Abstract
Objective
Several treatment options have proven effective for metastatic brain tumors, including surgery and stereotactic radiosurgery. Tumors with cystic components, however, are difficult to treat using a single method. We retrospectively assessed the outcome and efficacy of gamma knife radiosurgery (GKRS) for cystic brain metastases after stereotactic aspiration of cystic components to decrease the tumor volume.
Methods
The study population consisted of 24 patients (13 males, 11 females; mean age, 58.3 years) with cystic metastatic brain tumors treated from January 2002 to August 2008. Non-small cell lung cancer was the most common primary origin. After Leksell stereotactic frame was positioned on each patient, magnetic resonance images (MRI)-guided stereotactic cyst aspiration and GKRS were performed (mean prescription dose : 20.2 Gy). After treatment, patients were evaluated by MRI every 3 or 4 months.
Results
After treatment, 13 patients (54.2%) demonstrated tumor control, 5 patients (20.8%) showed local tumor progression, and 6 patients (25.0%) showed remote progression. Mean follow-up duration was 13.1 months. During this period, 10 patients (41.7%) died, but only 1 patient (4.2%) died from brain metastases. The overall median survival after these procedures was 17.8 months.
Conclusion
These results support the usefulness of GKRS after stereotactic cyst aspiration in patients with large cystic brain metastases. This method is especially effective for the patients whose general condition is very poor for general anesthesia and those with metastatic brain tumors located in eloquent areas.
Keywords: Cystic brain metastases, Gamma knife radiosurgery, Stereotactic cyst aspiration
INTRODUCTION
Many different treatments have proven effective for brain metastases, including surgery, stereotactic radiosurgery, whole brain radiation therapy (WBRT) and chemotherapy. Although no standard therapy has been defined, some general guidelines are available. The median survival is 1 month without treatment, with the administration of steroids increasing median survival to 2 months24). Median survival improves to about 4 months after WBRT4,7), to 6 months after gamma knife radiosurgery (GKRS) boost1), and to 1 year in patients with surgery prior to WBRT11). With either of the latter interventions, however, the survival benefit is attained only by patients with a single brain metastasis1,19).
Radiosurgery has gained increasing relevance for treatment of brain metastases and offers several advantages over resection. For example, radiosurgery can be used to treat multiple metastatic lesions and allows treatment of metastases in deep locations that would be considered surgically inaccessible9). Radiosurgery can also be used for patients with other major medical problems considered contraindications for general anesthesia and surgery.
Some brain metastases have large volumes because of cystic components. Sometimes complete control of the cystic component of the tumor may be a challenge for surgical removal. But, patient functional status, tumor multiplicity, and lesion location can be the real limitations for surgery. On the other hand, radiosurgery is not suitable for cystic metastatic tumors because of large volume. To treat such lesions by radiosurgery, it is necessary to decrease the volume of the cystic components.
We assessed the outcomes and efficacy of GKRS used to treat cystic brain metastases after stereotactic aspiration of cystic components to decrease the tumor volume.
MATERIALS AND METHODS
Between January 2002 and August 2008, 24 patients with large cystic brain metastases were referred for GKRS. Twenty-three of these patients had single large cystic brain metastases, whereas one patient had two large cystic lesions. All patients had a confirmed primary malignancy, based on pathologic examination of specimens obtained from primary extracranial sites. During the initial consultation, a detailed general and neurologic history was obtained, and physical and neurologic examinations were performed. The diagnosis of brain metastases were confirmed using magnetic resonance (MR) imaging. All patients were classified according to the recursive partitioning analysis (RPA) classification system of the Radiation Therapy Oncology Group (RTOG)21).
Of the 24 included patients, 13 were male and 11 female, with a mean age of 58.3 years (range : 39-71 years). Primary tumors included non-small cell lung cancer (11 patients, 45.8%), small cell lung cancer (2 patients, 8.3%), breast cancer (7 patients, 29.2%), colorectal cancer (2 patients, 8.3%), hepatocellular carcinoma (1 patient, 4.2%) and malignant melanoma (1 patient, 4.2%). Initial symptoms of brain metastasis included motor weakness (10 patients, 41.7%), headache (9 patients, 37.5%), gait disturbance (2 patients, 8.3%), visual disturbance (1 patient, 4.2%), seizure (1 patient, 4.2%), and incidental discovery (1 patient, 4.2%). The mean number of brain metastases was 2.7 (range : 1-13). Twenty-one lesions were located within the supratentorial area and 4 at the infratentorial area. The mean KPS score was 72.9 (range : 50-100). Thirteen patients (54.2%) were categorized as RPA class 1, 6 (25.0%) as RPA class 2, and 5 (20.8%) as RPA class 3. Patient characteristics are summarized in Table 1.
