Abstract
Objective
There is no definite adjustment protocol for patients shunted with programmable valves. Therefore, we attempted to find an appropriate method to adjust the valve, initial valve-opening pressure, adjustment scale, adjustment time interval, and final valve-opening pressure of a programmable valve.
Methods
Seventy patients with hydrocephalus of various etiologies were shunted with programmable shunting devices (Micro Valve with RICKHAM® Reservoir). The most common initial diseases were subarachnoid hemorrhage (SAH) and head trauma. Sixty-six patients had a communicating type of hydrocephalus, and 4 had an obstructive type of hydrocephalus. Fifty-one patients had normal pressure-type hydrocephalus and 19 patients had high pressure-type hydrocephalus. We set the initial valve pressure to 10-30 mmH2O, which is lower than the preoperative lumbar tapping pressure or the intraoperative ventricular tapping pressure, conducted brain computerized tomographic (CT) scans every 2 to 3 weeks, correlated results with clinical symptoms, and reset valve-opening pressures.
Results
Initial valve-opening pressures varied from 30 to 180 mmH2O (mean, 102 ± 27.5 mmH2O). In high pressure-type hydrocephalus patients, we have set the initial valve-opening pressure from 100 to 180 mmH2O. We decreased the valve-opening pressure 20-30 mmH2O at every 2- or 3-week interval, until hydrocephalus-related symptoms improved and the size of the ventricle was normalized. There were 154 adjustments in 81 operations (mean, 1.9 times). In 19 high pressure-type patients, final valve-opening pressures were 30-160 mmH2O, and 16 (84%) patients' symptoms had nearly improved completely. However, in 51 normal pressure-type patients, only 31 (61%) had improved. Surprisingly, in 22 of the 31 normal pressure-type improved patients, final valve-opening pressures were 30 mmH2O (16 patients) and 40 mmH2O (6 patients). Furthermore, when final valve-opening pressures were adjusted to 30 mmH2O, 14 patients symptom was improved just at the point. There were 18 (22%) major complications : 7 subdural hygroma, 6 shunt obstructions, and 5 shunt infections.
Conclusion
In normal pressure-type hydrocephalus, most patients improved when the final valve-opening pressure was 30 mmH2O. We suggest that all normal pressure-type hydrocephalus patients be shunted with programmable valves, and their initial valve-opening pressures set to 10-30 mmH2O below their preoperative cerebrospinal fluid (CSF) pressures. If final valve-opening pressures are lowered in 20 or 30 mmH2O scale at 2- or 3-week intervals, reaching a final pressure of 30 mmH2O, we believe that there is a low risk of overdrainage syndromes.
Keywords: Hydrocephalus, Programmable valve, Valve-opening pressure, Shunt
INTRODUCTION
In communicating hydrocephalus, shunting is the treatment of choice. Since Nulsen and Spitz first inserted a modern valve system in a hydrocephalus patient in 1949, more than 100 valves have been developed14). Most of them have fixed pressure settings17). If the valve-opening pressure does not match the cerebrospinal fluid (CSF) hydrodynamics of the patient, CSF overdrainage or CSF underdrainage symptoms can develop. When using a fixed pressure setting valve, postoperative changes in the opening pressure can be accomplished only by undertaking another operation to insert another pressure-setting valve7).
The use of programmable shunt devices offers many advantages in the management of patients with hydrocephalus, because of their adjustable-pressure valve systems10). However, there are few guidelines regarding valve adjustment in the treatment of hydrocephalic patients. Therefore, we attempted to suggest guidelines for adjusting shunt valves by determining the initial opening pressures, time intervals, criteria for changing pressure, and determining the optimal final pressure.
MATERIALS AND METHODS
From April 2002 to April 2005, 81 shunt operations on 70 hydrocephalus patients (63 adults and 7 children) were undertaken by using a programmable shunting device (Micro Valve with RICKHAM® Reservoir). Thirty-two patients were males and 38 were females, and the follow-up period ranged from 4 months to 60 months (mean 13 ± 3.5 months). Patient age at implantation ranged from 1 month to 83 years (mean, 58 ± 20.7 years).
