Abstract
The renin angiotensin system (RAS) exerts a tremendous influence over fluid balance and arterial pressure. Angiotensin II (Ang-II), the effector peptide of the RAS, acts in the CNS to regulate neurohumoral outflow and thirst. Dysregulation of Ang-II signaling in the CNS is implicated in cardiovascular diseases, however the mechanisms remain poorly understood. Recently we established that NADPH oxidase (Nox)-derived superoxide acting in the forebrain subfornical organ (SFO) is critical in the physiologic responses to central Ang-II. In addition, we have found that Nox2 and Nox4 are the most abundantly expressed Nox homologues within Ang-II-sensitive sites in the forebrain. To dissect out the functional importance and unique roles of these Nox enzymes in the pressor and dipsogenic effects of central Ang-II, we developed adenoviral vectors expressing siRNA to selectively silence Nox2 or Nox4 expression in the SFO. Our results demonstrate that both Nox2 and Nox4 are required for the full vasopressor effects of brain Ang-II, but that only Nox2 is coupled to the Ang-II-induced water intake response. These studies establish the importance of both Nox2- and Nox4-containing NADPH oxidases in the actions of Ang-II in the CNS, and are the first to reveal differential involvement of these Nox enzymes in the various physiologic effects of central Ang-II.
Keywords: hypertension, blood pressure, water intake, subfornical organ, adenovirus, siRNA
Introduction
Highly conserved throughout evolution, the renin-angiotensin system (RAS) is vital for maintaining fluid and arterial pressure homeostasis. Osmoregulation in the leech, water swallowing in euryhaline fish, and sodium appetite in pigeons all are mediated by the RAS1, 2. As cardiovascular systems evolved, the RAS has kept pace; in higher order animals the RAS is intricately involved with nearly every aspect of cardiovascular function. Angiotensin II (Ang-II), the primary effector peptide of the RAS, acts in the central nervous system (CNS) to modulate autonomic tone, increase thirst, and initiate the release of neurohormones3. Circulating Ang-II is detected by circumventricular organs (CVOs), specialized brain regions that lie outside of the blood-brain-barrier and connect to key cardio-regulatory regions within the CNS4. In addition, since most of these CNS cardiovascular nuclei possess the enzymatic machinery to generate Ang-II locally5, 6, this source of the peptide is also important in modulating neurotransmission. The tremendous impact of RAS-CNS interactions on normal cardiovascular physiology has prompted many investigations of their role in cardiovascular disease. Indeed, mounting evidence has implicated dysregulation of central Ang-II signaling in hypertension and heart failure both in the clinic and in experimental models7. However, despite a century’s worth of studies aimed at uncovering the physiology and pathophysiology of the RAS8, the precise neural pathways and molecular mechanisms involved in Ang-II signaling in the CNS remain unresolved.
Pioneering studies by Simpson and colleagues over 30 years ago implicated the subfornical organ (SFO), a prominent forebrain CVO, as a key interface between circulating Ang-II and cardio-regulatory centers of the brain9. Recently, we demonstrated a critical role for superoxide (O2•−) production in this brain region in mediating the classical pressor, bradycardic, and dipsogenic responses elicited by ICV delivery of Ang-II10. In addition, we have identified a Rac1-dependent NADPH oxidase (Nox), a multisubunit enzyme that catalyzes the one electron transfer from NADPH to molecular oxygen11, as a primary source of Ang-II-dependent O2•− production in the SFO12. Genetic inhibition of this enzyme complex in the SFO attenuated the cardiovascular and dipsogenic profiles to ICV administration of Ang-II12. Recent studies using pharmacological or peptide inhibitors (e.g. gp91dstat13) have also confirmed NADPH oxidase as a key player in the actions of Ang-II in other CNS cardiovascular nuclei14, 15.
