Abstract
Objective
In this study, the authors assessed the ability of rat bone marrow derived mesenchymal stem cells (BMDMSCs), in the presence of a growth factor, fibroblast growth factor-4 (FGF-4) and hydroxyapatite, to act as a scaffold for posterolateral spinal fusion in a rat model.
Methods
Using a rat posterolateral spine fusion model, the experimental study comprised 3 groups. Group 1 was composed of 6 animals that were implanted with 0.08 gram hydroxyapatite only. Group 2 was composed of 6 animals that were implanted with 0.08 gram hydroxyapatite containing 1 × 106/ 60 µL rat of BMDMSCs. Group 3 was composed of 6 animals that were implanted with 0.08 gram hydroxyapatite containing 1 × 106/ 60 µL of rat BMDMSCs and FGF-4 1 µG to induce the bony differentiation of the BMDMSCs. Rats were assessed using radiographs obtained at 4, 6, and 8 weeks postoperatively. After sacrifice, spines were explanted and assessed by manual palpation, high-resolution microcomputerized tomography, and histological analysis.
Results
Radiographic, high-resolution microcomputerized tomographic, and manual palpation revealed spinal fusion in five rats (83%) in Group 2 at 8 weeks. However, in Group 1, three (60%) rats developed fusion at L4-L5 by radiography and two (40%) by manual palpation in radiographic examination. In addition, in Group 3, bone fusion was observed in only 50% of rats by manual palpation and radiographic examination at this time.
Conclusion
The present study demonstrates that 0.08 gram of hydroxyapatite with 1 × 106/ 60 µL rat of BMDMSCs induced bone fusion. FGF-4, added to differentiate primitive 1 × 106/ 60 µL rat of BMDMSCs did not induce fusion. Based on histologic data, FGF-4 appears to induce fibrotic change rather than differentiation to bone by 1 × 106/ 60 µL rat of BMDMSCs.
Keywords: Bone marrow derived mesenchymal stem cell, Fibroblast growth factor-4, Hydroxyapatite, Spinal fusion
INTRODUCTION
Spinal fusion is a commonly performed spinal procedure and autograft placement remains the gold standard for achieving spinal fusion. However, the rate of bone fusion failure is nearly 45%, and the incidence of morbidity associated with autogenous iliac crest bone graft harvesting is -30%2,9,26). These non-unions frequently lead to clinical symptoms, high medical costs, morbidity, and the need for additional surgery. These problems have led surgeons to search for alternative solutions to stimulate bone formation. The most predictable bone fusion materials, recombinant BMPs, have also been used successfully in clinical trials3,32). However, large doses of BMPs are required to induce adequate bone repair, and the associated costs are too great a burden for patients24,25). Therefore, the development of alternative strategies is desired. Cell therapy, which can potentially replace BMP, has been introduced, and has the additional advantages of no donor site complications, and straightforward harvesting and application1,19,28,30,32,33).
Mesenchymal stem cells (MSCs) from bone marrow have been studied in the context of treating bone defects because of their ability to differentiate into the bony lineage under appropriate conditions4,6,10,26,29,30). However, the short life span of bone marrow derived mesenchymal stem cells (BMDMSCs) and reductions in their differentiation potentials in culture have limited the progress of MSC-based therapies. To overcome the limitations of cell-based therapy, growth factors and scaffolds have been examined to promote stable growth and the differentiation of BMDMSCs4-7,10,14).
We have used hydroxyapatite ceramic as a bone scaffold for extensive bone replacement. Hydroxyapatite is composed of calcium phosphate and is incorporated into bone as a physiological minera2,11,12,15,23,31). It is not antigenic, carcinogenic, or osteogenic. Furthermore, fibroblast growth factor-4 (FGF-4) has been introduced as a strong mitogen for bone derived cells, and also induces bone formation by stimulating the proliferation and differentiation of mesenchymal osteoprogenitor cells when administrated systemically or locally to fracture sites13,18,20). FGFs elicit a variety of biological responses, such as, cell proliferation, differentiation and the migration of mesenchymal stem cells13,18,20). FGF-4 has higher cell mitogenic activity than the other known FGFs, and thus, we hypothesized that FGF-4 promotes bone fusion by facilitating MSCs on hydroxyapatite to promote their proliferation and differentiation into osteoblasts.