The etiologies of these cases of hydrocephalus were subarachnoid hemorrhage (SAH) after aneurysmal rupture (26 cases), head trauma (subdural hematoma, SAH, and contusion) (19 cases), intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH) (11 cases), germinal matrix bleeding (3 cases), tumor (3 cases), arteriovenous malformation (2 cases), congenital hydrocephalus (2 cases), achondroplasia (1 case), parasitic infection (1 case), cerebral infarction (1 case), and post-meningitis (1 case). Sixty-six patients had communicating-type hydrocephalus and 4 patients had obstructive-type hydrocephalus. Fifty-one patients had normal pressure-type hydrocephalus (classified as such when lumbar or ventricular tapping pressure is <180 mmH2O, because the normal CSF pressure's upper limit is 180 mmH2O), and 19 patients had high pressure-type hydrocephalus (classified as such when lumbar or ventricular tapping pressure is >180 mmH2O and including the 4 patients with obstructive-type hydrocephalus).
Dilated ventricles, presence of periventricular lucency, effaced cortical sulci, and presence of hydrocephalus symptoms were our indications for shunt operations. In questionable patients, we performed a lumbar-tapping test or drainage test. If there was some improvement of symptoms after drainage of 20 mL CSF daily for 1-3 days or lumbar drainage of 100-200 mL CSF daily for 5-7 days, these were other indications for shunt operations.
For patients in the high-pressure group, we set the initial valve-opening pressure to 100-140 mmH2O; for patients in the normal-pressure group, we set the initial valve-opening pressure to 10-30 mmH2O lower than that of the preoperative lumbar tapping pressure or intraoperative ventricular tapping pressure.
Two to 3 weeks after the shunt operation, we evaluated the symptoms of the patients, performed CT, and used a skull X-ray to rule out spontaneous resetting of the valve. By using these results, we lowered the valve pressure by 30 mmH2O if there were no signs of CSF overdrainage or shunt malfunction. In some patients, the pressure adjustment scale was 10 mmH2O, because their final valve-opening pressures were lowered from 40 to 30 mmH2O. In the Micro Valve with RICKHAM® Reservoir programmable valve, the lowest valve-opening pressure is 30 mmH2O (10-mmH2O scale, from 30 to 200 mmH2O).
We repeated these procedures every 3 weeks, until the symptoms improved and the size of the ventricle normalized. If there were signs of overdrainage, such as slit ventricle or subdural fluid collection, we increased the valve pressure by 20-30 mmH2O. If symptoms of hydrocephalus improved and the size of the ventricle was normal, a follow-up study was carried out 3 months later (Fig. 7).
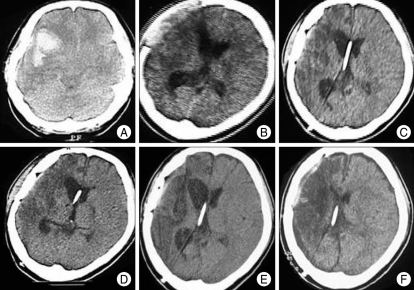
Fig. 7.
A 35-year-old female patient with aneurysmal subarachnoid hemorrhage received shunt surgery after aneurysmal neck-clipping and removal of intracerebral hemorrhage. A : Computerized tomographic (CT) scanning shows aneurysmal subarachnoid hemorrhage and severe brain edema. B : CT scanning 25 days after craniectomy and aneurysmal neck-clipping showing ventricular dilatation and brain-bulging through the craniectomy area. C : CT scanning 2 weeks after the first adjustment of the valve-opening pressure to 100 mmH2O; there is persistent ventricular dilatation (initial valve-opening pressure was 140 mmH2O). D : CT scanning 64 days after the fourth adjustment of the valve-opening pressure to 30 mmH2O; there is a slight decrease in ventricular dilatation. After the final valve adjustment from 50 to 30 mmH2O, she showed marked improvement; several months later, she could walk with assistance. E : CT scanning 6 months after the final adjustment of the valve-opening pressure to 30 mmH2O; there is persistent mild ventricular dilatation. F : CT scanning 22 months after the final adjustment of the valve-opening pressure to 30 mmH2O; there is a mild late subdural collection and collapsed ventricle, and she complained of a chronic postural headache. The valve-opening pressure was increased from 30 mmH2O to 50 mmH2O, and her headache then improved.
