Abstract
Objective
The purpose of this study was to analyze in detail the relationship between outcome and time course of effect in medically refractory primary cervical dystonia (CD) with phasic type that was treated by bilateral globus pallidus internus (Gpi) deep brain stimulation (DBS).
Methods
Six patients underwent bilateral implantation of DBS into the Gpi under the guide of microelectrode recording and were followed for 18.7 ± 11.1 months. The mean duration of the CD was 5.8 ± 3.4 years. The mean age at time of surgery was 54.2 ± 10.2 years. Patients were evaluated with the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) and relief scale using patient self-reporting.
Results
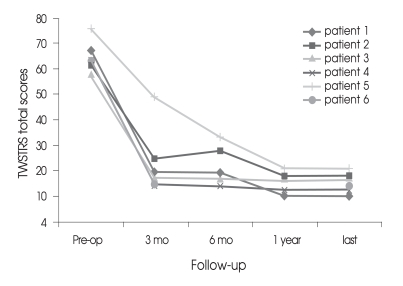
The TWSTRS total scores improved by 64.5%, 65.5%, 75.8%, and 76.0% at 3, 6, 12 months, and at the last available follow-up after surgery, respectively. Statistically significant improvements in the TWSTRS scores were observed 3 months after surgery (p = 0.028) with gradual improvement up to 12 months after surgery, thereafter, the improvement was sustained. However, there was no statistically significant difference between the scores at 3 and 12 months. Subjective improvement reported averaged 81.7 ± 6.8% at last follow-up. Mild dysarthria, the most frequent adverse event, occurred in 3 patients.
Conclusions
Our results show that the bilateral Gpi-DBS can offer a significant therapeutic effect from 3 months postoperatively in patients with primary CD with phasic type, without significant side effects.
Keywords: Globus pallidus, Cervical dystonia, Deep brain stimulation
INTRODUCTION
Idiopathic cervical dystonia (CD) is the most common form of focal dystonia in adult1). CD is characterized by abnormal posture of the head and neck, limitation of head movements, and hypertrophy of overactive muscles with or without involuntary head movements. The three basic types of CD are tonic, phasic and tremulous2). The phasic type of CD is characterized by an abnormal mobile attitude of the head. Chronic bilateral deep brain stimulation (DBS) of the globus pallidus internus (Gpi) has proven useful for patients with complex CD who are poor candidates for peripheral denervation procedures3,5-7,9). Such patients include those with continuous phasic movements, marked dystonic head tremor or myoclonus, severe retrocollis, sagittal and lateral translations, and anterocollis3,7). The literature reports only a few case series regarding pallidal stimulation for different types of CD3,6). Very few attempts have been made at a case series using only phasic type CD. In this report, we retrospectively assess the experiences of 6 patients with medically refractory primary CD with pure phasic type, who were treated by bilateral Gpi-DBS. The purpose of this study was to analyze in great detail the relationship between outcome and time course of effect in the patients.
MATERIALS AND METHODS
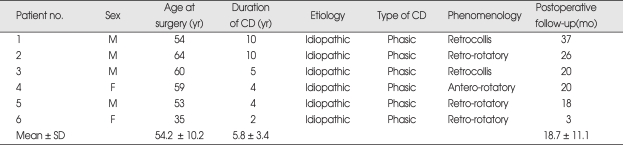
Between May 2006 and December 2008, six patients were identified who met the following criteria : 1) primary CD; 2) adult onset; 3) significant disability; 4) phasic-type CD; 5) medically insufficient benefit from other treatments, including botulinum toxin injection. None of the patients had major psychiatric disorders, dementia, or a familial history of dystonia. Magnetic resonance imaging (MRI) of the brain and a medical laboratory evaluation had not revealed abnormal findings, and neurological examination was normal with the exception of CD. The clinical features of patients are summarized in Table 1. Patient 3-6 had combined slight blinking eyelids without prolonged spasms of eye closure and grimacing. Patient 5 had combined upper-limb dystonia and had a history of bilateral microvascular decompression for the treatment of CD. All patients had received botulinum toxin and medication treatment with no remarkable improvement. Patients were evaluated with the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) before and every 3 months after surgery by one neurosurgeon and one neurologist. The relief scale was assessed by using patients' self-report of their subjective improvement in percentage every 3 months after surgery. Five patients with follow-up evaluations spanning more than 12 months were then retrospectively analyzed.
Table 1.