Table 1.
Clinical characteristics of the 24 study patients and the 25 study lesions
RPA : recursive partitioning analysis
MR imaging was performed after positioning of a Leksell stereotactic frame. MR T1-weighted images with gadolinium contrast were obtained, and slices were reconstructed every 2 mm in the axial plane. The SurgiPlan Electa Instruments system was used to plan surgical trajectory. Stereotactic cystic drainage, with or without Ommaya reservoir insertion, was performed aseptically in the operating room. At that time of stereotactic aspiration, the mean tumor volume was 23.2 cc (range : 7.9-100.9 cc). Twelve lesions (48.0%) were treated with additional Ommaya reservoir insertion after stereotactic drainage. All patients showed good reduction in cyst volume after stereotactic drainage.
After cystic drainage, a second set of MR images were obtained. GammaPlan Electa Instruments software was used to plan GKRS. At GKRS, mean tumor volume was reduced from 23.2 cc (range : 7.9-100.9 cc) to 4.3 cc (range : 0.2-19.0 cc) and mean tumor volume reduction rate was 77.9% (range : 31.4-98.3%). The mean prescription radiation dose to the tumor margin was 20.2 Gy (range : 13-25 Gy). GKRS was also performed to other multiple lesions without large cyst.
MR imaging was scheduled every 3 or 4 months (Fig. 1, 2). Tumor control was defined as a decrease in tumor size or little change after treatment. Local tumor progression was defined as an increase in size of a previously treated tumor, whereas remote tumor progression was defined as the appearance of new brain metastasis.
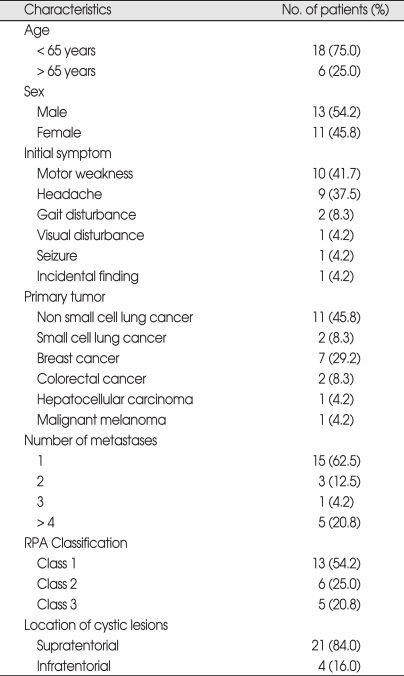
Fig. 1.
Axial contrast-enhanced T1-weighted magnetic resonance images of the brain of a 58-year-old woman with a large cystic brain metastasis, with hemorrhage, developing from hepatocellular carcinoma. A : Before aspiration : initial cyst volume, 28.8 cc. B : After cyst aspiration : cyst volume, 2.5 cc. The prescription dose at gamma knife radiosurgery was 25 Gy. C : 6 months postoperatively.
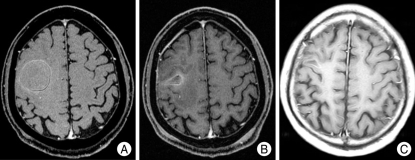
Fig. 2.
Axial contrast-enhanced T1-weighted magnetic resonance images of a 70-year-old woman with two large cystic brain metastases, developing from breast cancer. A : Before aspiration : initial cyst volume of lesion 1, 34.7 cc; of lesion 2, 11.0 cc. B : After cyst aspiration : cyst volume of lesion 1, 8.0 cc; of lesion 2, 0.9 cc. The prescription dose at G gamma knife radiosurgery was 23 Gy for lesion 1 and 25 Gy for lesion 2. C : 6 months postoperatively.
RESULTS
After treatment, 13 patients (54.2%) showed tumor control, and 11 (45.8%) showed tumor progression; 5 (20.8%) locally and 6 (25.0%) remotely. One patient (4.2%) who presented with remote tumor progression required WBRT, whereas the other 10 patients (41.7%) needed a second GKRS.