For all patients, the average follow-up time was 13 ± 3.5 months (minimum, 4 months; maximum, 60 months).
Outcome was determined by the effect of treatment on symptoms, signs, and radiological findings; it was graded as excellent (clear improvement, with no or only minor residual symptoms or signs and a return to independent living), good (improvement, but moderate residual symptoms or signs), unchanged, or worse20).
RESULTS
Initial valve-opening pressure
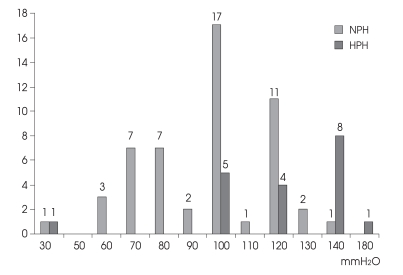
Initial valve-opening pressures varied from 30 to 180 mmH2O (mean, 100 ± 27.5 mmH2O) (Fig. 1). Among high pressure-type patients, initial valve-opening pressures were set to 100-140 mmH2O in all but 2 patients; one patient was a 2-year-old boy with choroid plexus carcinoma-associated hydrocephalus. His previous programmable valve was obstructed after a tumor removal operation. We set his initial valve-opening pressure to 30 mmH2O at the second operation. The other patient was a 61-year-old female with a previous craniectomy state due to a large hemispheric infarction. We set her initial valve-opening pressure to 180 mmH2O, because a large skull bone defect site was scheduled to be operated on, using cranioplasty, several days later.
Fig. 1.
Bar graph demonstrating the initial valve-opening pressure in the normal pressure-type hydrocephalus group and high pressure-type hydrocephalus group.
In normal-pressure types, initial valve-opening pressures varied from 30 to 140 mmH2O, depending on preoperative lumbar tapping pressure.
Adjustment time intervals
Pressure adjustments of valve-opening pressure were performed 154 times in 81 operations (mean, 2.2 times per patient and 1.9 times per operation). The typical pressureadjustment time intervals were from 2 to 4 weeks. In the normal-pressure group, the adjustment rate was 1.84/operation; in the high-pressure group, it was 2.04/operation.
Pressure adjustment scales
Most of the pressure adjustment scales were 20 mmH2O (19 times in the high-pressure group, 38 times in the normal-pressure group) and 30 mmH2O (21 times in the high-pressure group, 49 times in the normal-pressure group).
Final valve-opening pressure
Among 19 patients from the high-pressure group, the final valve-opening pressures varied from 30 to 160 mmH2O (mean, 84 ± 44 mmH2O). Interestingly, the final valve-opening pressures of 7 patients from the high-pressure group were < 60 mmH2O. Among the 51 patients from the normal-pressure group, the final valve-opening pressures were < 60 mmH2O in all but 9 patients; in these 9 patients, a final valve-opening pressure of > 60 mmH2O was due to subdural hematoma or collection (Fig. 2).
Fig. 2.
Bar graph demonstrating the final valve-opening pressure in the normal pressure-type hydrocephalus group and high pressure-type hydrocephalus group.
Outcomes of the high-pressure group
Among 19 patients from the high-pressure group, 16 (84%) improved (final valve-opening pressure : mean, 85 ± 39.8 mmH2O). Interestingly, final valve-opening pressures in 9 of the 19 high-pressure group patients were ≤ 60 mmH2O, while 5 patients showed final valve-opening pressures of 30 mmH2O, even though their initial CSF pressures were very high (Fig. 3). Among 19 patients from the high-pressure group, three (16%) did not improve. One patient whose final valve-opening pressure was 160 mmH2O and 2 patients whose final valve-opening pressures were 30 mmH2O did not improve because of initial severe brain damage. These three patients' valves functioning on shuntogram or lumbar-tapping pressure were good.
