluding endometrial cancer cases diagnosed during the first year of follow-up. Results from this analysis did not differ substantially from those for the entire cohort. RRs for the second and third category of coffee consumption as compared to the lowest one were 0.75 (95% CI 0.62–0.92) and 0.75 (95%CI 0.58–0.97), respectively.
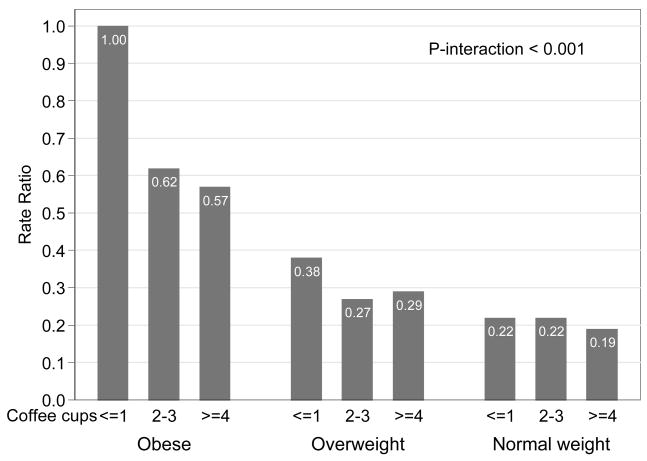
We also examined whether the observed association differed according to BMI status, by stratifying the cohort into groups with BMI 20–25, 25–30, >30 kg/m2. We observed a decreased risk associated with coffee intake among overweight and obese women, but not among women with normal weight (Table 3). There was a statistically significant interaction between coffee consumption and BMI, pinteraction <0.001 (Figure 2). Women drinking 4 or more cups of coffee per day and had a normal body weight (BMI 20–25 kg/m2) had a statistically significantly 81% lowered risk compared to those who were obese (BMI >30) and drank 1 cup of coffee or less per day.
Table 3.
Rate ratios (RRs) and 95% CIs of coffee consumption stratified by BMI in relation to endometrial cancer for women in the Swedish Mammography Cohort.
| Coffee consumption at baseline | No of cases | ≤1 cups | 2–3 cups | ≥4 cups | Ptrend | Per 1 cup† | |
|---|---|---|---|---|---|---|---|
| RR (95% CI)* | Normal weight (BMI 20–25) | 246 | 1.00 (ref) | 1.21 (0.84–1.73) | 1.13 (0.72–1.78) | 0.79 | 1.00 (0.88–1.15) |
| Overweight (BMI 26–30) | 219 | 1.00 (ref) | 0.65 (0.46–0.92) | 0.72 (0.46–1.14) | 0.07 | 0.88 (0.77–1.00) | |
| Obese (BMI >30) | 163 | 1.00 (ref) | 0.50 (0.34–0.74) | 0.54 (0.32–0.93) | 0.007 | 0.80 (0.69–0.93) | |
Pinteraction between coffee consumption and BMI <0.001.
Rate ratios from Cox proportional hazards models adjusted for age in months, BMI (continuously) and smoking (never/ever/missing).
Cup size 200gram
Figure 2.
Associations of coffee consumption stratified by BMI in relation to endometrial cancer for women in the Swedish Mammography Cohort.
Rate ratios (RR) from Cox proportional hazards models adjusted for age and smoking.
Pinteraction <0.001
All Pvalues from RRs were <0.05.
We examined the possible effect modification by postmenopausal hormone use, no evidence for effect modification was observed (Pinteraction = 0.38). The relative risk associated with each additional cup of coffee was RR 0.93 (95%CI 0.83–1.06) and 0.86 (95% CI 0.75–0.99) among postmenopausal hormone users and non-users respectively. We evaluated the possible modification by leisure time physical inactivity (sitting/watching TV 5 hours or more per day) and coffee consumption in a subset of the cohort (32 649 women and 240 cases, since information on physical inactivity was available for the second time period only). Due to limited power we combined the two upper categories of coffee consumption. Although the interaction did not reach statistical significance, there was a suggestion that the association was stronger among inactive women (pinteraction=0.06). Inactive women drinking 2 or more cups of coffee had a RR of 0.58 (95%CI 0.23–1.49) compared to inactive women drinking 1 cup of coffee or less per day. The corresponding estimate among active women was 1.09 (95% CI 0.82–1.44).
We also evaluated the association among women with diabetes. In the analysis including 2505 diabetic women and 47 endometrial cancer cases, we combined the two upper categories of coffee consumption. Women with diabetes drinking 2 or more cups of coffee per day had a RR of 0.48 (95%CI 0.22–1.05) compared to diabetic women drinking 1 cup of coffee or less per day.