MATERIALS AND METHODS
Isolation and culture of bone marrow MSCs
Bone marrow cell preparation
Bone marrow was harvested from the femurs and tibias of 4-week-old male Sprague Dawley (SD) rats. Briefly, marrow plugs resuspended in α-minimum essential medium (MEM) were centrifuged, and the resulting cell pellet was resuspended in α-MEM containing 10% fetal bovine serum (FBS, Gibco Invitrogen) (proliferation medium). Nucleated cells were seeded onto 150-mm culture dishes and incubated at 37℃ for 6 days. Non-adherent cells were removed, and the monolayer of adherent cells was further incubated in complete medium until they reached a semi-confluent condition. Cells were then trypsinized (0.25% trypsin-EDTA, Gibco), resuspended in complete medium, reseeded (8,000 cells/cm2), and cultured for 3 days. Resulting monolayers of cells, hereafter referred to as rat mesenchymal stem cells (rMSCs), were trypsinized and aliquots were either, frozen and stored or cultured further.
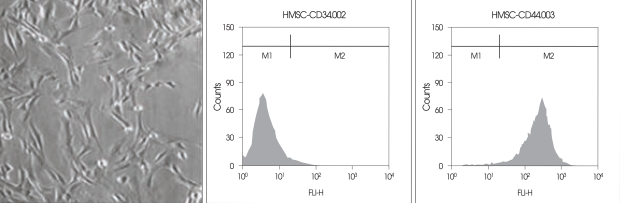
For phenotypic analysis of BMMSCs after passage 3, cells were lifted with 0.25% trypsin/EDTA (Gibco-BRL) and the trypsin/EDTA was then inactivated with fresh media. We resuspended approximately 3 × 104 BMMSCs per tube in 200 µL of phosphate buffered saline (PBS) + 2% FBS and fixed the solution with 2% paraformaldehyde for 10 min at room temperature. Cells were subsequently incubated for 20 min at 4℃ with the primary antibodies to CD34 and CD44 (both from BD Biosciences, Bedford, MA). After washing twice with PBS +2% FBS, cells were incubated with a fluorescein isothiocyanate (FITC)-conjugated with goat anti-rat antibody (Sigma-Aldrich, St. Louis, MO) for 15 min at 4℃. For control experiments, cells were stained with secondary antibodies only. After washing twice with PBS + 2% FBS, cells were analyzed by flow cytometry using a FACScaliber Flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) (Fig. 1).
Fig. 1.
Immunophenotyping of mesenchymal stem cells isolated from the bone marrow. The majority of cells (> 99.9%) were CD34 negative and CD44 positive
Surgical technique in rat
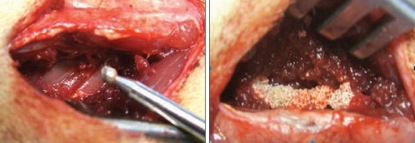
SD rats were anesthetized with intraperitoneal (i.p.) ketamine. The spine fusion model has been previously citation initially, a posterior midline incision was made, and this was followed by two separate paramedian incisions 3 mm from midline in the lumbar fascia. The transverse processes of L4 and L5 were then exposed. These processes were decorticated with a low-speed burr to produce a fusion material bed (Fig. 2). Group 1 was composed of 6 animals implanted with 0.08 gram of hydroxyapatite only. Group 2 was composed of 6 animals implanted with 0.08 gram hydroxyapatite containing 1 × 106/ 60 µL rat BMDSCs. Group 3 was composed of 6 animals implanted with 0.08 gram hydroxyapatite with 1 × 106/ 60 µL rat bone marrow derived stem cells and FGF-4 1 µG. The fascia and skin incisions were closed with a 3-0 absorbable suture. Rodents were housed in separate cages, allowed to eat and drink ad libitum, and their conditions were monitored on a daily basis.