Fig. 3.
Bar graph showing the outcomes of the high pressure-type group patients, compared to their final valve-opening pressures. Sixteen of 19 patients of the high pressure-type group improved; the final valve-opening pressures of 7 improved patients from among the 19 high pressure-type group patients were ≤ 60 mmH2O.
Outcomes of the low-pressure group
Among the 51 patients of the normal-pressure group, 31 (61%) showed improvement in their clinical symptoms during or after adjustment; the final pressures of the majority of patients were ≤ 60 mmH2O (mean, 49.4 ± 34.7 mmH2O). Interestingly, 22 of the 31 improved patients in the normal-pressure group had final valve-opening pressures of 30 mmH2O (16 patients) and 40 mmH2O (6 patients) (mean, 32 ± 4.6 mmH2O) (Fig. 4). Twenty patients' symptoms did not improve; 13 of these patients showed no clinical improvements, despite multiple vigorous adjustments.
Fig. 4.
Bar graph showing the outcomes of the normal pressure-type group patients, compared to their final valve-opening pressures. Twenty-two of the 31 improved patients in the normal pressure-type group had final valve-opening pressures at 30 mmH2O and 40 mmH2O.
Complications
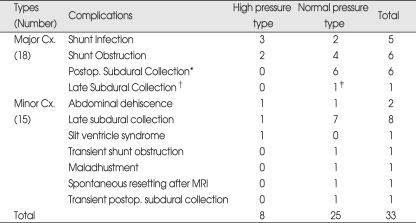
There were 33 complications (41%) : 18 major complications (22%) and 15 minor complications. Eleven patients needed shunt revisions (13.6%).
In the high-pressure group, 3 shunt infections and 2 shunt obstructions were the major complications (20%). In the high-pressure group, there were 3 minor complications : one abdominal wound dehiscence, one late subdural collection, and one slit ventricle syndrome. The late subdural collection and slit ventricle syndrome were improved only by valve adjustment; in these cases, the valve-opening pressure was increased by 20-30 mmH2O.
In the normal-pressure group, 6 postoperative subdural collections, 4 shunt obstructions, 2 shunt infections, and 1 late subdural collection were the major complications (23%). One of the subdural collections was not corrected by valve adjustment, and a burr hole trephination operation was therefore required. Five postoperative subdural collections and one late subdural collection were not improved by valve adjustment; however, the number of subdural collections was small and the patients' clinical conditions were stable; therefore, they received no further treatment. There were 12 minor complications : 7 transient late subdural collections, one transient shunt malfunction due to IVH after operation, one CSF leakage at abdominal wound, one maladjustment of valve system by reverse direction, one spontaneous resetting after a 1.5T magnetic resonance imaging study, and one transient postoperative subdural collection. The postoperative subdural collection and 7 late subdural collections were improved only by valve adjustment; the valve-opening pressure was increased by 20-30 mmH2O (Table 1).
Table 1.
Summary of complications in 81 shunt operations
*Postoperative subdural collection; developed within a 3-week postoperative period. †Late subdural collection; developed from the first adjustment to long-term follow-up periods. ‡Burr hole and drainage operation. Cx : complication, Postop. : postoperative, MRI : magnetic resonance imaging
Case 1
This patient was a 65-year-old male, and his initial disease was traumatic ICH and IVH (Fig. 5A). He received a craniectomy and hematoma removal operation, and he developed communicating hydrocephalus 9 months after surgery (Fig. 5B). His initial lumbar tapping pressure was 120 mmH2O and intraoperative ventricular pressure was 100 mmH2O. We set the initial valve-opening pressure at 80 mmH2O and lowered the pressure according to the protocol (Fig. 6). After the first valve adjustment, the patient showed no improvement and was still in a bedridden state (Figs. 5C, D). However, after the final adjustment to 30 mmH2O, the patient showed a dramatic improvement and he could walk by himself (Fig. 5E). Eleven months later, he could live independently without overdrainage syndrome, and CT scanning showed a normal-sized ventricle (Fig. 5F).
