Abstract
Since the start of the antibiotic era, syphilis has become rare. However, in recent times, it has tended to be prevalent concomitantly with human immunodeficiency virus (HIV) infection and coinfection in North America and Europe. Now, such cases are expected to increase in elsewhere including Korea. A 40-year-old male patient visited hospital complaining of a headache for about one month. Brain computed tomography and magnetic resonance imaging, showed leptomeninged enhancing mass with edema an right porisylvian region, which was suspected to be glioma. Patient underwent a blood test and was diagnosed with syphilis and acquired immune deficiency syndrome. Partial cortical and subcortical resection were performed after small craniotomy. The dura was thick, adhered to the brain cortex, and was accompanied by hyperemic change of the cortex. The pathologic diagnosis was meningovascular syphilis (MS) in HIV infection. After the operation, the patient was treated with aqueous penicillin G. Thereafter, he had no neurological deficit except intermittent headache. At first, this case was suspected to be glioma, but it was eventually diagnosed as MS in HIV coinfection. At this point the case was judged to be worth reporting.
Keywords: HIV, AIDS, Neurosyphilis
INTRODUCTION
Although neurosyphilis has become an uncommon disease among the general population, people who live with human immunodeficiency virus (HIV) have a greater tendency to develop it. In fact, at least one third of HIV-infected people are coinfected with syphilis.
The immunodeficiency induced by HIV results in more frequent, more rapid progression to severe neurosyphilis, which is less responsive to therapy and more prone to frequent relapse.
The frequency of neurosyphilis in the HIV-positive population is probably 3-35%3). The number of acquired immune deficiency syndrome (AIDS) patients is on the increase even in Korea, therefore the number of patients with neurosyphilis is also expected to increase. A signific problem is that radiologic findings are nonspecific in the case of neurosyphilis, and furthermore there are very few physicians experienced in the diagnosis of neurosyphilis. It is on account of this that at first the case was suspected to be glioma after the initial radiologic evaluation, although the final pathologic diagnosis was meningovascular syphilis (MS) in HIV infection.
Following is a report of this case of meningoencephalitic type neurosyphilis with AIDS.
CASE REPORT
A 40-year-old man was referred to hospital with a one month history of progressive headache. He didn't complain any focal weakness, visual disturbance, hearing loss, nausea, vomiting or fever. There was no past history of trauma, transfusion or surgery. He was married but had been separated from his wife for 10 years. He had traveled frequently to Southeast Asia on business.
On admission the patient did not look ill. Neurological examination revealed an awake, well-oriented patient. Other focal abnormal neurological signs were not noted. Routine complete blood count was within normal range except an elevated erythrocyte sedimentation rate (94 mm/hr). Liver function and serum electrolytes were within the normal range. Among the serological tests, serum rapid plasma reagin was positive with a titer of 1 : 128.
The fluorescent treponemal antibody-absorption test (FTA-ABS) was positive. Laboratory test for HIV were as follows; Treponema pallidum latex agglutination increased up to 230T.U, strong positive anti-HIV 1/2 antibody test (enzyme-linked immunosorbent assay, ELISA) and western immunoblot assay confirmed HIV infection. CD4 cell test was 567/mL. (34.9%) with HIV ribonucleic acid 150,000 Copy/mL.
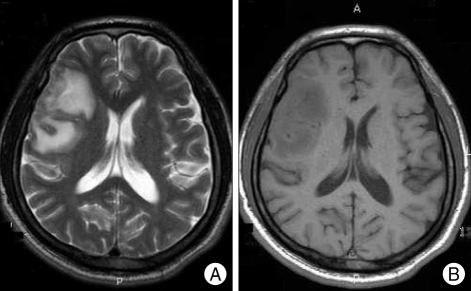
The brain computed tomography (CT) with contrast enhancement, showed a leptomeningeal enhancing lesion with edema on the right perisylvian region (Fig. 1). The magnetic resonance image of the brain, more clearly demonstrated the leptomeningeal enhancement with dural thickening and edema around right sylvian fissure (Fig. 2).
Fig. 1.
Brain computer tomography shows low density lesion (A) which enhanced heterogeneously at right perisylvian region (B).
Fig. 2.
Brain magnetic resonance image shows high signal in T2 weighted image (A) and low signal at T1 weighted image (B) heterogeneously at same site as computer tomography image.