An entire family of Nox enzymes has now been described, each containing a unique homologue of the catalytic subunit of the oxidase16. Peripheral cardiovascular tissues express distinct complements of Nox1, Nox2, and Nox4, and these different Nox enzymes have been shown to subserve diverse roles in the pathogenesis of numerous cardiovascular diseases11. While the studies described above clearly demonstrate the general importance of NADPH oxidase in central Ang-II signaling, such broad inhibition of the enzyme through targeting either assembly or activation does not provide information about the molecular identity of the oxidase(s) involved. Recently we reported that the Nox homologues exhibit a differential expression pattern across key cardio-regulatory regions in the CNS, with Nox2 and Nox4 being the most abundantly expressed homologues in forebrain CVOs17. While little information exists regarding the functional roles of the Nox homologues in the CNS, a few recent studies implicate Nox2 in Ang-II signaling within brainstem cardiovascular nuclei. In the rostral ventrolateral medulla, Zucker’s group reported upregulation of Nox2 expression in response to central Ang-II treatment18. In addition, Wang et al. showed that Nox2 co-localizes with Ang-II receptors (AT1 subtype) in the nucleus tractus solitarius (NTS)19, and further demonstrated that Ang-II-dependent responses are significantly impaired in neurons cultured from the NTS of animals lacking Nox220. Much less is known regarding the role of Nox4 in central Ang-II or other signaling pathways of the brain.
Given the importance of NADPH oxidase-derived O2•− in the actions of Ang-II in the brain, our knowledge that the various Nox enzymes serve distinctive physiological roles, and our findings that both Nox2 and Nox4 are expressed in forebrain CVOs at high levels, here we sought to identify the functional significance of Nox2- and Nox4-containing NADPH oxidases in brain Ang-II-elicited physiological responses. To dissect out the relative contributions of these Nox homologues, we utilized adenoviral-mediated delivery of siRNA to the SFO of mouse brain to induce stable and localized knockdown of Nox2 or Nox4. We then examined the impact of selectively silencing these Nox homologues on central Ang-II-induced vasopressor and dipsogenic responses, as well as on Ang-II-induced ROS formation in cultured neurons.
Materials and Methods
What follows is a brief summary of the experimental protocols. A detailed description of all methods can be found in the expanded Materials and Methods section in the online data supplement available at http://hyper.ahajournals.org.
Adenoviral Vectors
Adenoviral vectors expressing siRNA targeted against Nox2 (AdsiNox2), Nox4 (AdsiNox4), or the control message eGFP (AdsiGFP) were constructed, purified and provided by the University of Iowa Gene Vector Core as previously described21. In brief, 21 base pair short hairpin RNAs representing sequences directed against Nox2, Nox4, or eGFP were placed under the control of the mouse U6 promoter. A separate CMV promoter drives expression of a reporter gene (GFP for siNox2 and siNox4 expressing constructs, LacZ for the siGFP expressing construct).
Measurement of Nox homologue expression in vitro
Primary neonatal rat cardiomyocytes were treated with vehicle, AdsiGFP (100pfu/cell), AdsiNox2 (100pfu/cell), or AdsiNox4 (100pfu/cell). In separate experiments, M-1 kidney cortical duct cells were also treated with vehicle, AdsiGFP or AdsiNox4 at the same concentrations as above. After 48 hours, total RNA was isolated and relative expression levels of Nox mRNAs were analyzed using real time RT-PCR with Sequence Detection Software v1.4 (Applied Biosystems) and expressed relative to vehicle-treated controls using the ΔΔCt method22.
Detection of superoxide production in primary cell culture
Primary neuronal cultures were treated with vehicle (saline) or infected with AdsiGFP, AdsiNox2 alone, AdsiNox4 alone, or AdsiNox2 and AdsiNox4 together 24 hours prior to loading with dihydroethidium (DHE, 5µM). DHE fluorescence was imaged using confocal microscopy as described10, 12. After obtaining baseline images (0 minutes), cells were stimulated with Ang-II (1µM) and reimaged after 30 minutes. DHE fluorescence was quantified using ImageJ analysis software (version 1.33, NIH) and expressed relative to baseline fluorescence in individual cells as described10.