Fig. 5.
This patient was a 65-year-old male, and his initial disease was traumatic intracerebral hemorrhage and intraventricular hemorrhage. He underwent a craniectomy and hematoma removal operation, and developed communicating hydrocephalus 9 months after surgery. A : Computerized tomographic (CT) scanning shows traumatic intracerebral and intraventricular hemorrhage. B : CT scanning 9 months after a hematoma removal operation shows communicating hydrocephalus. C : CT scanning 3 weeks after shunt operation showing persistent ventricular dilatation (initial valve-opening pressure was 80 mmH2O). D : CT scanning 24 days after the first adjustment of the valve-opening pressure to 50 mmH2O; there is a slight decrease in ventricular dilatation, but the patient did not show any improvement. E : CT scanning 24 days after the second adjustment of the valve-opening pressure to 30 mmH2O; there is no ventricular dilatation. After the final adjustment to 30 mmH2O, the patient showed a dramatic improvement. F : CT scanning 11 months after the second adjustment of the valve-opening pressure to 30 mmH2O; there is no ventricular dilatation or subdural collection. Patient could live independently without overdrainage syndromes.
Fig. 6.
Pressure adjustment protocol for programmable valves.
Case 2
A 35-year-old female patient with aneurysmal SAH underwent shunt surgery after aneurysmal neck-clipping and removal of ICH (Fig. 7A). The initial opening pressure was set at 140 mmH2O, because her lumbar tapping pressure was greater than 180 mmH2O (Fig. 7B). Her valve was adjusted 3 times, but there was no definite improvement in her condition (Fig. 7C). After the final valve adjustment from 50 to 30 mmH2O, she showed a marked improvement; several months later, she could walk with assistance (Fig. 7D, E). Twenty-six months after the shunt operation, her neurological status was the same, except for chronic postural headache. A slight subdural collection developed on follow-up CT scanning (Fig. 7F). The valve-opening pressure was increased from 30 to 50 mmH2O, and her headache improved.
DISCUSSION
Several points must be considered when selecting the type of valve to include with the implanted shunt system. One of these is the opening pressure, which is the pressure that opens the valve, allowing the CSF to drain20).
In 1973, Hakim8) proposed the use of a shunt system with an adjustable valve to regulate opening pressure and later suggested that the valve-opening pressure was the most important factor in determining CSF drainage. A pro-grammable valve has the clear advantage of enabling the surgeon to make transcutaneous changes to the valve-opening pressure as a patient's postimplantation clinical course changes20).
The first commercially available programmable valve was the Sophy SU3, introduced in 198417). It offered 3 pressure settings by manual rotation of an external magnet. The succeeding Sophy SU8 allowed the selection of 8 different opening pressures between 50 and 170 mmH2O3). This shunt eliminated the need for reoperations that are otherwise performed to treat CSF overdrainage and underdrainage symptoms. However, this system had a bulky design and there were several cases of malfunctions, spontaneous pressure-setting changes, and inconstant opening pressures15,17).
Since 1989, the programmable Hakim valve has been available. Rohde et al.17) recommend a higher initial opening pressure to prevent overdrainage syndrome. The range of 100-140 mmH2O for valve-opening pressure is the medium to high range for a fixed-pressure valve; therefore, we usually set this range in the high-pressure group and then adjusted it as necessary7). In normal-pressure types, initial valve-opening pressures varied from 30 to 140 mm H2O, depending on the preoperative lumbar tapping pressure.
Adjustments to the valve-opening pressure were made at an average of 2.2 times per patient and 1.9 times per operation. Our protocol requires more frequent adjustments to determine the adequate lowest final valve-opening pressure.
