Abstract
Objective
Combined hyperative dysfunction syndrome (HDS) defined as the combination of HDSs such as trigeminal neuralgia (TN), hemifacial spasm (HFS) and glossopharyngeal neuralgia (GPN), which may or may not occur simultaneously on one or both sides. We reviewed patients with combined HDS and demonstrated their demographic characteristics by comparing them with those of patients with a single HDS.
Methods
Between October 1994 and February 2006, we retrospectively studied a series of 1,720 patients who suffered from HDS and found 51 patients with combined HDSs. We analyzed several independent variables in order to evaluate the prevalence and etiologic factors of combined HDS.
Results
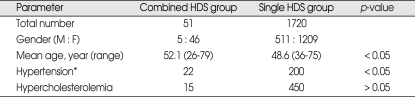
The combined HDS group accounted for 51 of 1,720 (2.97%) patients with HDS; 27 cases of bilateral HFS, 10 cases of bilateral TN and 14 cases of HFS with TN. Their mean age was 52.1 years (range, 26-79 years). There were 5 men and 46 women. Seven patients had synchronous and 44 patients metachronous onset of HDSs. By comparison of combined and single HDS groups, we found that age and hypertension were closely associated with the prevalence of combined HDS (p < 0.05).
Conclusion
This study revealed that combined HDS was very rare. Hypertension and age might be the most important causative factors to evoke combined HDS.
Keywords: Combined hyperactive dysfunction syndrome, Prevalence, Hemifacial spasm, Trigeminal neuralgia, Etiology
INTRODUCTION
A pathological condition caused by vascular compression at the root entry/exit zone of the cranial nerve such as trigeminal neuralgia (TN), hemifacial spasm (HFS), glossopharyngeal neuralgia (GPN) was defined hyperactive dysfunction syndrome (HDS) of the cranial nerve12,13). TN and HFS are the most common disease of HDS, whereas GPN is rare disease, with a relative frequency of TN ranging from 0.01% to 0.75%1,22,24). Furthermore, the combination of such diseases is extremely rare. Combined HDS was defined as the combination of HDSs which may or may not occur on one or both sides16). These symptoms, sometimes, can occur synchronously or metachronously. In the literature, arterial hypertension has been known to be closely associated with the HFS, however, there have been no comprehensive reports related to the incidence and etiologic factors of the combined HDS of cranial nerves6,14,21,28). Therefore, we reviewed the patients who visited our hospital to treat HDSs between October 1994 and February 2006, and analyzed those who exhibited a combination of HDSs retrospectively to study the incidence and etiological factors.
MATERIALS AND METHODS
Patients
Between October 1994 and February 2006, we searched the database registry at the department of neurosurgery to identify patients with newly diagnosed HDS who had been treated at our hospital, which is a tertiary referral institution. During this period, 1,720 patients visited the department of neurosurgery and neurology and were diagnosed as TN, HFS or GPN by thorough neurological examination and magnetic resonance imaging (MRI).
Inclusion criteria
Clinical diagnosis which satisfied the following diagnostic criteria for bilateral HDS was used : those with sequential onset of involuntary and asynchronous facial muscle contractions affecting one or more muscle groups which may or may not occur simultaneously on one or both sides. For TN with HFS or bilateral TN among the combined HDS, we included the synchronous or metachronous onset of both HDS.
Exclusion criteria
Other causing lesions such as arteriovenous malformation, brain tumor, facial tics, dystonia, hemimasticatory spasm, tardive dyskinesia, focal seizures, other forms of facial or oromandibular dystonic movements, a history of Bell's palsy, blepharospasm and metabolic diseases were excluded in this study. To differentiate these lesions, brain magnetic resonance angiography (MRA), MRI and electrophysiologic study as well as thorough and detailed clinical examination were examined10,11).
Evaluation of etiologic factors
We reviewed medical and surgical records for patients who exhibited combined HDS and compared the possible etiological factors with patients who had single HDS. Data from patients with single HDS were obtained from the data registry during the same period. Independent variables included age, sex, hypertension and hypercholesterolemia. Data were evaluated by X2 test to compare the characteristics of both subgroups. p value less than 0.05 was judged as a significant association. Statistical analysis used SPSS software program (version 11.0, Chicago, Illinois)19).
