Abstract
Objective
This is retrospective study of clinical and radiological outcomes of anterior cervical fusion using Bongros-HA™ (BioAlpha, Seongnam, Korea) which is a type of synthetic hydroxyapatite (HA) spacer to evaluate the efficacy in its clinical application and usefulness as a reliable alternative to autograft bone.
Methods
Twenty-nine patients were enrolled in this study and 40 segments were involved. All patients were performed anterior cervical interbody fusion using HA spacer and plating system. Indications for surgery were radiculopathy caused by soft-disc herniation or spondylosis in 18 patients, spondylotic myelopathy in 1 patient, and spinal trauma in 10 patients. Cervical spine radiographs were obtained on postoperative 1day, 1week, and then at 1, 2, 6, and 12 months in all patients to evaluate intervertebral disc height, and the degrees of lordosis. Cervical computed tomography was done at postoperative 12 month in all patients to confirm the fusion status. The mean period of clinical follow-up was 17 months.
Results
Complete interbody fusion was achieved in 100% of patients. Preoperative kyphotic deformities were corrected in all cases after surgery. Intervertebral disc height was well maintained during follow up period. There were no cases of graft extrusion, graft deterioration and graft fracture.
Conclusion
HA spacer is very efficient in achieving cervical fusion, maintaining intervertebral disc height, and restoring lordosis. When combined with the placement of a cervical plate, immediate stability can be achieved and graft related complication can be prevented.
Keywords: Hydroxyapatite, Spacer, Anterior cervical discectomy, Fusion, Graft, Plate
INTRODUCTION
Anterior cervical discectomy and interbody fusion (ACDF) is a reliable procedure commonly used to treat a variety of degenerative and posttraumatic conditions of the cervical spine resulting in pain, instability, and/or radiculopathy or myelopathy6,13,17,19-21). The objectives of anterior decompression and fusion of the cervical spine are decompression of the neural structures, restoration of stability, and maintenance of physiological alignment1,4,6,8,18-20).
Since in 1950' early descriptions of interbody fusion material were reported by Cloward and by Smith and Robinson7,9,24), many methods of grafting and fusion have been devised for these purposes. The ideal device or bone substitute would provide adequate stability and optimized fusion bed, restore disc height and cervical lordosis, preserve the integrity of the vertebral body endplates and must be an atraumatic method as possible. The harvesting bone graft material from the anterior iliac crest is associated with significant short- and long-term morbidity such as infection, pelvic fracture, meralgia paresthetica, wound hematoma, loss of sensation and especially postoperative pain in up to about 20% of cases3). And, the quality of the autologous graft material can be a concern in patients with osteoporosis, metabolic disorder, neoplasia, chronic inflammatory disease, infection, and bone fractures of the donor site. The surgeons who want to achieve a rigid cervical interbody fusion have sought materials to serve as an alternative to the patient's own tricorticate bone. To avoid complications, particularly donor site pain, related to the harvesting of iliac crest bone, numerous materials have been developed such as allografts, methylmethacrylate, biocompatible osteoconductive polymer, and coralline grafts or cages16,22,23,25,26). Some of these materials, however, are inefficient for fusion or are associated with specific complications29).
Many basic and clinical studies have highlighted the hydroxyapatit (HA) spacer as a possible alternative to either iliac bone grafts or allografts for anterior cervical interbody fusion material7,15,18,19,21,29). The clinical use of HA has varied in oral11), plastic12), otologic14), and orthopedic surgery28) in the past 20 years. Its biocompatibility and safety have been accepted by surgeons in different fields. Regarding the long-term clinical results of the HA spacer for the alternative graft in ACDF, there have been limited numbers of reports. Senter et al.21) reported that HA provided better long-term relief of radicular symptoms, less need for a second operation at the same or an adjacent level, comparable spinal alignment and stability, and the elimination of complications at the iliac crest donor site in comparison with autologous iliac crest interbody fusion. They also reported that the incidence of spacer dislodgement was 4% and breakage was 2%. Kim et al.15) reported that 3% dislodgement, no spacer breakage, and no neurologic complications related to the site of fusion occurred during a follow-up period of more than 1 year.
Therefore, we had performed ACDF using HA spacer and plate system together to evaluate clinical and radiological outcomes of anterior cervical fusion using synthetic HA spacer and to evaluate the efficacy in its clinical application and usefulness as a reliable alternative to autograft bone.