Fig. 2.
Transverse processes were decorticated with a low-speed burr to produce a fusion material bed. Groups 1, 2 and 3 materials were implanted 0.08 gram hydroxyapatite, 0.08 gram hydroxyapatite plus 1×106/ 60 µL rat of rat bone marrow derived mesenchymal stem cells and 0.08 gram hydroxyapatite plus and Fibroblast growth foctor-4 (FGF-4) 1 µG.
Radiographic analysis
Fusion between L4 and L5 was quantified using plain radiographs obtained at 4, 6, and 8-weeks postoperatively. Fusion between L4 and L5 transverse processes was recorded together with the percentage of total area between L4 and L5 filled with new bone. Three examiners unaware of experimental details evaluated whether fusion as was solid or not between top and bottom transverse processes, based on increases in radio-opacities versus initial evaluations. Fusion was accepted only when the three examiners agreed.
Manual assessment of fusion
Manual palpation has been determined to be the most sensitive and specific means of assessing spinal fusion in this model. Three blinded observers assessed spines in this manner. At 8 weeks postoperatively, all spines were explanted and subjected to manual palpation to assess fusion at L4-L5. The spinous processes of L4 and L5 were identified and motion was assessed at this level. The absence of motion (right and left) was considered successful fusion. The spines were scored as either fused or not fused.
MicroCT analysis
Individual spines were attached on a sample holder with a consistent superior-inferior orientation and scanned using a microCT unit (Skyscan 1072, Aartselaar, Belgium) operating in the fan beam mode. Images were obtained at 80 V and 100 µA with a 1-mm aluminum filter, at a magnification of × 11.23 (pixel size = 21.31 µm in all three spatial directions). A rotation of 0.90° was used between each image acquisition. Three-dimensional modeling and bone reconstruction were performed using ANT software (release 2.05; Skyscan). This program allows the reconstruction of objects from a stack of 2D sections, and allows structural parameters to be analyzed, after interactive thresholding. The reconstructed 3D models were obtained using a surface-rendering algorithm.
Histologic analysis
After death, spines were dissected, and specimens were fixed in 40% ethanol, decalcified using standard RDO solution (AEPC, Aurora, IL, USA) in a specimen container, washed with running tap water, and then transferred to 75% ethanol. Serial sagittal sections near transverse processes were cut at the level of the transverse process. The specimens were embedded in wax and sectioned. The sections were stained with hematoxylin and eosin.
Statistical Methods
Radiographs were scored by three blinded independent observers. Fusion rates in the three groups as determined by radiographic and manual palpation were compared using Fisher's exact test. K-statistics were calculated to measure interobserver reliabilities.
RESULTS
Radiographic analysis, manual palpation, and microCT
Radiographs of rat spines were obtained at 4, 6, and 8 weeks postoperatively. At 4 weeks, rat spines in each group were not sufficiently fused between the transverse processes of L4 and L5. At 8 weeks, 5 (83%) of the 6 rats in Group 2 achieved fusion by plain radiography. In contrast, only 3 (60%) of 5 rats (one rat expired) in Group 1, and 3 (50%) of 6 rats in Group 3 showed minimal evidence of new bone formation by 8 weeks (Fig. 3). Good agreement (K = 0.667) was found between the three observers. All spines were manually palpated by 3 observers. All five rat spines in Group 1 were assessed for fusion. However, two rats of three with fusion radiographically were found not to have achieved fusion due by palpation, and thus, only one (20%) was found to have achieved bone fusion in Group 1. Manual palpation findings in Group 3 agreed with radiographic findings. Fisher's exact test revealed a significantly different fusion rate between Group 1 and both of the other groups (p < 0.001). Three-dimensional microCT was performed after rats were sacrificed at week 8. Five spines from Group 1 exhibited new bone formation. The new bone masses solidly fused the L4 and L5 transverse processes, and no gaps were detected between the L4 and L5 transverse processes (Fig. 4). Multiple cut sections were reconstructed to determine the presence of a bony bridge between transverse processes. The presence of trabeculae bridging of L4 and L5 transverse processes on cut plane images was consistently observed for all spine samples that were assessed as fused by manual palpation (Table 1).