The mean follow-up duration was 13.1 months (range : 1-39 months). Finally, 14 patients (58.3%) are alive, whereas 10 patients (41.7%) died during follow-up. Only one patient (4.2%) died from the progression of brain metastasis. Three patients (12.5%) died from unrelated illnesses and 6 patients (25.0%) died from primary cancer progression. The 19 patients (79.2%) who underwent GKRS after stereotactic cyst drainage showed improvement in symptoms of brain metastasis (Table 2).
Table 2.
Outcomes of the 24 study patients
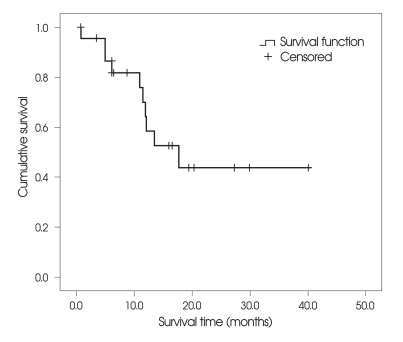
The overall median survival after GKRS was 17.8 months (range : 1-39 months); 17.8 months for patients in RPA class 1, 10.9 months for patients in RPA class 2, and 6.1 months for patients in RPA class 3. One patient died 1 month after GKRS because of disseminated intravascular coagulation during chemotheraphy. Median progression-free survival after treatment was 14.1 months (Fig. 3).
Fig. 3.
Kaplan-Meier survival plot shows the overall patient survival rate over 39 months after cyst aspiration and gamma knife radiosurgery. Surviving patients (n = 13) were censored.
There was no procedure-related mortality or morbidity at the surgery and GKRS or during follow-up periods.
DISCUSSION
Primary and metastatic brain tumors often have associated cystic components6,11,20), but the cause of cyst formation was not established clearly yet. Stem23) suggested that the relatively large amounts of protein in brain cyst fluid resembled that present in inflammatory exudates, which could be the result of increased permeability of pathologic vessels and mesodermal reactive processes. Cumings6) also suggested that formation of cyst fluid could be explained by tumor degeneration followed by transudation of fluid from blood vessels. Alternatively, Gardner et al.10) suggested that fluid accumulating in brain tumors is merely interstitial fluid without its normal drainage route because of the absence of lymphatics in the surrounding brain.
Conventionally, the presence of a single, large, and cystic brain tumor has been regarded as an indication for surgery9). Yoshida and Morii26) advocated surgical treatment for patients with large cystic lesions, providing rapid relief of neurological symptoms caused by mass effect. However, if the lesions are deep within the brain or located adjacent to eloquent areas, surgical procedures may result in severe neurologic deficits. In addition, surgical procedures are not effective or safe for patients in poor general condition or those with multiple lesions.
During the past decade, stereotactic radiosurgical procedure, particularly GKRS, have gained increasing relevance in the combined treatment of cerebral metastatic disease2,5,8). The rise in use of this technique is attributable to minimal invasiveness, a substantial reduction in hospitalization time and cost, an excellent local tumor control rate (even in putatively radioresistant oncotypes), and a very low associated morbidity16,22). Moreover, although there have been no direct randomized clinical comparisons of GKRS with other surgical-radiation protocols, the results of GKRS in patients with solitary lesions were similar or superior to those obtained using other methods8,13,15,17,19).
Large cystic brain metastases do not appear suitable for radiosurgery. Because the volume of the lesion is the limiting factor for radiosurgery given that it correlates with radionecrosis risk. Because the cystic component of a metastatic lesion is unresponsive to radiation, the therapeutic effect of GKRS is reduced9). Pan et al.18) reported that tumors with large cystic components (> 10 mL) were not effectively controlled by GKRS alone. Microsurgical resection or a combination of stereotactic cyst aspiration and GKRS, applied to the solid component of the tumor, was shown to result in a 1-year survival rate of 60%18). Franzin et al.9) reported that 39.1% of the patients did not experience local or remote tumor progression, the overall median survival was 15 months, and 26.3% of patients died from neurological progression after stereotactic drainage and GKRS of cystic brain metastases. In our study, 54.2% of patients showed tumor control, the overall median survival was 17.8 months and 4.2% of the patient died from progression of the brain metastases after treatment. But, Tendulkar et al.21) reported that median survival was reported to be 8.7 months after subtotal resection for single brain metastasis and 10.6 months after gross total resection of a single brain metastasis. Although it is difficult to directly compare these findings, the results of GKRS after stereotactic cyst aspiration of large cystic metastases were as good as that of gross total surgical resection. If the tumors locate in the eloquent areas or in the deep locations, or have large cystic volume, it is difficult to remove the tumor completely. In such situations, GKRS after stereotactic cyst aspiration could be the better treatment modality than surgical resection.