RESULTS
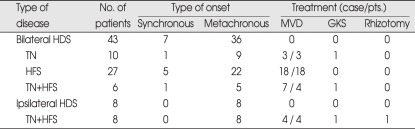
The combined HDS group accounted for 51 of 1,720 patients with HDS. Fourteen patients exhibited synchronously or metachronously HFS with TN. Twenty-seven patients had initially HFS on the ipsilateral side, and metachronously showed another HFS on the contralateral side. Ten patients demonstrated bilateral trigeminal neuralgia. There were 5 men and 46 women. The mean age at the time of treatment of patients with the combined HDS was 52.1 years (range, 26-79 years). According to the onset of involvement, 7 patients demonstrated synchronous onset and 44 patients had metachronous onset. Synchronous onset of combined HDS included 5 bilateral HFS, one bilateral TN and one bilateral TN with HFS (Table 1). For metachronous onset, symptom interval between the first and second rhizopathy was 37 months (range, 12-228 months). Twenty-nine of these patients underwent a total of 32 microvascular decompression procedures. Three patients received gamma knife radiosurgery for TN.
Table 1.
Clinical summary for comparison of bilateral and ipsilateral HDS
HDS : hyperactive dysfunction syndrome, TN : trigeminal neuralgia, HFS : hemifacial spasm, MVD : microvascular decompression, GKS : gamma-knife surgery, Pts. : patients
We also analyzed the affecting factors to the involvement of combined HDS and compared these possible variables with those who had single HDS group. By analyzing the affecting factors, we found that age and hypertension was closely associated with the involvement of combined HDS, compared with the single HDS group (p < 0.05) (Table 2).
Table 2.
Comparison of combined HDS with single HDS
*patients who exhibited elevated blood pressure (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) or receiving antihypertensive medication were categorized as the hypertensive group. HDS : hyperactive dysfunction syndrome
DISCUSSION
This cross-sectional study is the largest series published in the literature. In Norwegian study, the total prevalence of hemifacial spasm was 9.8 per 100,000. The prevalence increased with age to 39.7 among those older than 70 years23). TN has a prevalence of 0.1-0.2 per thousand and incidence ranging from about 4-5/100,000/year up to 20/100,000/year after age 60. A review of several case series shows that pain is more predominant on the right side, but the difference is not statistically significant8,9,27). We found a low prevalence of combined HDS among a large cohort of HFS patients, compatible with the 0.6% to 3% observed in some Caucasian series20). In our study, combined HDS was 51 of 1,720 HDS patients (2.97%) including 27 bilateral HFS.
This study demonstrated that hypertension and aging in single HDS group are the risk factors for developing combined symptom, but dyslipidemia has no correlation between combined and single HDS. The aging process and arteriosclerotic changes of the vessel, together with hemodynamic stress caused by hypertension, facilitate elongation and redundancy of vessels which begin to compress the root entry zone of the cranial nerves more consistently to cause HDS3,6,7,14,24).
Although hypertension and aging are proven as risk factors for developing combined HDS, other risk factors have to be identified such as individual susceptibility (lower threshold), familial history and races15). One hypothesis for the more frequent occurrence of HDS in Asians could be due to the relatively smaller posterior fossa amongst some of these races. In a computed tomographic study, the cerebellopontine angle cistern of Japanese patients with HFS was narrower, resulting in more crowded neurovascular structures compared to controls8). It has been suggested that such anatomic restriction may be a possible factor in facilitating neurovascular compression (NVC) in HFS8). The mean latency between the onset of single HDS and combined HDS was 36 months, within the range of 0-228 months. microvascular decompression (MVD) in bilateral HFS is done only for first onset site, but some patients who had HFS with TN or TN with TN underwent MVD surgery due to uncontrolled trigeminal neuralgia2,4).