Fig. 3.
Radiographs of Sprague Dawley rats at 8-week postoperatively. A : Group 1 : rats were implanted with 0.08 gram hydroxyapatite only. B : Group 2 : rats were implanted with 0.08 gram hydroxyapatite containing 1×106/ 60 µL rat bone marrow derived mesenchymal stem cells. C : Group 3 : rats were implanted with 0.08 gram hydroxyapatite with 1×106/ 60 µL rat bone marrow derived stem cells and fibroblast growth factor-4 1 µG. The arrowhead indicates the transverse process of L4-5.
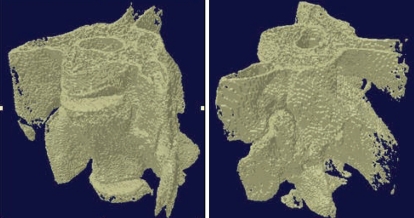
Fig. 4.
At week 8 postoperatively, micro-computed tomography was used to confirm bone fusion group 1 and group 2. On comparing groups 1 and 2, we were able to observe better bone bridging in groups implanted with mesenchymal stem cells.
Table 1.
Fusion rates in each group
*significantly different from HA plus MSC 1×106 group (Fisher exact test, p < 0.05).
Histologic analysis
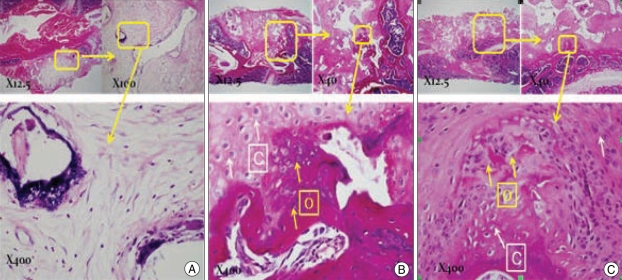
In Group 1, the histologic findings showed that the area between transverse processes was filled with hydroxyapatite. Despite some absorption of hydroxyapatite, bony replacement was not observed between the L4-L5 transverse process. Histologic analysis of Group 2 specimens confirmed bone fusion and demonstrated bone formation bridging the L4-L5 transverse processes (Fig. 5). This bone formation was localized to a region between the transverse processes and appeared mature at 8 weeks postoperatively. Furthermore, the pores of grafted hydroxyapatite fragments were filled with new bone. In un-fused cases of Group 3, fibroblast cells and cartilage predominated in grafted areas, and no new bone formation was observed.
Fig. 5.
Modified H&E findings. A : Group 1 H.A only. B : Group 2 H.A + mesenchymal stem cell (MSC) (1×106). C : Group 3 H.A + MSC + fibroblast growth factor-4 (FGF-4). Some calcified lesions in the control group were visible in inter transverse process space, but in most cases no bone material was observed. Osteocytes were observed in the MSC group and much cartilage formation was observed on surrounding areas, suggesting a high probability of bone formation given time. Groups treated with FGF-4 showed a predominance of fibroblasts.
DISCUSSION
BMDMSCs have the capacity for self-renewal and differentiation into a variety of cell types,4,7,25,27,29) and represent an attractive source of cells for spinal fusion therapy. However, the life span of BMMSCs is short and their low differentiation potentials in culture have limited the progress of MSC-based therapies25). To improve fusion rates, some researchers have found that it was important to implant larger cells that have differentiated into the osteogenic lineage21,22,29). Thus, we hypothesized that greater differentiation of BMDMSCs into osteogenic cells and their increased proliferation would increase the likelihood of achieving successful spinal fusion.
