Abstract
Objective
The definition of empty sella syndrome is 'an anatomical entity in which the pituitary fossa is partially or completely filled with cerebrospinal fluid, while the pituitary gland is compressed against the posterior rim of the fossa'. Reports of this entities relating to the brain tumors not situated in the pituitary fossa, have rarely been reported.
Methods
In order to analyze the incidence and relationship of empty sella in patients having brain tumors, the authors reviewed preoperative magnetic resonance imaging (MRI) of 72 patients with brain tumor regardless of pathology except the pituitary tumors. The patients were operated in single institute by one surgeon. There were 25 males and 47 females and mean patient age was 53 years old (range from 5 years to 84 years). Tumor volume was ranged from 2 cc to 238 cc.
Results
The overall incidence of empty sella was positive in 57/72 cases (79.2%). Sorted by the pathology, empty sella was highest in meningioma (88.9%, p = 0.042). The empty sella was correlated with patient's increasing age (p = 0.003) and increasing tumor volume (p = 0.016).
Conclusion
Careful review of brain MRI with periodic follow up is necessary for the detection of secondary empty sella in patients with brain tumors. In patients with confirmed empty sella, follow up is mandatory for the management of hypopituitarism, cerebrospinal fluid (CSF) rhinorrhea, visual disturbance and increased intracranial pressure.
Keywords: Empty sella, Brain tumor, Increased intracranial pressure
INTRODUCTION
The term 'empty sella' was first used by Busch1) in 1951 to indicate a peculiar anatomical condition, observed in human cadavers, particularly females, characterized by a sella turcica only partially filled by a pituitary gland severely flattened against the sellar floor.
The term 'primary empty sella' is used to refer to the conditions when it is unrelated to previous surgical, pharmacological, or radiotherapeutic treatment of the sellar region. In this category, congenital diaphragmatic defect is included5). Secondary empty sella syndrome occurs following successful transsphenoidal removal of a pituitary tumor and also occurs after pharmacological or radiotherapeutic treatment of the sellar region5).
Empty sella is also found in patients with brain tumors not situated in the pituitary fossa. While cases of empty sella resulting from primary pituitary tumors and it's surgical treatment is frequently reported2-4), report of the incidence and it's relationship to other brain tumors has been rarely reported. We would like to temporarily define these cases as 'empty sella syndrome secondary to the brain tumors' as it seems to be caused by increased intracranial pressure from existing brain tumors.
In order to analyze the incidence and relationship of empty sella in patients having brain tumors, the authors reviewed preoperative MRI of 72 patients with brain tumors regardless of pathology except pituitary tumors.
MATERIALS AND METHODS
The authors retrospectively reviewed and analyzed. The patients were operated in our institute from January 2000 to January 2009. Patients were categorized by initial tumor volume (range from 2 cc to 238 cc, average tumor volume was 70 cc). Mean patient age was 53 years old (ranged from 5 years to 84 years). There were 25 males and 47 females. The supratentorial tumors were 56 cases and infratentorial tumors were 16 cases. These patients were never treated or diagnosed for pituitary pathology such as pituitary adenoma, and so we could differentiate these patients from primary or conventional secondary empty sella patients.
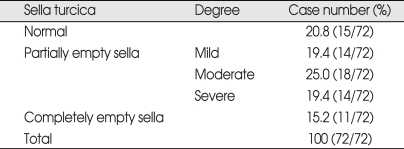
To determine the severity of the empty sella, the loss of pituitary height (concavity) on the mid-sagittal T1-weighted image was classified into five categories : I = normal, II = superior concavity that was mild (< 1/3 the height of the sella), III = moderate (between 1/3 and 2/3 concavity of height of the sella), IV = severe (> 2/3 concavity of height of the sella), and V = empty sella (no appreciable pituitary parenchyma observed). And, defined category II to category V as empty sella patients.
In this study, we excluded tumors such as pituitary adenomas and parasellar tumors which directly invaded the sella that were related to the secondary empty sella syndrome. In view of these findings, we sought to reveal the nature, the possible causative factors for the empty sella.