Clinical features of patients with primary cervical dystonia with phasic type
CD : cervical dystonia, SD : standard deviation
Six patients underwent simultaneous bilateral implantation of quadripolar DBS leads (DBS Model 3387, Medtronic, Brooklyn, NY, USA) into the Gpi under local anesthesia. Electrode implantation was based on indirect and direct targeting using 1.5-tesla MRI (General Electric, USA) and a Neurosurgery Simulator (Dimos, Seoul, Korea) as surgical planning tools with refinement using microelectrode recording and stimulation (Fig. 1). The initially planned mean target was 3.0 ± 0.3 mm anterior, 22.3 ± 0.6 mm lateral to the midcommissural point, and 3.9 ± 0.2 mm below the commissural plane. The mean distance between the anterior and posterior commissure was 23.5 ± 1.1 mm. The mean arc and sliding angles were 62.8 ± 4.2 and 8.1 ± 3.2 degrees, respectively. The mean length of Gpi in the final target was measured at 6.5 ± 1.4 mm in the left and 6.1 ± 1.2 mm in the right. Intraoperative test stimulation using a microelectrode was performed at 1.0 to 4.0 V, 60 to 200 µs and 130 Hz and motor, sensory and visual responses were checked. The final mean target was 3.2 ± 0.9 mm anterior, 22.7 ± 0.9 mm lateral, and 4.4 ± 1.1 mm inferior to the midcommissural point (Fig. 2). All patients underwent a MRI or CT postoperatively for assessment of the electrode's position and possible surgical complications. After 1 to 3 days (mean 2.4) of a trial test (Model 3625 external tester, Medtronic Inc.) in the hospital ward, the stimulation device (Soletra, Medtronic Inc.) was implanted subcutaneously in the subclavicular area while the patient was under general anesthesia. The stimulation parameters of the test stimulation were : frequency, 130 Hz; pulse width, 60 (in two patients) or 210 (in four patients) µs; and amplitude, 2.5 V. During test stimulation, all patients showed marked improvement, described themselves as very satisfied, and reported that CD was improved more than 80%. An implantable pulse generator was programmed on the first postoperative day. Electrode contacts were used with a setting of monopolar 0- at first programming except that the left side of the patient 5 was set monopolar 1-. The mean amplitude was 2.5 ± 0.53 V, the pulse width was 160 ± 77.4 µs, and the frequency was 130 Hz in all patients. Initially, the stimulators were set at lower amplitude to induce therapeutic effects without side effects.
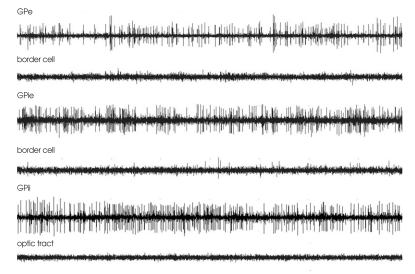
Fig. 1.
Microelectrode recordings in the globus pallidus of patient 3 at different depths along the stereotactic trajectory. GPe : globus pallidus externa, GPie : external segment of globus pallidus internus, GPii : internal segment of globus pallidus internus.
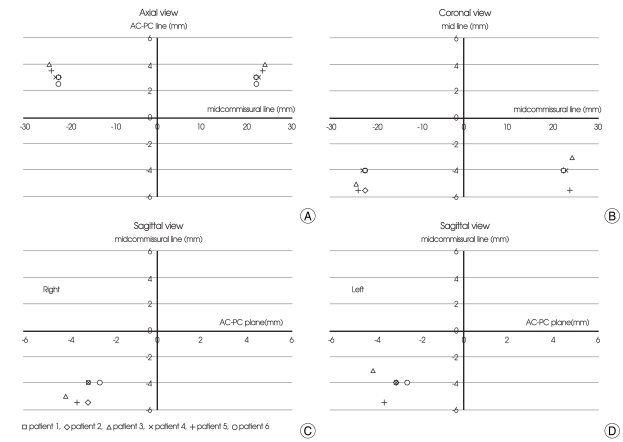
Fig. 2.
Scatterplots of the lowest electrode position (the final target) at the axial, coronal and sagittal planes. A, AC-PC plane. B, anterior 3.5 mm to midcommissural point. C, right lateral 22 mm to mid-line. D, left lateral 22 mm to mid-line. AC : anterior commissure, PC : posterior commissure.
Statistical analysis was used. The Wilcoxon signed-rank test and the Repeated measures analysis of variance compared the preoperative to postoperative TWSTRS scores using the statistical package for the social sciences software (SPSS) (Ver. 17.0; Inc, Chicago, IL,USA). P values less than 0.05 were considered to be statistically significant.