Recent studies have demonstrated that FGFs play a crucial role in self-renewal, maintenance, and the proliferation of hematopoietic stem cells, MSCs, neural stem cells, and embryonic stem cells7,8,13,16,18,20,22). Fibroblast growth factor (FGF) belongs to a family of heparin-binding growth factors, and promotes the proliferation, migration, survival, and differentiation of many different cell types8,13,16,18,20,22). FGF-2 functions as a mitogen, and increases the cellular proliferations of mesenchymal stem cells and stromal cells derived from various tissues, such as, bone marrow, adipose, and heart tissues5,13,20). Furthermore, FGF-2 was found to suppress the cellular senescence of human MSCs and to sustain the proliferative and osteogenic potentials of adipose tissue-derived stromal cells5,13,20). FGF-4 has been reported to increase the proliferation of neural progenitors, the survival of trophoblast stem cells, and the numbers of human hematopoietic colony-forming cells5,7,8,13,17,18,20). Others have reported that FGF-4 plays important roles in bone development during embryogenesis17). When 1 µG of rhFGF-4 was injected into the tibia of 10-week-old SD rats, they produce an osteoblastic population. At days 4 and 7, sets of animals were subjected to tibial bone marrow cell culture in an osteogenic medium. Basic FGF is a strong mitogen for bone derived cells, and also induces bone formation by stimulating the proliferation and differentiation of mesenchymal osteoprogenitor cells when administrated systemically or locally to fracture site. Basic FGF stimulates osteogenic differentiation of dexamethasone-treated bone marrow mesenchymal stem cell as evidenced by enhanced by enhanced alkaline phosphatase activity, osteocalcin production, and bone nodule formation22). In the present study, we failed to find that bone formation was promoted by the incorporation of FGF-4. Rather, we found fibrotic tissue in grafted areas. Accordingly, based on our findings, it appears that FGF-4 may promote fibroblast formation and inhibit the osteogenic differentiation of grafted BMDMSCSc. Our experimental results were similar to that of basic FGF induced proliferation. Choi et al.5) reported that rat bone marrow-derived mesenchymal stem cells treated with mitogenic factor basic FGF and differentiation factor BMP-2 stimulated osteogenesis more so than exposure to these factors alone. Bone formation is a complex set of events that are regulated by the extracellular matrix, systemic hormones, mechanical stress, and by the local productions of cytokines and growth factors. The levels of osteoinductive bone proteins in implants and their concentrations at interface points between implants and host bone must be in the physiologic range (which often requires high initial doses), to enable responding cells to come into sufficient contact with the proteins to initiate bone induction. When bone marrow-derived mesenchymal stem cells were combined with basic FGF, spinal fusion was achieved when a small dose of BMP was used, as an osteoinductor, or dexamethasone was co-administered to augment the osteogenic differentiation induced by FGF-47,13,17). We believe that FGF-4 promotes the proliferation and differentiation of mesenchymal stem cells into more osteogenic cells under suitable conditions in vitro, but the present study demonstrates that FGF-4, added to differentiate primitive BMDMSCs did not induce fusion in vivo. We regard the reason of unexpected result as inadequate dose of FGF-4 for fusion. We should have studied larger number and various different doses of FGF. However, the present study demonstrates that the osteogenic and proliferated mesenchymal stem cells and hydroxyapatite particles composite would promote new bone formation in the grafted area without dose adjustment or dexamethasone.
CONCLUSION
The present study demonstrates that 0.08 gram of hydroxyapatite with 1×106/ 60 µL rat of BMDMSCs induced bone fusion. However, FGF-4, added to differentiate primitive 1×106/ 60 µL rat of BMDMSCs did not induce fusion. Based on histologic data, FGF-4 appears to induce fibrotic change rather than differentiation to bone by 1×106/ 60 µL rat of BMDMSCs.