Physiological Studies
Adult C57Bl/6 mice (Harlan, Indianapolis, IN) underwent intracerebroventricular (ICV) microinjection of saline, AdsiGFP, AdsiNox2, AdsiNox4, or AdsiNox2 + AdsiNox4 for targeted gene delivery to the SFO as described previously21, 23. Mice were then instrumented with ICV cannulae for central delivery of Ang-II, and radiotelemeters were implanted for conscious recordings of mean arterial pressure (MAP) and heart rate (HR) as described10, 12. After 7 days of recovery, MAP, HR, and dipsogenic responses were recorded following ICV Ang-II (200ng, 200nl) as described10, 12. All procedures conformed to the guidelines set forth by the NIH and were approved by the University of Iowa and Cornell University Animal Care and Use Committees.
Viral transduction efficiency assessed by reporter gene expression
One week after virus injections, mice were sacrificed and perfused with 4% paraformaldehyde. Coronal sections from AdsiGFP(LacZ)-treated mice were processed for β-galactosidase activity and analyzed by light microscopy as described21. Sections from AdsiNox2(GFP)- and AdsiNox4(GFP)-treated mice were analyzed for GFP expression by confocal microscopy (Zeiss 510).
Immunoblot analysis for Nox homologue expression in the SFO
Brains were removed from animals 7 days after SFO-targeted delivery of saline, AdsiGFP, AdsiNox2, or AdsiNox4, and micropunches from the SFO and immediately surrounding tissue10, 12, 21, 23, were pooled from 3 animals in each group. Western analysis was performed using antigp91phox antibody (BD Biosciences, 1:1000) or anti-Nox4 antibody (kind gift of Dr. David Lambeth, Emory University).
Results
Adenoviral-mediated delivery of siRNA effectively silences Nox homologue expression
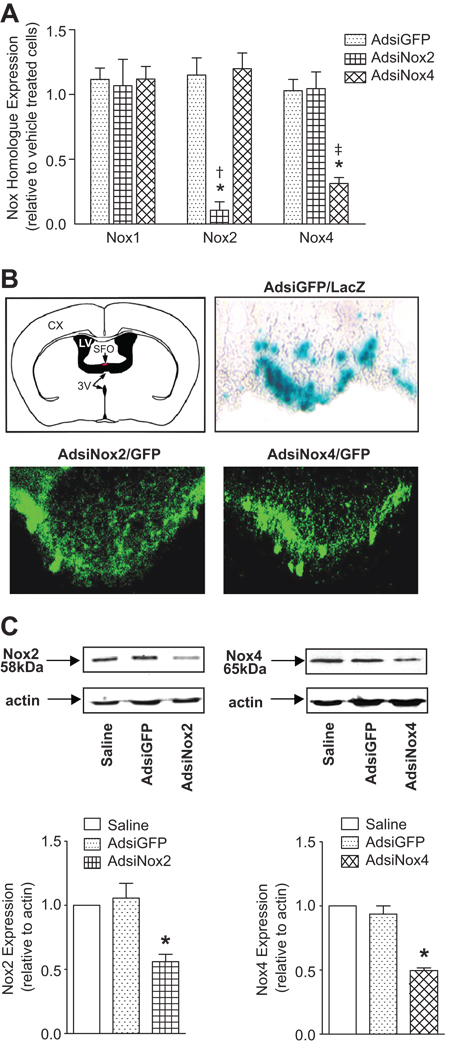
Virally-mediated delivery of RNAi has emerged as a valuable tool for stable and localized knockdown of specific genes in isolated brain regions24. We recently demonstrated that adenoviral-mediated delivery of siGFP (AdsiGFP) targeted to the SFO of GFP transgenic mice silenced GFP fluorescence selectively in this brain region25, demonstrating the feasibility of using virally-mediated delivery of siRNA to study targeted molecules within isolated cardio-regulatory regions of the CNS. In this study, we sought to utilize this strategy to evaluate the functional significance of individual Nox homologues in the SFO. To achieve this, we developed adenoviral vectors expressing siRNA targeted against either Nox2 (AdsiNox2) or Nox4 (AdsiNox4) and tested their ability to efficiently and selectively silence Nox2 or Nox4 expression, respectively. For these studies, we used primary rat neonatal cardiomyocytes since both Nox2 and Nox4 are expressed endogenously at high levels in these cells22. Cardiomyocytes were treated with vehicle, AdsiNox2, AdsiNox4, or the control vector AdsiGFP and Nox1, Nox2 and Nox4 homologue transcript levels were assessed by real-time RT-PCR. As shown in Figure 1A, AdsiNox2 and AdsiNox4 markedly diminished each of their respective targeted Nox homologues, but had no effect on the other homologues. For