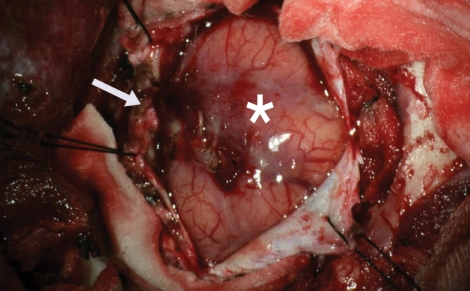
Two weeks after admission, an open biospsy was performed. The tentative diagnosis before the operation was low grade glioma or herpes encephalitis. After a small craniotomy, the dura was exposed. The dura was thick and adhered to the cortex, which showed hyperemic change (Fig. 3). Partial cortical and subcortical resection was carried out at the lesion site.
Fig. 3.
Gross appearance of hyperemic and edematous cortex (*), and thickened dura mater (arrow).
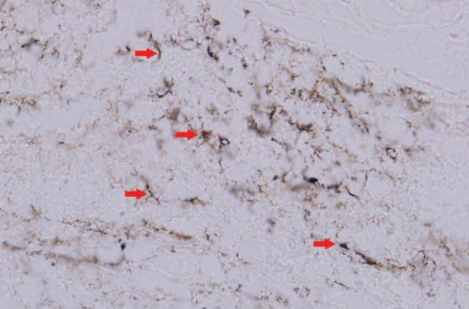
In the histopathological finding, submitted tissue disclosed a widening of meninges by proliferation of blood vessels, infiltration of many inflammatory cells, and deposition of collagen fibers. This inflammatory process extended into the brain parenchyma with obliteration of the meningocerebral junction. Vascular lumens were partially or neartotally obliterated by proliferation of endothelial cells and dense perithelial infiltration of inflammatory cells, predominantly plasma cells admixed with lymphocytes, resulting in endarteritis obliterans (Fig. 4). There was widespread reactive gliosis surrounding the inflammatory mass. However, there were no neoplastic cells. Warthin-Starry stain exhibited spirochetal organisms (Fig. 5). The pathological diagnosis was meningovascular syphilis.
Fig. 4.
High power view of specimen shows diffuse infiltration of mononuclear cells, predominantly plasma cells (arrow), with perithelial concentration is in the both meninges and brain parenchyma (H-E, ×400).
Fig. 5.
Special stain of specimen demonstrates many spiral organisms (arrow) (Warthin-Starry stain, ×400).
The patient received an intravenous administration of 24 million units of aqueous penicillin G every day for 2 weeks.
After operation and antibiotic treatment, the patient had no neurological deficits except intermittent headache, at a much reduced level of severity.
DISCUSSION
Syphilis is still a major cause of morbidity in most developing countries and is endemic in some areas of North America and Europe, particularly Eastern Europe8). In 1999, the overall incidence rate of primary and secondary syphilis in the U.S. was 2.5 per 100,000 population, but some cities showed incidence rates as high as 104.2 per 100,000 population6). Globally, there are more than 900,000 cases of pregnant women infected with syphilis every year, resulting in 360,000 fetal or perinatal deaths and in the birth of 270,000 infants with serious or permanent impairment owing to mother-to-fetus transmission5).
Coinfection of syphilis with HIV is an important issue for global HIV control strategies. A recent literature review including 30 studies on HIV prevalence among patients with primary diagnosis of syphilis showed a mean coinfection seroprevalence of 15.7%2). Such observations highlight the need to provide HIV testing for every patient diagnosed with syphilis. Several studies have demonstrated that syphilis constitutes an independent risk factor for HIV infection and the risk seems to be a result of a breakdown in the mucocutaneous barrier due to genital ulceration10). Treatment of syphilis in HIV positive people can be an important means of preventing HIV transmission. The incidence of syphilis among HIV-positive individuals is difficult to estimate, but seroprevalence studies have shown evidence of previous exposure to Treponema pallidum in 3% to 35% of HIV-positive patients3,4,12).
Concurrent HIV infection may alter the progression of syphilis by increasing the propensity of the disease to progress to neurosyphilis, decreasing the latency period before the onset of neurosyphilis, increasing the severity of the manifestations of neurosyphilis, or rendering standard therapy for primary and secondary syphilis inadequate13).
Asymptomatic neurosyphilis is common during untreated primary and secondary syphilis16,19) and during latent syphilis in HIV patients12).