References
- 1.Aslan H, Sheyn D, Gazit D. Genetically engineered mesenchymal stem cells : applications in spine therapy. Regen Med. 2009;4:99–108. doi: 10.2217/17460751.4.1.99. [DOI] [PubMed] [Google Scholar]
- 2.Boden SD, Martin GJ, Jr, Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine. 1999;24:320–327. doi: 10.1097/00007632-199902150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 2001;68:87–94. [PubMed] [Google Scholar]
- 5.Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ, Lim DS. Fibroblast growth factor-2 and -4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem Cells Dev. 2008;17:725–736. doi: 10.1089/scd.2007.0230. [DOI] [PubMed] [Google Scholar]
- 6.Dumont RJ, Dayoub H, Li JZ, Dumont AS, Kallmes DF, Hankins GR, et al. Ex vivo bone morphogenetic protein-9 gene therapy using human mesenchymal stem cells induces spinal fusion in rodents. Neurosurgery. 2002;51:1239–1244. doi: 10.1097/00006123-200211000-00020. discussion 1244-1245. [DOI] [PubMed] [Google Scholar]
- 7.Farré J, Roura S, Prat-Vidal C, Soler-Botija C, Llach A, Molina CE, et al. FGF-4 increases in vitro expansion rate of human adult bone marrow-derived mesenchymal stem cells. Growth Factors. 2007;25:71–76. doi: 10.1080/08977190701345200. [DOI] [PubMed] [Google Scholar]
- 8.Franke Stenport V, Johansson CB, Sawase T, Yamasaki Y, Oida S. FGF-4 and titanium implants : a pilot study in rabbit bone. Clin Oral Implants Res. 2003;14:363–368. doi: 10.1034/j.1600-0501.2003.00846.x. [DOI] [PubMed] [Google Scholar]
- 9.Fu TS, Chen WJ, Chen LH, Lin SS, Liu SJ, Ueng SW. Enhancement of posterolateral lumbar spine fusion using low-dose rhBMP-2 and cultured marrow stromal cells. J Orthop Res. 2009;27:380–384. doi: 10.1002/jor.20644. [DOI] [PubMed] [Google Scholar]
- 10.Hasharoni A, Zilberman Y, Turgeman G, Helm GA, Liebergall M, Gazit D. Murine spinal fusion induced by engineered mesenchymal stem cells that conditionally express bone morphogenetic protein-2. J Neurosurg Spine. 2005;3:47–52. doi: 10.3171/spi.2005.3.1.0047. [DOI] [PubMed] [Google Scholar]
- 11.Heise U, Osborn JF, Duwe F. Hydroxyapatite ceramic as a bone substitute. Int Orthop. 1990;14:329–338. doi: 10.1007/BF00178768. [DOI] [PubMed] [Google Scholar]
- 12.Itoh S, Kikuchi M, Koyama Y, Takakuda K, Shinomiya K, Tanaka J. Development of a hydroxyapatite/collagen nanocomposite as a medical device. Cell Transplant. 2004;13:451–461. doi: 10.3727/000000004783983774. [DOI] [PubMed] [Google Scholar]
- 13.Kavanagh E, Church VL, Osborne AC, Lamb KJ, Archer CW, Francis-West PH, et al. Differential regulation of GDF-5 and FGF-2/4 by immobilization in ovo exposes distinct roles in joint formation. Dev Dyn. 2006;235:826–834. doi: 10.1002/dvdy.20679. [DOI] [PubMed] [Google Scholar]
- 14.Koga A, Tokuhashi Y, Ohkawa A, Nishimura T, Takayama K, Ryu J. Effects of fibronectin on osteoinductive capability of fresh iliac bone marrow aspirate in posterolateral spinal fusion in rabbits. Spine. 2008;33:1318–1323. doi: 10.1097/BRS.0b013e3181732a5d. [DOI] [PubMed] [Google Scholar]
- 15.Konishi S, Nakamura H, Seki M, Nagayama R, Yamano Y. Hydroxyapatite granule graft combined with recombinant human bone morphogenic protein-2 for solid lumbar fusion. J Spinal Disord Tech. 2002;15:237–244. doi: 10.1097/00024720-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda S, Kasugai S, Oida S, Iimura T, Ohya K, Ohyama T. Anabolic effect of aminoterminally truncated fibroblast growth factor 4 (FGF4) on bone. Bone. 1999;25:431–437. doi: 10.1016/s8756-3282(99)00193-3. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda S, Kondo H, Ohya K, Kasugai S. Bone increase in rat tibiae by local administration of amino-terminally truncated rhFGF-4(73-206) Tissue Eng. 2007;13:415–422. doi: 10.1089/ten.2006.0112. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kim JY, Kang IY, Kim HK, Han YM, Kim J. The EWS-Oct-4 fusion gene encodes a transforming gene. Biochem J. 2007;406:519–526. doi: 10.1042/BJ20070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration-Bench to bedside. J Biomed Mater Res B Appl Biomater. 2009;87:252–263. doi: 10.1002/jbm.b.31199. [DOI] [PubMed] [Google Scholar]