RESULTS
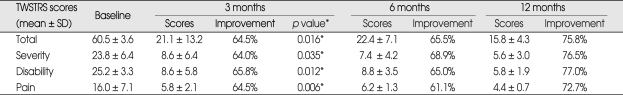
The follow-up period was 18.7 ± 11.1 months (range 3-35). Five of six patients were available for analysis of more than one year. All patients showed marked improvement in CD at the immediate postoperative period of test stimulation and CD relapsed in four patients (patients 1, 2, 5, 6) within 1 week after surgery. The improvement of CD continued up to the last follow-up without transient exacerbation in patients 3 and 4. The TWSTRS total scores improved by 64.5%, 65.5%, 75.8%, and 76.0% at 3, 6, 12 months, and at the last available follow-up after surgery, respectively (Table 2). TWSTRS scores was reduced significant improvements at 3 months postoperatively (p = 0.016, Repeated measures analysis of variance) and it was not improved significantly after 3 months postoperatively (p = 0.109, Wilcoxon signed-raked test). The TWSTRS subscale scores, including measurements of severity, disability and pain also improved at a similar rate compared to the individual subscores and the total scores (Table 2). Individual postoperative TWSTRS total scores improved significantly and gradually, compared to the preoperative TWSTRS total scores (Fig. 3). Subjective improvement reported by the patient averaged 81.7 ± 6.8% at the last follow-up. Patient 2 reported mild exacerbation of CD at the 6 months follow-up, and was reset the stimulation parameter. His clinical improvement was similar to other patients at 9 months and last follow-up. Extracervical dystonia was nearly abolished in the patients. The stimulation parameters at the last follow-up were : frequency, 130 ± 0 Hz; pulse width, 200 ± 15 (the right) or 185 ± 45 (the left) µs and amplitude, 3.4 ± 0.7 V. The electrode contacts were set according to the depth of the electrode and 0- in all patients except the left lead of patient 5. The frequency was set to 130 Hz as relatively high frequency all along. No significant peri-operative complication occurred. No patient had a permanent major complication due to a hardware-related problem or to chronic stimulation. Patients sometimes experienced transient reversible side effects such as mild gait disturbance (33%) and difficulty in speaking and swallowing (50%) during adjustment of the DBS settings. CD suddenly relapsed in patient 1 at 21 months after surgery. His batteries had run out, so the batteries were replaced. Subsequently, his stimulation system turned on after replacement and his CD stabilized.
Table 2.
Clinical outcomes following pallidal deep brain stimulation
TWSTRS total scores are significantly better at 3 months (p=0.016, Repeated measures analysis of variance) postoperatively in comparison to baseline scores. Statistically significant difference was not observed between the scores at 3 and 12 months (p=0.109, Wilcoxon signed-rank test). significant p value (< 0.005). *Repeated measures analysis of variance. TWSTRS : Toronto Western Spasmodic Torticollis Rating Scale, SD : standard deviation
Fig. 3.
Postoperative course showing dramatic but gradual and sustained improvement in the Toronto Western Spasmodic Torticollis Rating Scale(TWSTRS). Pre-op. : preoperation
DISCUSSION
The present retrospective analysis of 6 patients with medically refractory primary phasic-type CD with mean follow-up period of 18.7 months contributes to our understanding of the outcome and time course of effect of treatment by bilateral Gpi-DBS. The Gpi-DBS provided statistically significant improvements in TWSTRS scores at 3 months postoperatively, and gradual improvement up to 12 months of follow-up duration, thereafter, the improvement was sustained. However, there was no statistically significant difference between the TWSTRS scores at 3 and 12 months. These results correspond with the results of earlier studies which reported that phasic movement improves earlier than tonic symptoms3,7,9). Studies involving different types of CD also reported that TWSTRS scores were significantly better at 6 and 12 months or the last follow-up of 31.9 months3,6). In one recent report, patients reached maximum improvement at about 2 years postoperatively for spasmodic torticollis12). Previous case series often combined different types of CD, and assessing the outcome of each type based on the pallidal DBS was limited3,6,7,12). The mean improvement in TWSTRS total scores in their reports ranged from 54.4% to 65.4%, with a mean follow-up period of 12 months to 31.9 months. In our study, TWSTRS total scores improved up to 76.0% (severity = 75.5%, disability = 77.5%, and pain = 74.4%) with a mean follow-up period of 18.7 months. These results suggest that the patients with CD with phasic movements showed better improvement than those with tonic or tremulous movements. However, these