NVC of the facial nerve may not always leads to symptoms as NVC has been demonstrated in normal controls without HFS and in the contralateral asymptomatic side in HFS18,27). Tan and Jankovic27). examined 40 HFS patients with unilateral symptoms, and found a 15% (n = 6) prevalence of contralateral NVC, but as high as 95% ipsilateral NVC symptom5,27). This suggests that mere presence of contralateral NVC has poor predictive value of bilateral HFS. It is unclear how many of the six HFS patients with bilateral NVC would develop bilateral symptoms eventually. Only long-term follow-up of these patients could address this question25,26). It is conceivable that higher cortical modulation of the facial motor nucleus may partly explain why only some patients with NVC develop symptoms and others do not. In a case control transcranial magnetic stimulation study, electrophysiological evidence of a cortical influence of HFS could be demonstrated29). Clinical observations have shown that stress and emotional factors are known to precipitate HFS5). Genetic susceptibility could also determine whether NVC results in clinical symptoms. Familial HFS with presumed autosomal mode of inheritance has been described17).
CONCLUSION
This study revealed that combined HDS was very rare. Hypertension and age might be the most important causative factors to evoke combined HDS. Although hypertension and aging are proven as risk factors for developing combined HDS, other risk factors have to be identified such as individual susceptibility (lower threshold), familial history and races.
References
- 1.Ballantyne ES, Page RD, Meaney JF, Nixon TE, Miles JB. Coexistent trigeminal neuralgia, hemifacial spasm, and hypertension : preoperative imaging of neurovascular compression. Case report. J Neurosurg. 1994;80:559–563. doi: 10.3171/jns.1994.80.3.0559. [DOI] [PubMed] [Google Scholar]
- 2.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The Long-Term Outcome of Microvascular Decompression for Trigeminal Neuralgia. N Engl J MED. 1996;334:1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 3.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995;82:201–210. doi: 10.3171/jns.1995.82.2.0201. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71:359–367. doi: 10.3171/jns.1989.71.3.0359. [DOI] [PubMed] [Google Scholar]
- 5.Carter JB, Patrinely JR, Jankovic J, McCrary JA, III, Boniuk M. Familial hemifacial spasm. Arch Ophthalmol. 1990;108:249–250. doi: 10.1001/archopht.1990.01070040101040. [DOI] [PubMed] [Google Scholar]
- 6.Defazio G, Berardelli A, Abbruzzese G, Coviello V, De Salvia R, Federico F, et al. Primary hemifacial spasm and arterial hypertension : a multicenter case-control study. Neurology. 2000;54:1198–1200. doi: 10.1212/wnl.54.5.1198. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda H, Ishikawa M, Okumura R. Demonstration of neurovascular compression in trigeminal neuralgia and hemifacial spasm with magnetic resonance imaging : comparison with surgical findings in 60 consecutive cases. Surg Neurol. 2003;59:93–99. doi: 10.1016/s0090-3019(02)00993-x. [DOI] [PubMed] [Google Scholar]
- 8.Girrard N, Poncet M, Caces F. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology. 1997;39:46–51. doi: 10.1007/s002340050366. [DOI] [PubMed] [Google Scholar]
- 9.Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain : the UK primary care perspective. Pain. 2006;122:156–162. doi: 10.1016/j.pain.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Huang CI, Chen IH, Lee LS. Microvascular decompression for hemifacial spasm : analyses of operative findings and results in 310 patients. Neurosurgery. 1992;30:53–57. doi: 10.1227/00006123-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa M, Ohira T, Namiki J, Ajimi Y, Takase M, Toya S. Abnormal muscle response (lateral spread) and F-wave in patients with hemifacial spasm. J Neurol Sci. 1996;137:109–116. doi: 10.1016/0022-510x(95)00308-o. [DOI] [PubMed] [Google Scholar]