MATERIALS AND METHODS
Between May 2007 and August 2007, 19 patients with cervical spondylotic radiculopathy or myelopathy and 10 patients with trauma related disorders underwent anterior cervical discectomy and fusion using Bongros-HA™ (BioAlpha, Seongnam, Korea) (Fig. 1) which is one kind of synthetic HA spacer combined reinforced with rigid plate system.
Fig. 1.
Photographs showing the porous hydroxyapatite bone fusion spacer used for cervical interbody fusion; Bongros-HA™ (BioAlpha, Seongnam, Korea) has a property of rectangular shape with a threaded design that prevent dislodgement of graft and maximize bony contact surface of graft.
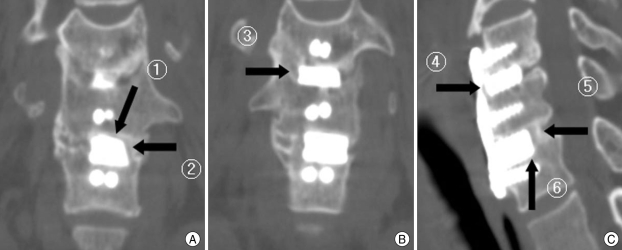
Cervical plain X-ray, CT scan and MRI were obtained in all patients preoperatively and postoperatively. The follow-up period ranged from 12 months to 22 months with a mean of 17 months. We evaluated the bone fusion rate. All patients were followed for a mean of postoperative 3, 6, 12 months using serial anteroposterior and lateral plain radiographs. Lateral flexion-extension radiography and cervical CT scan were obtained at postoperative 12 month to evaluate bone fusion state, intervertebral disc height, and the degree of lordosis. A rigid external orthosis was applied postoperatively for 2 months and bone fusion rate was examined by two radiologists. The authors proposed a 6-point scoring system as an indicator to confirm the bone fusion (Fig. 2). We considered a bridging bone between HA spacer and adjacent endplate of vertebral body in six surfaces (anterior, posterior, superior, inferior and both lateral sides) around the spacer as one indicator point. The bridging bone formation more than two surfaces is sufficient for bone fusion with stability30). But, to confirm much more solid and firm bone fusion without instability, we considered that if radioopaque stripes more than 3 interspaces appear, the instability of fusion segment cannot develop and the stability of the graft was assured. Formation of bridging bone was examined on six surfaces around graft using axial, coronal, and sagittal CT images performed postoperative 12 months and we confirmed no instability in flexion-extension radiograph of patients. We also measured intervertebral disc height and the degree of lordosis according to the method proposed by Bishop et al.5) to evaluate changes in intervertebral height and in cervical lordotic angle.
Fig. 2.
Evaluation of bone fusion rate; 6-point scoring system. Imaging studies (A, B and C) of a patient with C5-6-7 anterior cervical discectomy and fusion with hydroxyapatite spacer. Bone fusion check point (black arrow). Bone fusion check points (black arrow 1-6) represent bridging bone of six surfaces (anterior, posterior, superior, inferior and both lateral sides) around the spacer.
A conventional anterior cervical approach was used. After the completion of discectomy and decompression for a neural structure under microscope, the cartilaginous endplates were removed but the bony endplates were preserved. After intervertebral space was widened sufficiently using Caspar screw, an appropriate-size HA spacer was inserted without impact on HA spacer itself to avoid the breakage or crack of HA spacer. And, a rigid anterior cervical spine plate (Medtronic, Memphis, TN, USA) was applied in all cases. After removing Caspar screw, screws in anterior plating were inserted to the vertebral body in oblique upward and downward fashion respectively.
RESULTS
Indications for surgery were radiculopathy (caused by soft-disc herniation or spondylosis) in 18 cases, spondylotic myelopathy in 1 case, and spinal trauma in 10 cases. Total of 40 segments were involved.
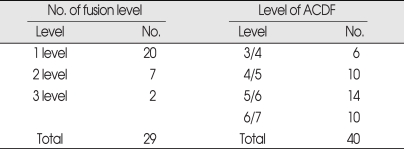
The patients were 22 men and 7 women. Their ages ranged from 32 to 73 years, with an average age of 56 years. Twenty of the patients had a one-level fusion; 7 patients had a two-level fusion; and 2 patients had a three-level fusion. Numbers according to fusion segment were 6 in C3-C4, 10 in C4-C5, 14 in C5-C6, and 10 in C6-C7. The clinical and radiological characteristics of patients are shown in Table 1.