Illustrative cases
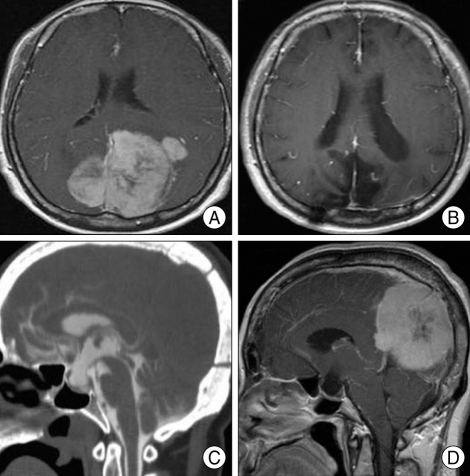
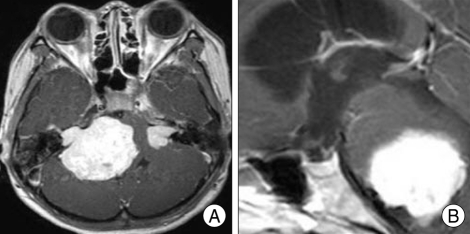
A patient visited our institute complaining chronic headache of 10 years duration and decreasing visual acuity of 1 year duration. The patient was a 59-year-old female and brain MRI image revealed a huge posterior falx meningioma (Fig. 1A).
Fig. 1.
Preoperative and postoperative images of the patient. A : Preoperative T1-weighted enhanced axial image. Huge posterior falx meningioma. B : Postoperative T1-weighted enhanced sagittal image reveals total removal of tumor. C : Postoperative CT-cisternography shows contrast leakage through pituitary fossa. D : Postoperative T1-weighted enhanced mid-sagittal image. Huge posterior falx meningioma associated with complete empty sella filled with cerebrospinal fluid.
Parieto-occipital craniotomy was done and the Simpson grade I tumor removal was achieved. Postoperative brain MRI revealed total removal of tumor (Fig. 1B). On the postoperative day 7, however, spontaneous cerebrospinal fluid (CSF) rhinorrhea was observed. Computerized tomography (CT)-cisternography (Fig. 1C) was performed and CSF leakage point detected, which was obliterated by transsphenoidal fat packing. We could find empty sella secondary to the brain tumor in reviewing of the preoperative mid-sagittal MRI (Fig. 1D).
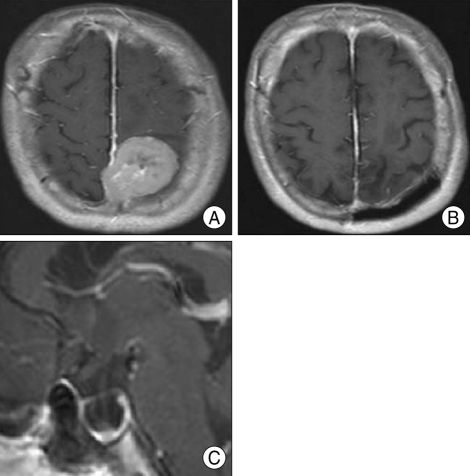
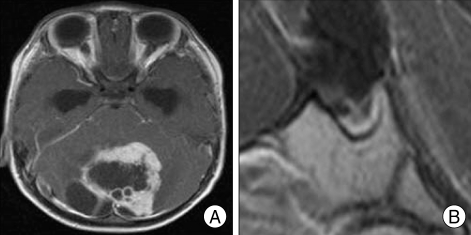
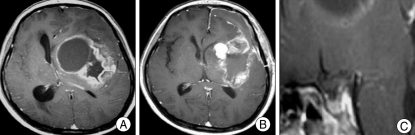
Review of MRI of the various brain tumor patients also revealed empty sella secondary to the brain tumors. For example, 63-year-old woman whose biopsy was confirmed for parasagittal meningioma, showed MRI of severe concavity of height of the sella (Fig. 2). Preoperative MRI of 3-year-old male patient whose pathology was confirmed for cerebellar pilocytic astrocytoma showed severe degree of empty sella (Fig. 3). A 57-year-old glioblastoma patient's MRI revealed mild concavity of height of the sella (Fig. 4), and 25-year-old neurofibromatosis type 2 (NF-2) patient's preoperative MRI revealed moderate concavity of height of the sella (Fig. 5).
Fig. 2.
Preoperative and postoperative images of the patient. A : Preoperative T1-weighted enhanced axial magnetic resonance image (MRI) shows left parasagittal meningioma. B : T1-weighted enhanced axial MRI after 18 months postoperatively. Through parieto-occipital interhemispheric approach, tumor was removed totally. C : The patient's preoperative T1-weighted enhanced mid-sagittal image shows severe concavity of height of the sella.
Fig. 3.
T1-weighted enhanced magnetic resonance image of pilocytic astrocytoma patient. A : Preoperative axial image. B : Preoperative mid-sagittal image shows severe concavity of height of the sella.
Fig. 4.
Magnetic resonance image (MRI) of the glioblastoma patient. A : Preoperative T1-weighted enhanced axial image shows peripheral rim-enhancing mass in left basal ganglia. B : MRI of 1 month after surgery. C : Preoperative mid-sagittal MRI revealed mild concavity of height of the sella.