Discussion
In this population-based prospective cohort study we found that daily coffee consumption was associated with a lower risk for endometrial cancer. More specifically, women drinking 2 or more cups of coffee a day had a statistically significant risk reduction for endometrial cancer. Each additional cup of coffee (cup 200g) was associated with 10% reduced risk of endometrial cancer among all women. The risk reduction was largely confined to overweight and obese women, who are at increased risk for endometrial cancer. Among these women, each additional cup of coffee consumed daily decreased the risk of endometrial cancer by 12% and 20%, respectively.
The association between coffee consumption and endometrial cancer risk has been studied previously in only two small prospective cohort studies8, 9 (a Japanese study including 117 cases and one Norwegian study including 84 cases). Case-control studies investigating this association have also been limited 10–17. Our results deriving from a large prospective population-based cohort study confirm and extend data from the reported cohort studies8, 9 and case-control studies 10–15, showing a decreased risk of endometrial cancer in relation to high coffee consumption. One of the cohorts8 and five of the previously published case-control studies10–14 have reported a statistically significant inverse association, the other cohort9 and one case-control study observed a non-significant inverse association15 whereas two case-control studies showed a non-significant positive association 16, 17.
There are several biological mechanisms through which coffee might reduce risk of endometrial cancer development. Coffee has been shown to affect the absorption and metabolism of glucose 24–26 and may also protect against type 2 diabetes 2. Furthermore coffee has been shown to improve insulin sensitivity 1, 27. We have recently shown that coffee consumption is directly related to plasma levels of adiponectin, an endogenous insulin sensitizer 28. Lower levels of adiponectin have in turn been consistently related to both higher risk of endometrial cancer 29–32 and hyperinsulinemia. Hyperinsulinemia is a common feature in diabetes, obesity and physical inactivity, all states associated with hypoadiponectinemia. Hyperinsulinemia has been shown to stimulate the growth of endometrial stromal cells by binding to insulin receptors in endometrium33 and may also increase levels of free estrogens through decreasing concentrations of circulating sex hormone binding globulin (SHBG) 34, 35, and through decreasing levels of IGFBP-1 increase circulating free IGF-1. IGF-1 stimulates cell proliferation by binding and activating IGF-1 receptors in the endometrium 36–41. Mechanistically, adiponectin is thus upstream of hyperinsulinemia/hyperandrogenemia and elevated IGF-1 levels. Furthermore, high caffeine intake has been linked to decreased free estrogen levels through higher concentration of circulating SHBG 42, 43. Estrogens in turn have been shown to increase endometrial cancer risk by stimulating proliferation of endometrial cells 44. Coffee also contains phytoestrogens45 which have been suggested to decrease the risk of endometrial cancer46, 47. Finally, coffee contains antioxidants that may reduce oxidative stress, an important factor for cancer development48. In our study, the strongest effect of coffee was observed among overweight and obese women who are at the highest risk for endometrial cancer; this observation is consistent with the notion that hypoadiponectinemia, insulin resistance and hyperinsulinemia may be involved in the process 49–52.
Major strengths of our study include its population-based design and the completeness of identification of endometrial cancer cases through the Swedish cancer registries. The prospective nature of the study makes it highly unlikely that the associations we observed were due to recall or selection biases that might lead to spurious associations in case-control studies. Furthermore, we had information on all major potential confounders. Although the possibility of uncontrolled or residual confounding can not be entirely eliminated we have adjusted for multiple potential confounders and we observed little difference between the age-adjusted and multivariable models. However, our study had several limitations. First, because coffee intake was assessed through self-administered food-frequency questionnaires, measurement errors are inevitable. However, results from comparisons of self-reported coffee intake in the questionnaire with dietary records suggest that we obtained a reasonable assessment of coffee consumption. This kind of non-differential misclassification would tend to attenuate the observed association between coffee intake and endometrial cancer risk and thus could not explain our results. Second, in our study the near absence of non coffee drinkers made it impossible to assess the effect of different amounts of coffee compared to no consumption. Finally, serum samples would have allowed us to test the hypotheses about mechanisms more directly. Future studies are needed in this regard.
In conclusion, our results show that coffee consumption is associated with decreased risk of endometrial cancer, especially among overweight and obese women. If confirmed by other studies and in other populations, these data may prove to be of major public health significance in Western societies.