Table 1.
Summary of demographic data and level of surgery
ACDF: anterior cervical discectomy and interbody fusion
In all patients, stability of the graft was confirmed by plain radiographs 3, 6, 12 months after surgery. Neurologic deterioration related to the fusion segment was not observed in any patient. On final clinical assessment, 23 patients presenting with radicular symptoms including traumatic spondylopathy showed improvement of visual analogue scale (VAS) from preoperative 8.7 to postoperative 3.4. Six patients with myelopathy showed improvement of motor power by one grade in each upper and lower extremity with motor weakness. Formation of bridging bone on the surface of the grafts became evident in all patients. Mean point score by 6-point scoring system that measure the rate of bone fusion around HA spacer was 5.43 and bony formation was found at least 4 points in all patients. So, the authors concluded that bone fusion was achieved successfully. Twenty-three of 40 fusion levels showed 6 points. The bone fusion rate is shown in Table 2. Complete interbody fusion was achieved in 100% of all cases. The average intervertebral space height in an index level changed from 5.8 mm before operation, to 6.7 mm right after operation, and maintained 6.4 mm and the angle of postoperative lordotic curvature made by anterior plate fixation was maintained during follow-up. There are no cases of graft extrusion, graft deterioration, subsidence and graft fracture (Fig. 3).
Table 2.
Results of bone fusion rate scored by 6-point scoring system
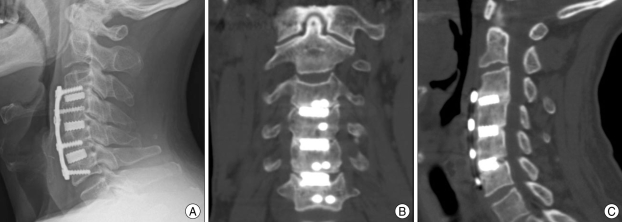
Fig. 3.
Imaging studies of a 61-year old male patient with C3-6 anterior cervical discectomy and fusion with hydroxyapatite graft. The postoperative 6 month follow-up lateral X-ray (A) and postoperative 12 month follow-up coronal (B) and sagittal (C) computed tomography show lordotic alignment and formation of bridging bone.
DISCUSSION
Since 1991, many neurosurgeons have used synthetic HA as a substitute for autologous bone grafting in cervical interbody fusions7,15,18,29). HA bone fusion spacer with which autologous bone was replaced has the property that is purely synthetic and porous, mimics the physical structure and the chemical composition of human cancellous bone and provides ideal scaffold for long term support and rapid new bone formation. HA is a hydroxyl compound of calcium phosphate [Ca10 (PO4) 6 (OH) 2] and is the main constituent (65%) of the naturally occurring bone matrix2,10-12). Experimental studies have reproducibly demonstrated bioactive properties of HA. Formation of direct bonding with the bone has been confirmed by electron microscopy, and growth of bone on the surface of HA spacer (osteoconduction) has been observed27). Theoretically, these properties make the use of HA advantageous as a construct material in cervical interbody fusion. Unlike autogenous or allogenic bone, HA undergoes little absorption and maintains its initial compressive strength. With progression of osteoconduction, the implant becomes surrounded by autologous bridging bone, which eventually fuses with adjacent vertebrae. The uniform quality and composition of HA allow for its optimal biomechanical configuration, producing good resistance to collapse and a physiological shape. Moreover, it is safe from immune and foreign body reaction and has the advantages which include shorter operation time and no donor site morbidity than iliac crest harvest, which may lead to shorter hospital stay and therapy.
In spite of the biologic benefits of HA, Zdeblick et al.29) pointed its biomechanical weakness in compressive strength using coral-based HA in a goat model. They reported that 24% of the coral-based HA collapsed despite plate fixation. In the aspect of bone fusion, HA spacer can provide good clinical results. However, mechanical stability cannot be assured, especially in a convalescent period. Several articles have described a few complications of HA block in spinal reconstructive surgery, including block slippage and/or fracture18,25,26). Nakajima et al.18) reported a 2% rate of spacer breakage or fragmentation among 5,000 Japanese patients treated with HA spacers for cervical spine conditions in the past decade.