While symptomatic neurosyphilis usually manifests after 2 years of untreated infection, syphilitic meningitis, the earliest manifestation, usually occurs during the first two years following infection.
The most common clinical manifestations of meningeal involvement are headache, nausea, vomiting, stiff neck, delirium, cerebral and cranial nerve dysfunction (mainly of cranial nerves VII and VIII) and, less frequently, polyradiculopathy.
Radiologic findings in neurosyphilis are nonspecific and include multifocal infarction and multiple white matter lucencies. The increase in meningeal and parenchymal enhancement observed on brain CTs in HIV-infected patients with neurosyphilis most likely reflects the increased incidence of syphilitic meningitis in this group15).
Establishing a definitive diagnosis of neurosyphilis is difficult because there is no "gold standard" criterion. Cerebrospinal fluid (CSF) examination is indispensable for the initial diagnosis and for following the patient's response to treatment. The CSF opening pressure may be increased in cases of acute syphilitic meningitis. The traditional hallmarks for the diagnosis of neurosyphilis are the CSF-venereal disease research laboratory test (VDRL), the CSF white blood cell (WBC) count and CSF protein levels. Classical abnormalities are a reactive CSF-VDRL test, pleocytosis and elevated CSF protein levels. However, normal values of these three parameters have been found in neurosyphilis11,17,19,20,22). The CSF WBC count is generally abnormal in early meningovascular neurosyphilis, but it is not necessarily abnormal in late parenchymatous neurosyphilis21). The CSF immunoglobulin G index appears to have higher sensitivity than the CSF protein level14). CSF glucose is normal but occasionally it may be slightly decreased, especially in HIV coinfected patients15).
CSF VDRL, despite its high specificity, has limited application due to its low sensitivity, especially in asymptomatic neurosyphilis11,17,19,22). False-positive CSF VDRL results can occur occasionally11), although rarely, due to blood contamination. During lumbar puncture the CSF-FTA-ABS test is more sensitive than the CSF-VDRL test for the diagnosis of neurosyphilis, but it also has limitations when there is blood contamination or diffusion of serum immunoglobulin into the CSF.
The therapy of choice is intravenous penicillin G 12 to 24 million U per day for 10 to 14 days1). In special cases requiring use of an alternative therapy, oral doxycycline, 200 mg every 12 hours for 4 weeks or intravenous doxycycline are indicated. Ceftriaxone (1 g to 2 g IM daily, for 10 to 14 days) has been used18), but treatment failures have been reported, especially in HIV-infected patients7).
Parameters for the results of the treatment of neurosyphilis are clinical improvement (which generally does not improve in parenchymatous neurosyphilis and may sometimes progress relentlessly despite treatment) and laboratory findings. CSF WBC count should have decreased at 6 months and CSF should be normalized by 2 years. If not, retreatment should be considered. Cases of persistence of CSF pleocytosis recurrence and treatment failures of symptomatic neurosyphilis after treatment with IV penicillin have been reported in HIV coinfected patients9,15,19). In fact, it is recommended that treatment continue until the CSF WBC count is normalized, so CSF examination can be performed every 6 months1).
CONCLUSION
This report is of a case of MS in an HIV positive patient, mimicking glioma at brain CT and brain MRI.