AdsiNox2, this further verifies our earlier data showing that this construct is selective and efficient in silencing Nox222. To further confirm the effectiveness of AdsiNox4 in another cell type in which endogenous Nox4 is expressed at high levels, M-1 kidney cortical duct cells were transduced with AdsiNox4 or AdsiGFP. AdsiNox4 caused a ~90% decrease in Nox4 levels compared to AdsiGFP (8.7 ± 0.4 × 10−3 AdsiGFP vs 0.9 ± 0.1 × 10−3 AdsiNox4 fold-β-actin; n=3, p<0.05).
Figure 1. Ad-mediated delivery of RNAi effectively silences Nox homologue expression in the SFO.
A) Real-time RT-PCR data showing the effects of AdsiGFP, AdsiNox2, or AdsiNox4 treatment on endogenous Nox1, Nox2, and Nox4 transcript levels in primary neonatal cardiomyocytes. Levels of Nox1, Nox2, and Nox4 mRNA were first normalized to β-actin, and expressed relative to levels in vehicle-treated cells. Experiments were performed in triplicate. B) ICV viral delivery results in robust and widespread transgene expression in the SFO. One week after adenoviral delivery, coronal brain sections through the SFO were visualized for reporter gene expression. The SFO is depicted in the upper left panel. Representative light and fluorescence microscopy images of sections from AdsiGFP(LacZ) (upper right panel), AdsiNox2(GFP) (lower left panel), or AdsiNox4(GFP) (lower right panel) treated mice demonstrate robust LacZ or GFP expression in the SFO. C) SFO-targeted delivery of AdsiNox2 or AdsiNox4 significantly silences Nox2 and Nox4 expression, respectively. Representative western blot and summary data of Nox2 (left) and Nox4 (right) expression in the lamina terminalis of mice microinjected with saline, AdsiGFP, or AdsiRNA targeted against Nox2 or Nox4 one week earlier. Brain tissue was pooled from 3 animals per group. Experiment was performed in triplicate. Data are expressed as mean ±SEM. *P<0.05 vs. saline and AdsiGFP. †P<0.05 vs. AdsiNox4. ‡P<0.05 vs. AdsiNox2.
Next we tested the ability of AdsiNox2 and AdsiNox4 to effectively silence Nox homologue expression within the SFO in vivo. We first verified effective viral delivery of siRNA constructs to the SFO by visualizing reporter gene expression in this brain region following ICV delivery of AdsiNox2 or AdsiNox4. These constructs harbor CMV-driven GFP as a reporter gene, while AdsiGFP contains CMV-driven LacZ. As shown in Figure 1B, reporter gene expression was robust and localized to the SFO one week following stereotaxic gene transfer of AdsiNox2, AdsiNox4, or AdsiGFP, verifying efficient viral transduction of this region. To confirm effective silencing of Nox2 or Nox4 in the SFO with these viruses, we performed western blot analysis on micropunches taken from the SFO and immediately surrounding tissue one week after injection. As shown in Figure 1C, Nox2 and Nox4 protein levels were significantly diminished in mice treated with AdsiNox2 or AdsiNox4, respectively. Together, these results confirm that Ad-mediated delivery of siNox2 or siNox4 results in effective transduction and inhibition of Nox homologue expression in targeted brain regions.