Acknowledgments
Funding/Support: This work was supported by research grants from World Cancer Research Fund International, The Swedish Cancer Foundation, The Swedish Research Council for infrastructure, by AICR, NIH grants DK58785, DK79929, DK 081913, DK58845 and a discretionary grant from BIDMC.
Footnotes
Novelty: The first large prospective cohort. This is also the first study assessing long-term coffee consumption.
References
- 1.Arnlov J, Vessby B, Riserus U. Coffee consumption and insulin sensitivity. JAMA. 2004;291:1199–201. doi: 10.1001/jama.291.10.1199-b. [DOI] [PubMed] [Google Scholar]
- 2.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 3.IARC. Weight control and physical activityed. Lyon: IARC Press; 2002. [Google Scholar]
- 4.Modesitt SC, van Nagell JR., Jr The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstetrical & gynecological survey. 2005;60:683–92. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 5.Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst. 2004;96:1635–8. doi: 10.1093/jnci/djh291. [DOI] [PubMed] [Google Scholar]
- 6.Friberg E, Mantzoros CS, Wolk A. Physical activity and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:2136–40. doi: 10.1158/1055-9965.EPI-06-0465. [DOI] [PubMed] [Google Scholar]
- 7.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–74. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 8.Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Kurahashi N, Yamaji T, Tsugane S. Coffee consumption and risk of endometrial cancer: a prospective study in Japan. Int J Cancer. 2008;123:2406–10. doi: 10.1002/ijc.23760. [DOI] [PubMed] [Google Scholar]
- 9.Stensvold I, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 1994;5:401–8. doi: 10.1007/BF01694753. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi T, Nakaya N, Okamura C, Sato Y, Shimazu T, Nagase S, Niikura H, Kuriyama S, Tase T, Ito K, Tsubono Y, Okamura K, et al. Case-control study of coffee consumption and the risk of endometrial endometrioid adenocarcinoma. Eur J Cancer Prev. 2008;17:358–63. doi: 10.1097/CEJ.0b013e3282f0c02c. [DOI] [PubMed] [Google Scholar]
- 11.Petridou E, Koukoulomatis P, Dessypris N, Karalis D, Michalas S, Trichopoulos D. Why is endometrial cancer less common in Greece than in other European Union countries? Eur J Cancer Prev. 2002;11:427–32. doi: 10.1097/00008469-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hirose K, Niwa Y, Wakai K, Matsuo K, Nakanishi T, Tajima K. Coffee consumption and the risk of endometrial cancer: Evidence from a case-control study of female hormone-related cancers in Japan. Cancer science. 2007;98:411–5. doi: 10.1111/j.1349-7006.2007.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCann SE, Yeh M, Rodabaugh K, Moysich KB. Higher regular coffee and tea consumption is associated with reduced endometrial cancer risk. Int J Cancer. 2009;124:1650–3. doi: 10.1002/ijc.24125. [DOI] [PubMed] [Google Scholar]
- 14.Bravi F, Scotti L, Bosetti C, Zucchetto A, Talamini R, Montella M, Greggi S, Pelucchi C, Negri E, Franceschi S, La Vecchia C. Food groups and endometrial cancer risk: a case-control study from Italy. Am J Obstet Gynecol. 2009;200:293, e1–7. doi: 10.1016/j.ajog.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: a nationwide case-control study in Sweden. Nutr Cancer. 2002;42:25–32. doi: 10.1207/S15327914NC421_4. [DOI] [PubMed] [Google Scholar]
- 16.Levi F, Franceschi S, Negri E, La Vecchia C. Dietary factors and the risk of endometrial cancer. Cancer. 1993;71:3575–81. doi: 10.1002/1097-0142(19930601)71:11<3575::aid-cncr2820711119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Goodman MT, Hankin JH, Wilkens LR, Lyu LC, McDuffie K, Liu LQ, Kolonel LN. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer research. 1997;57:5077–85. [PubMed] [Google Scholar]
- 18.Coffeeresearch. 2008 http://www.coffeeresearch.org/market/consumption.htm.