Considering that spacer breakage can be influenced by several factors, such as shape of spacer, height, and composition of HA, usage of HA spacer alone for ACDF may achieve solid fusion but complications such as implant dislocation have developed15). To avoid HA spacer breakage during or after the surgery, several cautions for its secure insertion have to be pointed out : creation of sufficient room for the spacer before insertion so that strong impact on the spacer is not needed, avoidance of grooves or ditches at the spacer recipient area so that stress concentration on the spacer from the bone can be avoided, and use of a soft surfaced impactor so that microcracks to the spacer during insertion maneuvers can be avoided.
In achieving cervical interbody fusion, a rigid anterior plating provides the advantages which ensure immediate stability, prevent graft extrusion or fracture by reducing the distortional forces. And, anterior plating contributes to restoring proper spinal alignment, reducing the risk of late-developing pseudoarthrosis. Moreover, we expect an increase in the rate of graft incorporation and a decrease in the rate of collapse.
CONCLUSION
Based on the postoperative results, we believe that HA spacers are very efficient in achieving cervical fusion, maintaining intervertebral disc height, and restoring lordosis if careful surgical procedures are made by atraumatic method as possible. Combination of HA with placement of a cervical plate can provide more immediate stability and solid fusion and prevent graft displacement better than usage of HA spacer alone for ACDF.
References
- 1.Abraham DJ, Herkowitz HN. Indications and trends in use in cervical spinal fusions. Orthop Clin North Am. 1998;29:731–744. doi: 10.1016/s0030-5898(05)70044-4. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez JA, Hardy RW., Jr Anterior cervical discectomy for one- and two-level cervical disc disease : the controversy surrounding the question of whether to fuse, plate, or both. Crit Rev Neurosurg. 1999;9:234–251. doi: 10.1007/s003290050138. [DOI] [PubMed] [Google Scholar]
- 3.Aronson N, Filtzer DL, Bagan M. Anterior cervical fusion by the smith-robinson approach. J Neurosurg. 1968;29:396–404. [PubMed] [Google Scholar]
- 4.Bartels RH, Donk R, van Azn RD. Height of cervical foramina after anterior discectomy and implantation of a carbon fiber cage. J Neurosurg. 2001;95:40–42. doi: 10.3171/spi.2001.95.1.0040. [DOI] [PubMed] [Google Scholar]
- 5.Bishop RC, Moore KA, Hadley MN. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate : a prospective comparative analysis. J Neurosurg. 1996;85:206–210. doi: 10.3171/jns.1996.85.2.0206. [DOI] [PubMed] [Google Scholar]
- 6.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–1307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Böker DK, Schultheiss R, van Roost D, Osborn JF, Kaden B. Anterior cervical discectomy and vertebral interbody fusion with hydroxyapatite ceramic. Preliminary results. Acta Neurochir (Wien) 1993;121:191–195. doi: 10.1007/BF01809274. [DOI] [PubMed] [Google Scholar]
- 8.Chen BH, Natarajan RN, An HS, Andersson GB. Comparison of biomechanical response to surgical procedures used for cervical radiculopathy : posterior keyhole foraminotomy versus anterior foraminotomy and discectomy versus anterior discectomy with fusion. J Spinal Disord. 2001;14:17–20. doi: 10.1097/00002517-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602–617. doi: 10.3171/jns.1958.15.6.0602. [DOI] [PubMed] [Google Scholar]
- 10.Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion : outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202–206. [PubMed] [Google Scholar]
- 11.Denissen HW, de Groot K. Immediate dental root implants from synthetic dense calcium hydroxylapatite. J Prosthet Dent. 1979;42:551–556. doi: 10.1016/0022-3913(79)90253-1. [DOI] [PubMed] [Google Scholar]
- 12.el Deeb M, Waite DE, Mainous EG. Correction of the deficient alveolar ridge. Clin Plast Surg. 1989;16:733–748. [PubMed] [Google Scholar]