Fig. 5.
Magnetic resonance image of the neurofibromatosis type 2 patient. A : Preoperative T1-weighted enhanced axial image shows bilateral cerebellopontine angle mass. B : Preoperative T1-weighted enhanced mid-sagittal image revealed moderate concavity of height of the sella.
RESULTS
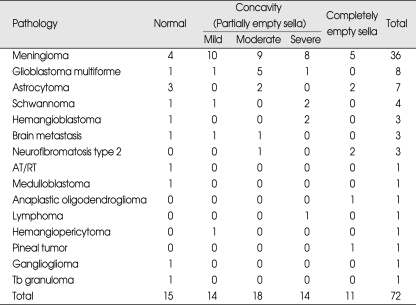
The incidence of secondary empty sella was positive in 57/72 cases (79.2 %) and negative in 15/72 cases (20.8%) (Table 1). Sorted by the pathology of tumor, incidence of empty sella in 1) meningioma was 32/36 cases (88.9%) except the 4 cases where the tumor had invaded the pituitary fossa, 2) glioma was in 7/8 cases, 87%, 3) astrocytoma was 4/7 cases, 57%, 4) shwannoma was 3/4 cases, 5) hemangioblastoma was 2/3 cases, 6) metastatic brain tumor was 2/3 cases, 7) NF-2 was 3/3 case, 8) atypical teratoid rhabdoid tumor (AT/ RT) was 0/1 case, 9) medulloblastoma was 0/1 cases, 10) anaplastic oligodendroglioma was 1/1 case, 11) lymphoma was 1/1 case, 12) hemangiopericytoma was 1/1 case, 13) pineal tumor was 1/1 case (Table 2).
Table 1.
Incidence of the empty sella (morphologic change) secondary to the brain tumors
*normal - The superior aspect of pituitary gland was either convex or flat Mild - h < 1/3 of H, Moderate - 1/3H < h < 2/3H, Severe - 2/3H < h. Empty sella - no observable pituitary parenchyma. H-height of the sella, h : height of the pituitary.
Table 2.
Qualitative analysis of pituitary morphology
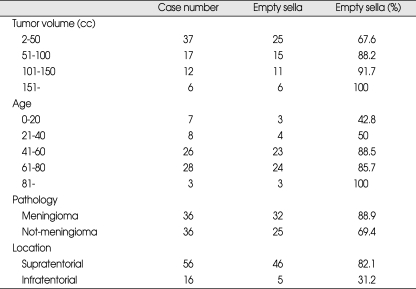
In regard to tumor volumes, average tumor volume was 70 cc in all patients and 38 cc (15 cases) in normal pituitary fossa, and 79 cc (57 cases) in empty sella patients. Case number was highest between 1 to 50 cc in volume and p-value was 0.016 between tumor volume and incidence of empty sella (Table 3).
Table 3.
Tumor volume, patient's age, pathology, tumor location and incidence of empty sella
The incidence of empty sella in relation to patient's age (p = 0.003) (Table. 3) and tumor volume (p = 0.016) was significant. But, the correlation according tumor pathology was difficult to evaluate due to too few cases for certain pathologies. Therefore. tumor pathologies were categorized as meningiomas or non-meningiomas and the correlation was significant (p = 0.042) (Table 3).
DISCUSSION
The incidence of morphologic changes of pituitary gland (partially empty sella + completely empty sella) varies markedly among studies (10% to 94%).
Yuh et al.7) investigated morphologic changes of pituitary gland, i.e., empty sella, in patients with the clinical diagnosis of idiopathic intracranial hypertension. They regarded moderate concavity (h > 1/3 the height of the sella) of the superior surface of pituitary fossa as the threshold of abnormality, and the incidence of empty sella was 85% in patients with idiopathic intracranial hypertension and 13.5% in patients with acute increased intracranial pressure caused by head trauma. And, the incidence of the completely empty sella (no appreciable pituitary parenchyma observed) was only 2.5% of idiopathic intracranial hypertension patients.
For empty sella secondary to the brain tumor, however, has never been discussed in the literatures and the incidence of the morphologic changes of pituitary gland in our paper was 79.2%. To ascertain the correlation between the empty sella and increased intracranial pressure caused by brain tumors, we regarded mild concavity (< 1/3 the height of the sella) of the superior surface of pituitary fossa as the threshold of abnormality.
The incidence of empty sella seemed to increase with the increase of tumor volume. It was postulated that the larger the tumor size, the more pressure it will exert on the sella compared to small sized tumors. And the incidence also seems to be increased with aging.