Among patients with unresectable brain metastases classified into RPA classes 1, 2, and 3, the median survival time was 7.1 months, 4.2 months, and 2.3 months, respectively21). We observed long median survival of 17.8 months, 10.9 months, and 6.1 months in RPA classes 1, 2 and 3, respectively. Moreover, patients in RPA classes 3 were too weak to permit general anesthesia. But, this modality does not require general anesthesia. These findings further reinforce the efficacy of GKRS after stereotactic cyst aspiration for unresectable cystic brain metastases.
Stereotactic cyst aspiration is a safe and effective procedure. Possible complications include hemorrhage, neurosurgical deficits, seizures, and infection14). The mortality rate in several large series has been less than 1%, and complication rates vary from 0% to 7%3,14). The complications of stereotactic radiosurgery are related to the effects of radiation on the brain and structures in proximity to the lesions. Significant early complications include seizures and worsening neurological deficits, but they are very rare. Approximately one-third of patients experiences mild transitory symptoms, including headache, nausea, and dizziness25). Late complications occur 6 to 9 months after the procedure and can include facial palsy, trigeminal neuropathy, and visual symptoms12). Patients may become symptomatic from radiation necrosis or local brain edema. Franzin et al.9) reported that there was no acute complication and 7.6% of the patients experienced radionecrosis after stereotactic aspiration and GKRS of cystic brain metastases. In our study, there were no procedure related mortality or morbidity.
This treatment method is composed of two stereotactic procedures with a single frame application. Stereotactic cyst aspiration with or without Ommaya reservoir insertion and GKRS are performed with a single frame application on the same day. So, GKRS after stereotactic cyst aspiration can contribute to a substantial reduction in hospitalization time, cost, and inconvenience of patients.
CONCLUSION
Cyst aspiration reduced tumor volume, permitting increase in radiation dose and decreasing the associated risks of radiation necrosis and post-radiation complications. Our findings support the usefulness and safety of GKRS after stereotactic cyst aspiration in patients with large cystic brain metastases. This method is especially applicable in patients whose overall condition is too poor to permit general anesthesia and in those with metastatic lesions deeply located.
References
- 1.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases : phase III results of the RTGO 9585 randomized trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Arnold SM, Patchell RA. Diagnosis and management of brain metastases. Hematol Oncol Clin North Am. 2001;15:1085–1107. doi: 10.1016/s0889-8588(05)70269-0. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein M, Parrent A. Complication of CT guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165–168. doi: 10.3171/jns.1994.81.2.0165. [DOI] [PubMed] [Google Scholar]
- 4.Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases : final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Bio Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 5.Coffey RJ. Stereotactic radiosurgical treatment of brain metastases. In: Cohen AR, Haines SJ, editors. Minimally invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995. pp. 139–143. [Google Scholar]
- 6.Cumings JN. The chemistry of cerebral cysts. Brain. 1950;73:244–250. doi: 10.1093/brain/73.2.244. [DOI] [PubMed] [Google Scholar]
- 7.Diener-West M, Dobbins TW, Phillips TL, Nelson DF. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTGO study 7916. Int J Radiat Oncol Bio Phys. 1989;16:669–673. doi: 10.1016/0360-3016(89)90483-5. [DOI] [PubMed] [Google Scholar]
- 8.Flickiner JC. Radiotherapy and radiosurgical management of brain metastases. Curr Oncol Rep. 2001;3:484–489. doi: 10.1007/s11912-001-0069-5. [DOI] [PubMed] [Google Scholar]