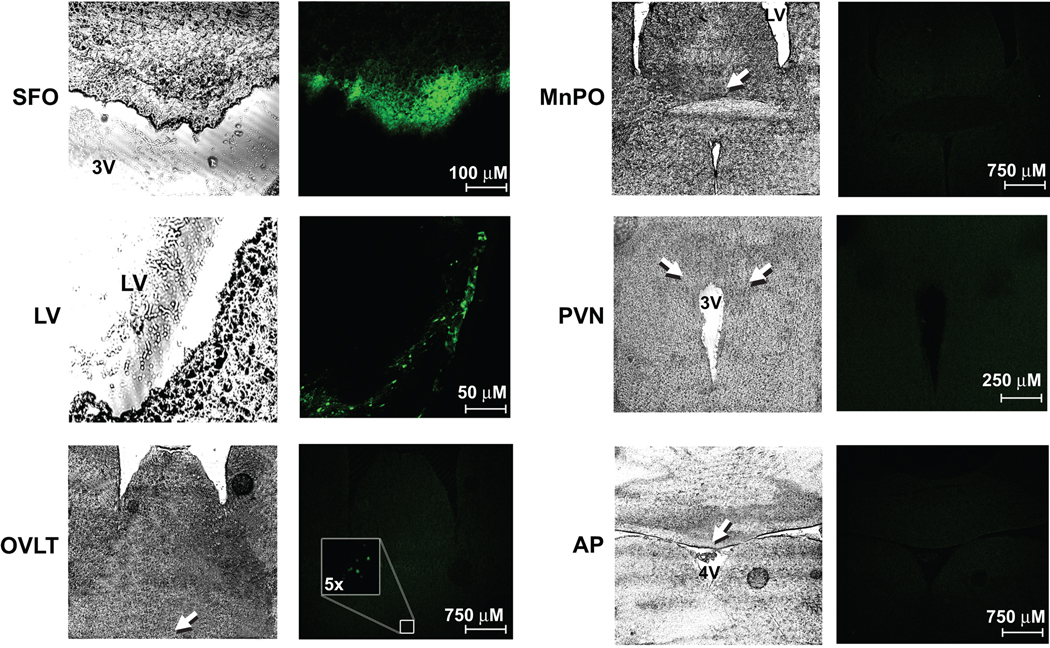
Previous studies in our laboratory have established that ICV delivery of adenoviruses is a highly effective and reliable method for targeting transgene expression to the SFO6, 10, 12, 21, 23, 26. To confirm these earlier findings, and to further explore the brain regions affected by ICV delivery of our AdsiRNAs, we examined various regions throughout the CNS for GFP expression in 4 C57Bl/6 mice one week following ICV delivery of AdsiNox2 and AdsiNox4 concomitantly. As shown in Figure 2, ICV injection of AdsiNox2 + AdsiNox4 resulted in highly robust GFP expression throughout the SFO. Similar to our previous studies27, GFP expression was also found along the ependymal layer of the lateral ventricles. There was sparse GFP expression in the organum vasculosum of the lamina terminalis (OVLT) in one animal (see Fig 2), but no expression was detected in the median preoptic nucleus, paraventricular nucleus of the hypothalamus, or area postrema (Fig 2). These findings provide additional evidence confirming our ability to target high levels of transgene expression selectively to the SFO by ICV delivery of viral vectors.
Figure 2. ICV delivery of virus results in robust and localized transgene expression in the SFO.