The magnitude of single adjustments was generally not > 30-40 mm H2O, given the risks inherent in larger (> 40 mmH2O) adjustments2). As has also been observed by other authors, fine-tuning or titrating the opening pressure more precisely than the traditional low-, medium-, or high-pressure settings offered by nonadjustable valves optimizes treatment by allowing for adaptation and evaluation9,18,20). Therefore, we usually adjusted by 20-30 mmH2O scales, and we consider this protocol to be safe.
Final valve-opening pressure and outcome in the high-pressure group
Among the 19 patients in the high-pressure group, the improvement rate was high (16 patients, 84%). Interestingly, the final valve-opening pressure was ≤ 60-mmH2O in 7 of the 16 improved cases in the high-pressure group and 30-mmH2O in 3 patients among the 7 patients (≤ 60 mmH2O), even though their initial pressures were very high. Because we lowered the valve-opening pressures if there were any residual hydrocephalus symptoms or signs, unexpected low final valve-opening pressures in the high-pressure group was seen. These final valve-opening pressures are much lower than those of several previous reports2,10,17). So, we suggest more dynamic changes to the valve-opening pressure, depending on a patient's symptoms and signs.
Final valve-opening pressures and outcomes in the normal-pressure group
Among the 51 patients of the normal-pressure group, the symptoms of 20 patients did not improve; of those 20 non-improved patients, 13 showed no clinical improvements, despite multiple vigorous adjustments. We speculate this lack of improvement to a normalized ventricle size not to be the result of hydrocephalus, but in some cases by initial brain damage. Further, some other patients showed dilated ventricle on CT scanning, despite a valve-opening pressure of 30 mmH2O, good shunt functioning on shuntogram, and a low lumbar-tapping CSF pressure (< 30 mmH2O). In this group, we thought that an extra-low valve pressure of < 30 mmH2O could be helpful for patients with extra-low CSF pressure.
Complications
In previous reports, the shunt complication rate varied from 6% to 28%1,4,7,10,11,19,20). In the current study, there were 18 major complications (22%) and 11 patients needed shunt revisions (13.6%). Shunt infections, shunt obstructions, and other complications-except overdrainage syndromes-did not differ from those found in previous reports1,4,7,10,11,19,20). There were 17 CSF overdrainage syndromes : 16 subdural collections and one slit ventricle syndrome. In the normal-pressure group, 15 subdural collections occurred. Seven subdural collections (postoperative subdural collections) developed within a 3-week postoperative period, and 8 from the first adjustment to long-term follow-up periods (late subdural collections).
Several authors reported CSF overdrainage after using a programmable valve and failed to manage chronic subdural hematoma by increasing the pressure setting5). The programmable valve is a differential pressure valve and drains an unphysiologically high quantity of CSF (436 mL/h) in the upright position, even if the opening pressure is set at 200 mmH2O17). Therefore, there is a risk of overdrainage syndrome (subdural collection or slit ventricle syndrome), especially during a long-term follow-up period. In our study, 7 patients (6 postoperative subdural collections and 1 late subdural collection) developed a large subdural collection and complained of overdrainage symptoms. Therefore, we had to increase the valve-opening pressure and, in these cases, further lowering of the valve-opening pressure was limited. In one case, subdural collection persisted, despite an increase in valve-opening pressure; an operation for drainage was required.
We also observed thin subdural hygroma in well-functioning shunt systems near 30 mmH2O, especially during long-term follow-up periods. Eight patients (7 late subdural collections and 1 postoperative subdural collection) had mild subdural collections but with no overdrainage symptoms, and their neurologic status were good. In these cases, observations alone were performed. However, if overdrainage symptoms appeared, we elevated the valve-opening pressure by 20-30 mmH2O. Three weeks later (first observation period), subdural collections disappeared. If there were any remaining hydrocephalus symptoms or signs, the valve-opening pressure was again lowered according to the protocol.