In this analysis, slowly growing meningiomas showed higher incidence (88.9%) of empty sella despite the exclusion of the cases that invaded pituitary fossa directly (cavernous sinus meningioma, tuberculum sellae meningioma, clinoidal meningioma). Compared with benign tumors, rapidly growing glial tumors (glioblastoma multiforme and astrocytoma) showed relatively low incidence (73%) of empty sella in comparison to meningiomas. The low incidence of empty sella in aggressive glial tumors could be explained by the nature of that tumors which has a tendency to invade adjacent structures opposed to the nature of more slowly growing tumors which increase the intracranial pressure. The relative incidence of empty sella for other pathologies can not be discussed because of the relatively few cases.
The morphologic changes of the pituitary gland in brain tumor patients (empty sella secondary to the brain tumors) may be explained by long-standing raised intracranial pressure and obstruction of the CSF pathway which give rise to weakening of the sellar diaphragm, resulting in the herniation of the basal cisternal arachnoid membrane into the sella.
Non-symptomatic cases require no treatment but periodic follow up is necessary. Empty sella is associated with neuroradiological and endocrine symptoms. Indications for surgical treatment of empty sella3) is, 1) CSF rhinorrhea, because of the risk for meningitis5,6), 2) Visual disturbance, 3) Severely increased intracranial pressure.
It is postulated that the empty sella can be symptomatic, including CSF rhinorrhea, at any time since the sella floor filled with CSF. Although we can not explain the exact mechanism how the empty sella can cause CSF rhinorrhea, the possible mechanism that we think is like that.
In the case which we presented, because of long standing IICP, microscopic arachnoid defect could be formed before surgery. Due to the tight equilibrium that was formed between sella floor and arachnoid membrane which was descended by long standing IICP, CSF rhinorrhea was not present before surgery. Through the tumor surgery, tight contact between sellar floor and arachnoid membrane may have caused loosened and weak microscoic arachnoid membrane defect enlarged and through that space, CSF rhinorrhea could have been developed.
In clinical conditions about handling brain tumors, surgeon should be aware of the possibility that the empty sella can be complicated. And, during treatment period as well as after treatment period, surgeon must appreciate and prepare for possibilities of these complications and treatment options of these complications.
There are a substantial number of literatures on primary empty sella and empty sella caused by surgery, radiation or chemotherapy for pituitary fossa. But, there has been no definitive reports about the empty sella secondary to the brain tumors in the literature.
CONCLUSION
The patient who complicated with secondary empty sella need periodic check up and treatment about hypopituitarism, CSF rhinorrhea, visual disturbance. In brain tumor patient, careful examination about the incidence of secondary empty sella using brain MRI is needed. Further investigation and accumulation of cases are required.
References
- 1.Busch W. [Die Morphologie der Sella Turcica and ihre Beziehungen zur Hypophyse.] Virchows Arch. 1951;320:437–458. doi: 10.1007/BF00957474. [DOI] [PubMed] [Google Scholar]
- 2.Cansiz H, Inci E, Sekercioglu N. Spontaneous cerebrospinal fluid rhinorrhea associated with empty sella : atransnasal-transsphenoidal repair of the fistula. Kulak Burun Bogaz Ihtis Derg. 2003;10:110–113. [PubMed] [Google Scholar]
- 3.Keyaki A, Makita Y, Nabeshima S, Motomochi M, Itagaki T, Tei T. [Secondary empty sella syndrome : report of three cases and review of the literature.] No Shinkei Geka. 1982;11:1189–1194. [PubMed] [Google Scholar]
- 4.Lee TC, Yang LC, Huang PL. Treatment of empty sella syndrome with ventriculoperitoneal shunt. J Clin Neurosci. 2005;12:201–205. doi: 10.1016/j.jocn.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Maira G, Anile C, Mangiola A. Primary empty sella syndrome in a series of 142 patients. J Neurosurg. 2005;103:831–836. doi: 10.3171/jns.2005.103.5.0831. [DOI] [PubMed] [Google Scholar]
- 6.Spetzler RF, Wilson CB. Management of recurrent CSF rhinorrhea of the middle and posterior fossa. J Neurosurg. 1978;49:393–397. doi: 10.3171/jns.1978.49.3.0393. [DOI] [PubMed] [Google Scholar]
- 7.Yuh WT, Zhu M, Quets JP, Maley JE, Muhonen MG, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging. 2000;12:808–813. doi: 10.1002/1522-2586(200012)12:6<808::aid-jmri3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Zagardo MT, Kelman SE, Rothman MI. Reversible empty sella in idiopathic intracranial hypertension : an indicator of successful therapy? AJNR Am J Neuroradiol. 1996;17:1953–1956. [PMC free article] [PubMed] [Google Scholar]