Serial coronal sections (30 µm) throughout the CNS from 4 mice injected ICV with AdsiNox2 + AdsiNox4 7 days earlier were processed for GFP expression using confocal microscopy. Both AdsiNox2 and AdsiNox4 harbor GFP as a reporter gene. Robust GFP expression was seen in the SFO (top left) and along the lining of the lateral ventricle (left middle). While sparse expression was found in the OVLT of one animal (left bottom), expression could not be detected in the median preoptic nucleus (MnPO), paraventricular nucleus (PVN), or area postrema (AP) (right). LV, lateral ventricle; 3V, 3rd ventricle.
Nox2 and Nox4 mediate Ang-II-induced ROS production in SFO neurons
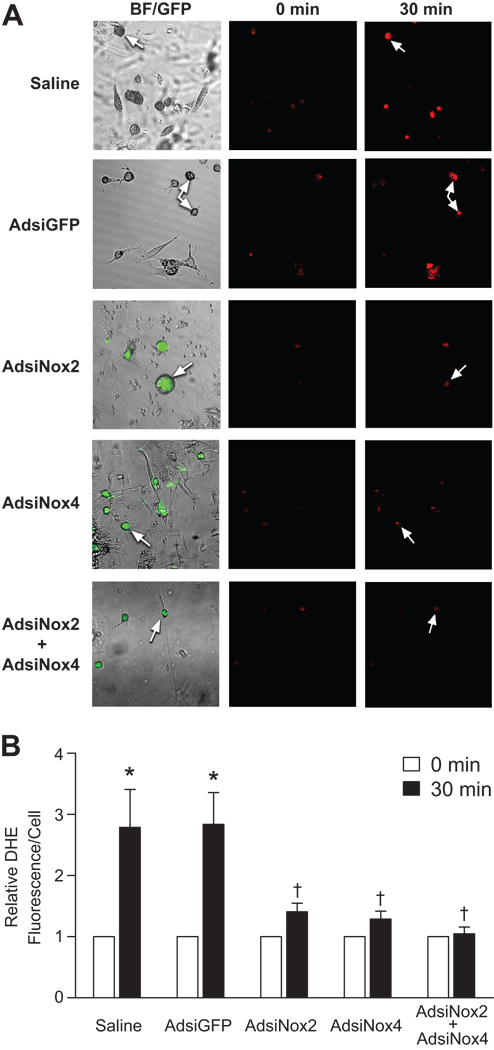
We have previously established a Rac1-dependent NADPH oxidase as the primary enzymatic source of Ang-II-induced ROS in neurons12, and have found that Nox2 and Nox4 are the most abundantly expressed Nox homologues within forebrain CVOs17. As a first step in identifying the importance of these Nox homologues in the actions of Ang-II in the CNS, we examined the impact of silencing Nox2 or Nox4 on Ang-II-dependent ROS production in neurons cultured from the lamina terminalis, a forebrain region that includes the SFO and is critical for the physiological actions of Ang-II in the CNS7, 17. In primary cultures treated with either vehicle or the control AdsiGFP vector, Ang-II induced nearly a three-fold increase in fluorescence from the ROS indicator dihydroethidium (DHE) by 30 minutes (Fig 3). However, treatment with either AdsiNox2 or AdsiNox4 alone significantly inhibited this response (Fig 3). Furthermore, concomitant treatment with both AdsiNox2 and AdsiNox4 abolished Ang-II-induced increases in DHE fluorescence (Fig 3), suggesting that both Nox2- and Nox4-containing NADPH oxidases are important sources of Ang-II-induced ROS in neurons from this brain region.
Figure 3. Silencing Nox2 or Nox4 prevents Ang-II-induced ROS production in neurons cultured from the lamina terminalis.