- 12.Jannetta PJ. Observations on the etiology of trigeminal neuralgia, hemifacial spasm, acoustic nerve dysfunction and glossopharyngeal neuralgia. Definitive microsurgical treatment and results in 117 patients. Neurochirurugia (Stuttg) 1977;20:145–154. doi: 10.1055/s-0028-1090369. [DOI] [PubMed] [Google Scholar]
- 13.Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977;47:321–328. doi: 10.3171/jns.1977.47.3.0321. [DOI] [PubMed] [Google Scholar]
- 14.Jannetta PJ, Segal R, Wolfson SK., Jr Neurogenic hypertension : etiology and surgical treatment. I. Observations in 53 patients. Ann Surg. 1985;210:391–398. doi: 10.1097/00000658-198503000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiguchi H, Ohira T, Ochiai M, Kawase T. Computed tomographic analysis of hemifacial spasm : narrowing of the posterior fossa as a possible facilitating factor for neurovascular compression. J Neurol Neurosurg Psychiatry. 1997;62:532–534. doi: 10.1136/jnnp.62.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo A. Follow-up results of microvascular decompression in trigeminal neuralgia and hemifacial spasm. Neurosurgery. 1997;40:46–52. doi: 10.1097/00006123-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kong DS, Park K. Hemifacial spasm : A Neurosurgical Perspective. J Korean Neurosurg Soc. 2007;42:355–362. doi: 10.3340/jkns.2007.42.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotterba S, Tegenthoff M, Malin JP. Hemifacial spasm or somatoform disorder-postexcitatory inhibition after transcranial magnetic cortical stimulation as a diagnostic tool. Acta Neurol Scand. 2000;101:305–310. doi: 10.1034/j.1600-0404.2000.90281a.x. [DOI] [PubMed] [Google Scholar]
- 19.Linskey ME, Jho HD, Jannetta PJ. Microvascular decompression for trigeminal neuralgia caused by vertebrobasilar compression. J Neurosurg. 1994;81:1–9. doi: 10.3171/jns.1994.81.1.0001. [DOI] [PubMed] [Google Scholar]
- 20.Manzoni GC, Torelli P. Epidemiology of typical and atypical craniofacial neuralgias. Neurol Sci. 2005;26:S65–S67. doi: 10.1007/s10072-005-0410-0. [DOI] [PubMed] [Google Scholar]
- 21.Møller AR, Jannetta PJ. Monitoring facial EMG responses during microvascular decompression operations for hemifacial spasm. J Neurosurg. 1987;66:681–685. doi: 10.3171/jns.1987.66.5.0681. [DOI] [PubMed] [Google Scholar]
- 22.Morales F, Albert P, Alberca R, de Valle B, Narros A. Glossopharyngeal and vagal neuralgia secondary to vascular compression of the nerves. Surg Neurol. 1977;8:431–433. [PubMed] [Google Scholar]
- 23.Nilsen B, Le KD, Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology. 2004;26:1532–1533. doi: 10.1212/01.wnl.0000142080.85228.e8. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira LD, Cardoso F, Vargas AP. Hemifacial spasm and arterial hypertension. Mov Disord. 1999;14:832–835. doi: 10.1002/1531-8257(199909)14:5<832::aid-mds1017>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64–69. doi: 10.1227/00006123-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Sanders DB. Ephaptic transmission in hemifacial spasm : a single fiber EMG study. Muscle Nerve. 1989;12:690–694. doi: 10.1002/mus.880120810. [DOI] [PubMed] [Google Scholar]
- 27.Tan EK, Jankovic J. Bilateral hemifacial spasm : a report of five cases and a literature review. Mov Disord. 1999;14:345–349. doi: 10.1002/1531-8257(199903)14:2<345::aid-mds1023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Tan EK, Jankovic J. Psychogenic hemifacial spasm. J Neuropsychiatry. Clin Neurosci. 2001;13:380–384. doi: 10.1176/jnp.13.3.380. [DOI] [PubMed] [Google Scholar]
- 29.Valls-Sole J. Electrodiagnostic studies of the facial nerve in peripheral facial palsy and hemifacial spasm. Muscle Nerve. 2007;36:14–20. doi: 10.1002/mus.20770. [DOI] [PubMed] [Google Scholar]