- 9.Franzin A, Vimercati A, Picozzi P, Serra C, Snider S, Gioia L, et al. Stereotactic drainage and Gamma Knife radiosurgery of cystic brain metastasis. J Neurosurg. 2008;109:259–267. doi: 10.3171/JNS/2008/109/8/0259. [DOI] [PubMed] [Google Scholar]
- 10.Gardner WJ, Collis JS, Lewis LA. Cystic brain tumors and the blood-brain barrier. Comparision of protein fractions in cyst fluids and sera. Arch Neurol. 1963;8:291–298. doi: 10.1001/archneur.1963.00460030075007. [DOI] [PubMed] [Google Scholar]
- 11.Kim MS, Lee SL, Sim SH. Brain tumors with cyst treated with Gamma Knife Radiosurgery : Is Microsurgery Indicated. Stereotact Funct Neurosurg. 1999;72(Suppl 1):38–44. doi: 10.1159/000056437. [DOI] [PubMed] [Google Scholar]
- 12.Kondziolka D, Firlik AD, Lunsford LD. Complications of stereotactic brain surgery. Neurol Clin. 1998;16:35–54. doi: 10.1016/s0733-8619(05)70366-2. [DOI] [PubMed] [Google Scholar]
- 13.Lohr F, Pirzkall A, Hof H, Fleckenstein K, Debus J. Adjuvant treatment of brain metastases. Semin Surg Oncol. 2001;20:50–56. doi: 10.1002/ssu.1016. [DOI] [PubMed] [Google Scholar]
- 14.Lunsford LD, Martines AJ. Streotactic exploration of the brain in the era of computed tomography. Surg Neurol. 1984;22:222–230. doi: 10.1016/0090-3019(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 15.Mandell L, Hilaris B, Sullvian M, Sundaresan N, Nori D, Kim JH, et al. The treatment of single brain metastasis from non-oat cell lung carcinoma : surgery and radiation versus radiation therapy alone. Cancer. 1986;58:641–649. [PubMed] [Google Scholar]
- 16.Mingione V, Oliveira M, Prasar D, Steiner M, Steiner L. Gamma surgery for melanoma metastases in the brain. J Neurosurg. 2002;96:544–551. doi: 10.3171/jns.2002.96.3.0544. [DOI] [PubMed] [Google Scholar]
- 17.Muacevic A, Kreth FW, Horstmann GA, Schmid-Elsaesser R, Wowra B, Steiger HJ, et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35–43. doi: 10.3171/jns.1999.91.1.0035. [DOI] [PubMed] [Google Scholar]
- 18.Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L. Gamma knife radiosurgery for brain metastases from lung cancer. J Neurosurg. 2005;102:128–133. doi: 10.3171/jns.2005.102.s_supplement.0128. [DOI] [PubMed] [Google Scholar]
- 19.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N England J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 20.Pullicino P, Thompson EJ, Moseley IF, Zilkha E, Shortman RC. Cystic intracranial tumors. Cyst fluid, biochemical changes and computerized tomographic findings. J Neurol Sci. 1979;44:77–85. doi: 10.1016/0022-510x(79)90225-9. [DOI] [PubMed] [Google Scholar]
- 21.Tendulkar RD, Liu SW, Barnett GH, Vogelbaum MA, Toms SA, Jin T, et al. RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys. 2006;66:810–817. doi: 10.1016/j.ijrobp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Sansur CA, Chin LS, Ames JW, Banegura AT, Aggarwal S, Ballesteros M, et al. Gamma knife radiosurgery for the treatment of brain metastases. Stereotact Funct Neurosurg. 2000;74:37–51. doi: 10.1159/000056462. [DOI] [PubMed] [Google Scholar]
- 23.Stem K. Chemical study of fluids obtained from cerebral cysts : report on 56 cases. Brain. 1939;62:88. [Google Scholar]
- 24.Weissman DE. Glucocorticoid treatment for brain metastases and epidural spinal cord compression : a review. J Clin Oncol. 1988;6:543–551. doi: 10.1200/JCO.1988.6.3.543. [DOI] [PubMed] [Google Scholar]
- 25.Werner-Wasik M, Rudoler S, Preston PE, Hauck WW, Downes BM, Leeper D, et al. Immediate side effects of stereotactic radiotherapy and radiosurgery. Int J Radiat Oncol Biol Phys. 1999;43:299–304. doi: 10.1016/s0360-3016(98)00410-6. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S, Morii K. The role of surgery in the treatment of brain metastasis : a retrospective review. Acta Neurochir (Wien) 2004;146:767–770. doi: 10.1007/s00701-004-0228-1. [DOI] [PubMed] [Google Scholar]