From these results, we can assert that a late subdural collection could frequently develop as a result of using a very low pressure over a long period, but not severely; in most cases, a late subdural collection could be easily treated by making a valve adjustment. However, early postoperative subdural collection, especially in the normal-pressure group, could not be treated easily. In such cases, when the preoperative lumbar pressure is < 100 mmH2O, it might be advisable for the initial opening pressure to be set at the same opening pressure selected at the time of lumbar puncture, to avoid an early postoperative subdural collection13).
Significance of 30-mmH2O final valve-opening pressure
Many patients (27%) showed an improvement of symptoms at very low pressure, not only in the normal-pressure group but also in the high-pressure group. Interestingly, 14 patients showed a marked improvement of symptoms just after lowering the valve-opening pressure to 30 mmH2O. In many cases from our study, a very low valve-opening pressure was necessary to treat the normal-pressure hydrocephalus group. Gradually setting the valve-opening pressure to low levels according to our protocol is both safe and effective.
The aforementioned results indicate that the ideal ventricular pressure was different in every hydrocephalus patient, especially those with normal-pressure hydrocephalus. Thus, shunting patients with pressure-prefixed valves might not effectively solve problems. The use of a programmable shunt device has been strongly recommended for all types of hydrocephalus, but especially for normal-pressure hydrocephalus. In the past, some authors who selected a medium-pressure valve may not have lowered the intraventricular pressure adequately, and may have found a higher rate of treatment failure following shunt operation6). Another author used a low-pressure valve in normal-pressure hydrocephalus, but there was a higher incidence of extra-axial hematoma formation13).
We suggest our protocol for valve adjustment to be safe and effective for hydrocephalic patients, but diligence in determining the optimal pressure for each patient should be undertaken to achieve the best results from shunting. Our adjustment suggestions are as follows : set initial valve pressure to a level 10-30 mmH2O lower than that of CSF pressure, and decrease the pressure by 30-mmH2O scale at 3-week intervals (can be shortened to 2 weeks, depending on symptoms or CT findings), until symptoms improve and the ventricle is normalized. We found that this method was both effective and safe and reduced the risk of complications related to overdrainage syndrome. We should emphasize that these results are preliminary and further research should be undertaken to obtain more reliable results. Finally, we believe that an extra-low valve pressure of < 30 mmH2O might be helpful for patients with extra-low CSF pressure. Adding an antisiphon device is a possibility, but it should be used carefully, because it could lower the rate of overdrainage syndrome while increasing the risk of underdrainage.
CONCLUSION
In normal pressure-type hydrocephalus, most patients improved at the point when the final valve-opening pressure was near 30 mmH2O. We suggest that all normal pressure-type hydrocephalus patients might to be shunted with a programmable valve and that initial valve-opening pressures might to be set to levels 10-30 mmH2O below their CSF pressures. If the final valve-opening pressures are set to 30 mmH2O by decreasing 20 or 30 mmH2O at intervals of 2 or 3 weeks, we think that there is a low risk of overdrainage syndrome.
References
- 1.Ahn KJ, Koh HS, Kim SH, Youm JY, Song SH, Kim Y. Clinical analysis of programmable valve versus differential pressure valve in hydrocephalus. J Korean Neurosurg Soc. 2003;34:230–233. [Google Scholar]
- 2.Ahn ST, Yoo DS, Cho KS, Kim JK, Huh PW, Kim DS, et al. The use of the programmable valve shunt system in the management of patients with hydrocephalus. J Korean Neurosurg Soc. 2002;31:139–144. [Google Scholar]