- 20.Lovinescu I, Koyama E, Pacifici M. Roles of FGF-10 on the development of diathrodial limb joints. Penn Dent J (Phila) 2003;103:5, 9. [PubMed] [Google Scholar]
- 21.Lu SS, Zhang X, Soo C, Hsu T, Napoli A, Aghaloo T, et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50–60. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Minamide A, Yoshida M, Kawakami M, Okada M, Enyo Y, Hashizume H, et al. The effects of bone morphogenetic protein and basic fibroblast growth factor on cultured mesenchymal stem cells for spine fusion. Spine. 2007;32:1067–1071. doi: 10.1097/01.brs.0000261626.32999.8a. [DOI] [PubMed] [Google Scholar]
- 23.Minamide A, Yoshida M, Kawakami M, Yamasaki S, Kojima H, Hashizume H, et al. The use of cultured bone marrow cells in type I collagen gel and porous hydroxyapatite for posterolateral lumbar spine fusion. Spine. 2005;30:1134–1138. doi: 10.1097/01.brs.0000162394.75425.04. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Sugiyama O, Tow B, Zou J, Morishita Y, Wei F, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008;21:372–379. doi: 10.1097/BSD.0b013e31814cf51d. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki M, Zuk PA, Zou J, Yoon SH, Wei F, Morishita Y, et al. Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine. 2008;33:863–869. doi: 10.1097/BRS.0b013e31816b45c3. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima T, Iizuka H, Tsutsumi S, Kayakabe M, Takagishi K. Evaluation of posterolateral spinal fusion using mesenchymal stem cells : differences with or without osteogenic differentiation. Spine. 2007;32:2432–2436. doi: 10.1097/BRS.0b013e3181573924. [DOI] [PubMed] [Google Scholar]
- 27.Orii H, Sotome S, Chen J, Wang J, Shinomiya K. Beta-tricalcium phosphate (beta-TCP) graft combined with bone marrow stromal cells (MSCs) for posterolateral spine fusion. J Med Dent Sci. 2005;52:51–57. [PubMed] [Google Scholar]
- 28.Riew KD, Wright NM, Cheng S, Avioli LV, Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998;63:357–360. doi: 10.1007/s002239900540. [DOI] [PubMed] [Google Scholar]
- 29.Risbud MV, Shapiro IM, Guttapalli A, Di Martino A, Danielson KG, Beiner JM, et al. Osteogenic potential of adult human stem cells of the lumbar vertebral body and the iliac crest. Spine. 2006;31:83–89. doi: 10.1097/01.brs.0000193891.71672.e4. [DOI] [PubMed] [Google Scholar]
- 30.Siddappa R, Fernandes H, Liu J, van Blitterswijk C, de Boer J. The response of human mesenchymal stem cells to osteogenic signals and its impact on bone tissue engineering. Curr Stem Cell Res Ther. 2007;2:209–220. doi: 10.2174/157488807781696267. [DOI] [PubMed] [Google Scholar]
- 31.Spivak JM, Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J. 2001;10(Suppl 2):S197–S204. doi: 10.1007/s005860100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szpalski M, Gunzburg R. Recombinant human bone morphogenetic protein-2 : a novel osteoinductive alternative to autogenous bone graft? Acta Orthop Belg. 2005;71:133–148. [PubMed] [Google Scholar]
- 33.Vadala G, Studer RK, Sowa G, Spiezia F, Iucu C, Denaro V, et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine. 2008;33:870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]