finding are inconsistent with the result of the earlier study, which reported that clinical types of CD are not a predictive factor of the outcome3,6,7). The published series reported that the patients with shorter disease duration might expect a better outcome4,9). In our study, however, we could not assess this factor yet. Although the posteroventral Gpi is now the preferred site of stimulation for most dystonia, the optimal site within Gpi for stimulation remains unclear3,11). Our permanent electrode was inserted 3.2 mm anterior to the midcommissural point, 22.7 mm lateral of the midline, and 4.4 mm below the commissural plane. The implanted target was slightly more anteriorly and laterally selected in the Gpi in our series because of the fear of irritation of the internal capsule compared to previous studies6,7,10). Accordingly, we expected to see stimulation in a wider area of the Gpi. Stimulation parameters in CD showed that a frequency of 130 Hz, together with tolerable highest voltage from the beginning stimulation, was associated with better dystonia improvement7-9). We usually started the stimulation using 130 Hz and 210 µs as lower amplitude to induce therapeutic effects without side effects compared to the previous studies6-9). If no benefit was noted, the amplitude increased step by step at regular follow-up evaluations. The most common complication in our experience was associated with stimulation-induced dysphasia and speech disturbance similar to that reported by other group3,6,7,9). CD patients may suffer a higher incidence of extension or lead fracture than other DBS patients7,13), although we have no hardware-related problems.
CONCLUSION
Gpi-DBS can improve phasic CD significantly from 3 months postoperatively compared to baseline. Also, the procedure can provide dramatic but gradual and sustained improvement of CD with phasic type. Further studies need to be performed to evaluate clinical outcome predictors and also to identify the optimal targets for Gpi-DBS of primary CD within the large homogenous group of the patients.
Fig. 4.
Postoperative course showing dramatic but gradual and sustained improvement in the Toronto Western Spasmodic Torticollis Rating Scale(TWSTRS). Pre-op. : preoperation
References
- 1.Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121:547–560. doi: 10.1093/brain/121.4.547. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Heinen F, Kleedorfer B, Wagner M, Lucking CH, Poewe W. Clinical and polymyographic investigation of spasmodic torticollis. J Neurol. 1992;239:9–15. doi: 10.1007/BF00839204. [DOI] [PubMed] [Google Scholar]
- 3.Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM, et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–459. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- 4.Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia : the role of disease duration. Brain. 2008;131:1895–1902. doi: 10.1093/brain/awn120. [DOI] [PubMed] [Google Scholar]
- 5.Jang KS, Park HK, Joo WI, Ji C, Lee KJ, Choi CR. Selective peripheral denervation for the treatment of spasmodic torticollis. J Korean Neurosurg Soc. 2005;37:350–353. [Google Scholar]
- 6.Kiss ZH, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- 7.Krauss JK, Loher TJ, Pohle T, Weber S, Taub E, Bärlocher CB, et al. Pallidal deep brain stimulation in patients with cervical dystonia and severe cervical dyskinesias with cervical myelopathy. J Neurol Neurosurg Psychiatry. 2002;72:249–256. doi: 10.1136/jnnp.72.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol. 2004;115:2431–2441. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 10.Laitinen LV, Bergenheim AT, Hariz MI. Leksell's posteroventral pallidotomy in the treatment of Parkinson's disease. J Neurosurg. 1992;76:53–61. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- 11.Vayssiere N, van der Gaag N, Cif L, Hemm S, Verdier R, Frerebeau P, et al. Deep brain stimulation for dystonia confirming a somatotopic organization in the globus pallidus internus. sonographic monitoring of electrode placement into the globus pallidus internus. J Neurosurg. 2004;101:181–188. doi: 10.3171/jns.2004.101.2.0181. [DOI] [PubMed] [Google Scholar]
- 12.Yianni J, Bain PG, Gregory RP, Nandi D, Joint C, Scott RB, et al. Post-operative progress of dystonia patients following globus pallidus internus deep brain stimulation. Eur J Neurol. 2003;10:239–247. doi: 10.1046/j.1468-1331.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 13.Yianni J, Nandi D, Shad A, Bain P, Gregory R, Aziz T. Increased risk of lead fracture and migration in dystonia compared with other movement disorders following deep brain stimulation. J Clin Neurosci. 2004;11:243–245. doi: 10.1016/j.jocn.2003.10.003. [DOI] [PubMed] [Google Scholar]