- 19.Kuskowska-Wolk A, Bergstrom R, Bostrom G. Relationship between questionnaire data and medical records of height, weight and body mass index. Int J Obes Relat Metab Disord. 1992;16:1–9. [PubMed] [Google Scholar]
- 20.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23:305–13. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 21.Grambsch PM, Thernau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in medicine. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 24.Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, Schubert G, Below P, Herling AW. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Archives of biochemistry and biophysics. 1997;339:315–22. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 25.Clifford MN. Chlorogenic acids and other cinnamates - nature, occurence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–43. [Google Scholar]
- 26.Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. The American journal of clinical nutrition. 2003;78:728–33. doi: 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- 27.Agardh EE, Carlsson S, Ahlbom A, Efendic S, Grill V, Hammar N, Hilding A, Ostenson CG. Coffee consumption, type 2 diabetes and impaired glucose tolerance in Swedish men and women. J Intern Med. 2004;255:645–52. doi: 10.1111/j.1365-2796.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 28.Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, Mantzoros CS. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes care. 2008;31:504–7. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM, Lu KH. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–81. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 30.Dal Maso L, Augustin LS, Karalis A, Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS, La Vecchia C. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–3. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 31.Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, Chrousos G, Trichopoulos D. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–7. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 32.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E, Tjonneland A, Olsen A, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–63. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- 33.Nagamani M, Stuart CA. Specific binding and growth-promoting activity of insulin in endometrial cancer cells in culture. Am J Obstet Gynecol. 1998;179:6–12. doi: 10.1016/s0002-9378(98)70244-3. [DOI] [PubMed] [Google Scholar]
- 34.Kazer RR. Insulin resistance, insulin-like growth factor I and breast cancer: a hypothesis. Int J Cancer. 1995;62:403–6. doi: 10.1002/ijc.2910620408. [DOI] [PubMed] [Google Scholar]
- 35.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–9. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 36.Irwin JC, de las Fuentes L, Dsupin BA, Giudice LC. Insulin-like growth factor regulation of human endometrial stromal cell function: coordinate effects on insulin-like growth factor binding protein-1, cell proliferation and prolactin secretion. Regul Pept. 1993;48:165–77. doi: 10.1016/0167-0115(93)90345-9. [DOI] [PubMed] [Google Scholar]
- 37.Murphy LJ. Growth factors and steroid hormone action in endometrial cancer. J Steroid Biochem Mol Biol. 1994;48:419–23. doi: 10.1016/0960-0760(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 38.Corocleanu M. Hypothesis for endometrial carcinoma carcinogenesis. Preventive prospects Clin Exp Obstet Gynecol. 1993;20:254–8. [PubMed] [Google Scholar]
- 39.Thiet MP, Osathanondh R, Yeh J. Localization and timing of appearance of insulin, insulin-like growth factor-I, and their receptors in the human fetal mullerian tract. Am J Obstet Gynecol. 1994;170:152–6. doi: 10.1016/s0002-9378(94)70401-5. [DOI] [PubMed] [Google Scholar]
- 40.Ordener C, Cypriani B, Vuillermoz C, Adessi GL. Epidermal growth factor and insulin induce the proliferation of guinea pig endometrial stromal cells in serum-free culture, whereas estradiol and progesterone do not. Biol Reprod. 1993;49:1032–44. doi: 10.1095/biolreprod49.5.1032. [DOI] [PubMed] [Google Scholar]
- 41.Weiderpass E, Brismar K, Bellocco R, Vainio H, Kaaks R. Serum levels of insulin-like growth factor-I, IGF-binding protein 1 and 3, and insulin and endometrial cancer risk. Br J Cancer. 2003;89:1697–704. doi: 10.1038/sj.bjc.6601312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.London S, Willett W, Longcope C, McKinlay S. Alcohol and other dietary factors in relation to serum hormone concentrations in women at climacteric. The American journal of clinical nutrition. 1991;53:166–71. doi: 10.1093/ajcn/53.1.166. [DOI] [PubMed] [Google Scholar]
- 43.Ferrini RL, Barrett-Connor E. Caffeine intake and endogenous sex steroid levels in postmenopausal women. The Rancho Bernardo Study. American journal of epidemiology. 1996;144:642–4. doi: 10.1093/oxfordjournals.aje.a008975. [DOI] [PubMed] [Google Scholar]
- 44.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–19. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 45.Mazur W. Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab. 1998;12:729–42. doi: 10.1016/s0950-351x(98)80013-x. [DOI] [PubMed] [Google Scholar]
- 46.Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst. 2003;95:1158–64. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- 47.Bandera EV, Williams MG, Sima C, Bayuga S, Pulick K, Wilcox H, Soslow R, Zauber AG, Olson SH. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Strom EC, Jacobs DR, Jr, Ose L, Blomhoff R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. The Journal of nutrition. 2004;134:562–7. doi: 10.1093/jn/134.3.562. [DOI] [PubMed] [Google Scholar]
- 49.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–43. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 50.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–60. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 51.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, Jacobs DR., Jr Serum adiponectin in young adults--interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol. 2004;14:492–8. doi: 10.1016/j.annepidem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]