- 13.Geer CP, Papadopoulos SM. The argument for single-level anterior cervical discectomy and fusion with anterior plate fixation. Clin Neurosurg. 1999;45:25–29. discussion 21. [PubMed] [Google Scholar]
- 14.Grote JJ. Reconstruction of the ossicular chain with hydroxyapatite prostheses. Am J Otol. 1987;8:396–401. [PubMed] [Google Scholar]
- 15.Kim P, Wakai S, Matsuo S, Moriyama T, Kirino T. Bisegmental cervical interbody fusion using hydroxyapatite implants : surgical results and long-term observation in 70 cases. J Neurosurg. 1998;88:21–27. doi: 10.3171/jns.1998.88.1.0021. [DOI] [PubMed] [Google Scholar]
- 16.Lee CJ, Rhee DY, Heo W, Yoon JW, Park HS. The advantages of rectangular titanium cage (RABEA) fusion after anterior cervical discectomy : comparative study of fibula allograft. J Korean Neurosurg Soc. 2004;36:448–453. [Google Scholar]
- 17.Madawi AA, Powell M, Crockard HA. Biocompatible osteoconductive polymer versus iliac graft. A prospective comparative study for the evaluation of fusion pattern after anterior cervical discectomy. Spine (Phila Pa 1976) 1996;21:2123–2129. doi: 10.1097/00007632-199609150-00013. discussion 2129-2130. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima T, Tominaga Y, Yamaguchi K. Design and clinical results of hydroxyapatite vertebral spacer in cervical anterior surgery; Proceedings of the 19th Annual Orthopedic Ceramic Implant Meeting; November 27, 1999. [Google Scholar]
- 19.Pintar FA, Maiman DJ, Hollowell JP, Yoganandan N, Droese KW, Reinartz JM, et al. Fusion rate and biomechanical stiffness of hydroxylapatite versus autogenous bone grafts for anterior discectomy. An in vivo animal study. Spine (Phila Pa 1976) 1994;19:2524–2528. doi: 10.1097/00007632-199411001-00006. [DOI] [PubMed] [Google Scholar]
- 20.Schneider JR, Bright RW. Anterior cervical fusion using preserved bone allografts. Transplant Proc. 1976;8:73–76. [PubMed] [Google Scholar]
- 21.Senter HJ, Kortyna R, Kemp WR. Anterior cervical discectomy with hydroxylapatite fusion. Neurosurgery. 1989;25:39–42. doi: 10.1097/00006123-198907000-00007. discussion 42-33. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro S. Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg. 1996;84:161–165. doi: 10.3171/jns.1996.84.2.0161. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro S, Connolly P, Donnaldson J, Abel T. Cadaveric fibula, locking plate, and allogeneic bone matrix for anterior cervical fusions after cervical discectomy for radiculopathy or myelopathy. J Neurosurg. 2001;95:43–50. doi: 10.3171/spi.2001.95.1.0043. [DOI] [PubMed] [Google Scholar]
- 24.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40 A:607–624. [PubMed] [Google Scholar]
- 25.Thalgott JS, Fritts K, Giuffre JM, Timlin M. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine (Phila Pa 1976) 1999;24:1295–1299. doi: 10.1097/00007632-199907010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Toth JM, An HS, Lim TH, Ran Y, Weiss NG, Lundberg WR, et al. Evaluation of porous biphasic calcium phosphate ceramics for anterior cervical interbody fusion in a caprine model. Spine (Phila Pa 1976) 1995;20:2203–2210. doi: 10.1097/00007632-199510001-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tracy BM, Doremus RH. Direct electron microscopy studies of the bone-hydroxylapatite interface. J Biomed Mater Res. 1984;18:719–726. doi: 10.1002/jbm.820180702. [DOI] [PubMed] [Google Scholar]
- 28.Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990;72:298–302. doi: 10.1302/0301-620X.72B2.2155908. [DOI] [PubMed] [Google Scholar]
- 29.Zdeblick TA, Cooke ME, Kunz DN, Wilson D, McCabe RP. Anterior cervical discectomy and fusion using a porous hydroxyapatite bone graft substitute. Spine (Phila Pa 1976) 1994;19:2348–2357. doi: 10.1097/00007632-199410150-00017. [DOI] [PubMed] [Google Scholar]
- 30.Zdeblick TA, Ghanayem AJ, Rapoff AJ, Swain C, Bassett T, Cooke ME, et al. Cervical interbody fusion cages. An animal model with and without bone morphogenetic protein. Spine. 1998;23:758–765. doi: 10.1097/00007632-199804010-00002. discussion 766. [DOI] [PubMed] [Google Scholar]