References
- 1.Centers for Disease Control and Prevention. 1998 guidelines for treatment of sexually transmitted diseases. MMWR Recomm Rep. 1998;47:1–111. [PubMed] [Google Scholar]
- 2.Blocker ME, Levine WC, St Louis ME. HIV prevalence in patients with syphilis, United States. Sex Transm Dis. 2000;27:53–59. doi: 10.1097/00007435-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Bordón J, Martínez-Vázquez C, Alvarez M, Miralles C, Ocampo A, de la Fuente-Aguado J, et al. Neurosyphilis in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1995;14:864–869. doi: 10.1007/BF01691492. [DOI] [PubMed] [Google Scholar]
- 4.Brandon WR, Boulos LM, Morse A. Determining the prevalence of neurosyphilis in a cohort co-infected with HIV. Int J STD AIDS. 1993;4:99–101. doi: 10.1177/095646249300400208. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Global Disease Elimination and Eradication as Public Health Strategies. Proceedings of a conference. Atlanta, Georgia, USA. 23-25 February 1998. MMWR Morb Mortal Wkly Rep. 1999;48 Suppl:1–208. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Primary and secondary syphilis-United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:113–117. [PubMed] [Google Scholar]
- 7.Dowell ME, Ross PG, Musher DM, Cate TR, Baughn RE. Response of latent syphilis or neurosyphilis to ceftriaxone therapy in persons infected with human immunodeficiency virus. Am J Med. 1992;93:481–488. doi: 10.1016/0002-9343(92)90574-u. [DOI] [PubMed] [Google Scholar]
- 8.Genest DR, Choi-Hong SR, Tate JE, Qureshi F, Jacques SM, Crum C. Diagnosis of congenital syphilis from placental examination : comparison of histopathology, Steiner stain, and polymerase chain reaction for Treponema pallidum DNA. Hum Pathol. 1996;27:366–372. doi: 10.1016/s0046-8177(96)90110-0. [DOI] [PubMed] [Google Scholar]
- 9.Gordon SM, Eaton ME, George R, Larsen S, Lukehart SA, Kuypers J, et al. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection. N Engl J Med. 1994;331:1469–1473. doi: 10.1056/NEJM199412013312201. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt RM, Lukehart SA, Plummer FA, Quinn TC, Critchlow CW, Ashley RL, et al. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988;2:47–50. doi: 10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Hart G. Syphilis tests in diagnostic and therapeutic decision making. Ann Intern Med. 1986;104:368–376. doi: 10.7326/0003-4819-104-3-368. [DOI] [PubMed] [Google Scholar]
- 12.Holtom PD, Larsen RA, Leal ME, Leedom JM. Prevalence of neurosyphilis in human immunodeficiency virus-infected patients with latent syphilis. Am J Med. 1992;93:9–12. doi: 10.1016/0002-9343(92)90673-y. [DOI] [PubMed] [Google Scholar]
- 13.Johns DR, Tierney M, Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987;316:1569–1572. doi: 10.1056/NEJM198706183162503. [DOI] [PubMed] [Google Scholar]
- 14.Jones HD, Urquhart N, Mathias RG, Banerjee SN. An evaluation of oligoclonal banding and CSF IgG index in the diagnosis of neurosyphilis. Sex Transm Dis. 1990;17:75–79. doi: 10.1097/00007435-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Katz DA, Berger JR, Duncan RC. Neurosyphilis. A comparative study of the effects of infection with human immunodeficiency virus. Arch Neurol. 1993;50:243–249. doi: 10.1001/archneur.1993.00540030009006. [DOI] [PubMed] [Google Scholar]
- 16.Lukehart SA, Hook EW, 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum : implications for diagnosis and treatment. Ann Intern Med. 1988;109:855–862. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- 17.MacLean S, Luger A. Finding neurosyphilis without the Venereal Disease Research Laboratory test. Sex Transm Dis. 1996;23:392–394. doi: 10.1097/00007435-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Marra CM, Boutin P, McArthur JC, Hurwitz S, Simpson PA, Haslett JA, et al. A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2000;30:540–544. doi: 10.1086/313725. [DOI] [PubMed] [Google Scholar]
- 19.Marra CM, Critchlow CW, Hook EW, 3rd, Collier AC, Lukehart SA. Cerebrospinal fluid treponemal antibodies in untreated early syphilis. Arch Neurol. 1995;52:68–72. doi: 10.1001/archneur.1995.00540250072015. [DOI] [PubMed] [Google Scholar]
- 20.Tomberlin MG, Holtom PD, Owens JL, Larsen RA. Evaluation of neurosyphilis in human immunodeficiency virus-infected individuals. Clin Infect Dis. 1994;18:288–294. doi: 10.1093/clinids/18.3.288. [DOI] [PubMed] [Google Scholar]
- 21.van Voorst Vader PC. Syphilis management and treatment. Dermatol Clin. 1998;16:699–711. xi. doi: 10.1016/s0733-8635(05)70035-8. [DOI] [PubMed] [Google Scholar]
- 22.Wolters EC, Hische EA, Tutuarima JA, van Trotsenburg L, van Eijk RV, Bos JD, et al. Central nervous system involvement in early and late syphilis : the problem of asymptomatic neurosyphilis. J Neurol Sci. 1988;88:229–239. doi: 10.1016/0022-510x(88)90220-1. [DOI] [PubMed] [Google Scholar]