- 3.Aschoff A, Krämer P, Benesch C, Klank A, Kunze S. Shunt-technology and overdrainage. A critical review of hydrostatic programmable and variable-resistance valves and flow reducing devices. Eur J Pediatr Surg. 1991;1(Suppl):49–50. [PubMed] [Google Scholar]
- 4.Black PM. Idiopathic normal-pressure hydrocephalus. Results of shunting in 62 patients. J Neurosurg. 1980;52:371–377. doi: 10.3171/jns.1980.52.3.0371. [DOI] [PubMed] [Google Scholar]
- 5.Black PM, Hakim R, Olsen BN. The use of the Codman-Medos programmable Hakim valve in the management of patients with hydrocephalus : illustrative cases. Neurosurgery. 1994;34:1110–1113. doi: 10.1227/00006123-199406000-00040. [DOI] [PubMed] [Google Scholar]
- 6.Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer HA, et al. Dutch normal-pressure hydrocephalus study : randomized comparison of low- and medium-pressure shunts. J Neurosurg. 1998;88:490–495. doi: 10.3171/jns.1998.88.3.0490. [DOI] [PubMed] [Google Scholar]
- 7.Han YM, Yoo DS, Kim DS, Huh PW, Cho KS, Kang JK. A clinical analysis of the ventriculoperitoneal shunt with programmable shunt device. J Korean Neurosurg Soc. 1999;28:75–81. [Google Scholar]
- 8.Hakim S. Hydraulic and mechanical mis-matching of valve shunts used in the treatment of hydrocephalus : The need for a servo-valve shunt. Dev Med Child Neurol. 1973;15:646–653. doi: 10.1111/j.1469-8749.1973.tb05175.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamano S, Nakano Y, Imanishi T, Hattori Ml. Management with a programmable pressure valve of subdural hematomas caused by a ventriculoperitoneal shunt : case report. Surg Neurol. 1991;35:381–383. doi: 10.1016/0090-3019(91)90050-j. [DOI] [PubMed] [Google Scholar]
- 10.Kim KJ, Kang JS, Suh BS, Lee HS, Lee JS. Clinical experience with the programmable valve for hydrocephalus patients. J Korean Neurosurg Soc. 2003;33:170–174. [Google Scholar]
- 11.Larsson A, Wikkelso C, Bilting M, Stephensen H. Clinical parameters in 74 consecutive patients shunt operated for normal pressure hydrocephalus. Acta Neurol Scand. 1991;84:475–482. doi: 10.1111/j.1600-0404.1991.tb04998.x. [DOI] [PubMed] [Google Scholar]
- 12.Marmarou A, Young HF, Aygok GA, Sawauchi S, Tsuji O, Yamamoto T, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus : a prospective study of 151 patients. J Neurosurg. 2005;102:987–997. doi: 10.3171/jns.2005.102.6.0987. [DOI] [PubMed] [Google Scholar]
- 13.Mori K. Management of idiopathic normal-pressure hydrocephalus : a multiinstitutional study conducted in Japan. J Neurosurg. 2001;95:970–973. doi: 10.3171/jns.2001.95.6.0970. [DOI] [PubMed] [Google Scholar]
- 14.Nulsen FE, Becker DP. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1951;2:399–403. [PubMed] [Google Scholar]
- 15.O'Reilly G, Williams B. The Sophy valve and the el-Shafei shunt system for adult hydrocephalus. J Neurol Neurosurg Psychiatry. 1995;59:621–624. doi: 10.1136/jnnp.59.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery. 1994;35:643–656. doi: 10.1227/00006123-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Rohde V, Mayfrank L, Ramakers VT, Gilsbach JM. Four-year experience with the routine use of the programmable Hakim valve in the management of children with hydrocephalus. Acta Neurochir (Wien) 1998;140:1127–1134. doi: 10.1007/s007010050226. [DOI] [PubMed] [Google Scholar]
- 18.Sutcliffe JC, Battersby RD. Do we need variable pressure shunts? Br J Neurosurg. 1992;6:67–70. doi: 10.3109/02688699209002904. [DOI] [PubMed] [Google Scholar]
- 19.Vanneste J, Augustijn P, Dirven C, Tan WF, Goedhart ZD. Shunting normal-pressure hydrocephalus : do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42:54–59. doi: 10.1212/wnl.42.1.54. [DOI] [PubMed] [Google Scholar]
- 20.Zemack G, Romner B. Seven years clinical experience with the Codman Hakim programmable valve : a retrospective study of 583 patients. J Neurosurg. 2000;92:941–948. doi: 10.3171/jns.2000.92.6.0941. [DOI] [PubMed] [Google Scholar]