A) Representative confocal images of DHE (5µM, 30 min)–loaded cells cultured from the lamina terminalis showing the effects of Ang-II (1µM, 30 min) on production of reactive oxygen species in noninfected cells and cells treated with AdsiGFP, AdsiNox2 alone, AdsiNox4 alone, or AdsiNox2 and AdsiNox4 together. Effective transduction of AdsiNox2 and/or AdsiNox4 was verified by visualization of expression of the reporter gene GFP. B) Summary of relative DHE fluorescence in individual cells before Ang-II stimulation (0 min) and after 30 minutes of Ang-II stimulation. Cells were stimulated with Ang-II 24 hours after treatment with AdsiGFP, AdsiNox2, AdsiNox4, or AdsiNox2 + AdsiNox4 together. Vehicle-treated cells served as a control. Data are expressed as mean±SEM (n=10–14 cells/group over 3 separate experiments) and expressed relative to DHE fluorescence prior to Ang-II treatment (0 min). *P<0.05 vs. 0 minutes. †P<0.05 vs. 30 min saline & 30 min & AdsiGFP.
Both Nox2 and Nox4 are required for the full vasopressor effects of central Ang-II
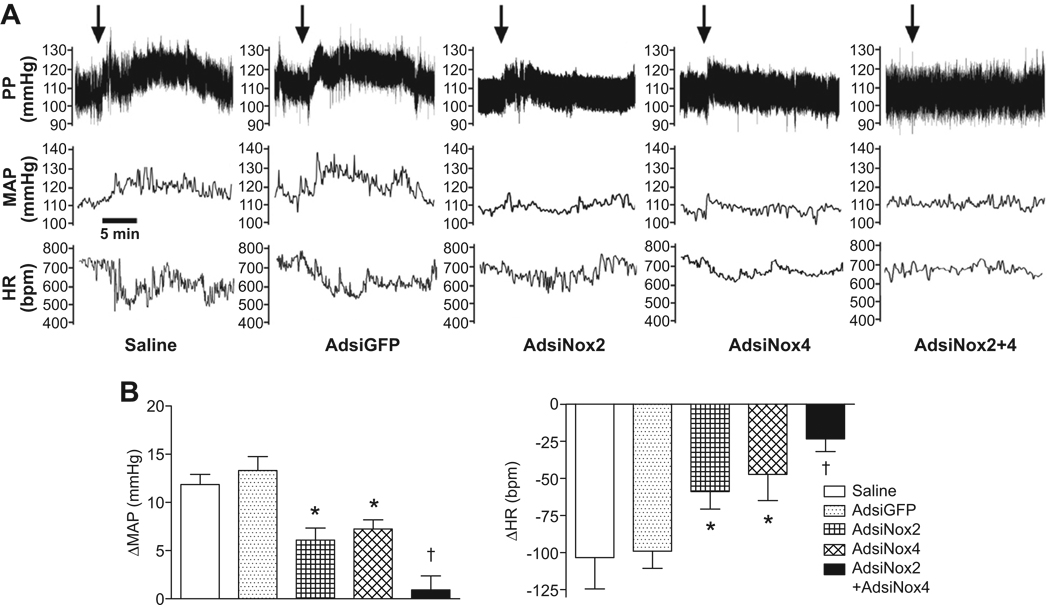
Having established the utility of Ad-mediated delivery of siRNA for selective silencing of Nox homologues in vivo, we next examined the impact of inhibiting Nox2 and/or Nox4 expression in the SFO on the physiologic profile elicited by central Ang-II. Mean arterial pressure (MAP) and heart rate (HR) responses to ICV Ang-II were recorded in conscious mice 7 days after implantation of radiotelemeters and gene transfer of AdsiNox2, AdsiNox4, AdsiNox2 and AdsiNox4 concomitantly, AdsiGFP, or saline injection. As shown in the representative tracings (Fig 4A), mice treated previously with either saline or AdsiGFP showed the classic pressor and bradycardic responses to ICV injection of Ang-II. It should be noted that ICV treatment with vehicle had no effect on blood pressure or heart rate (ΔMAP −0.4±1.0 mmHg; ΔHR −2±7 bpm, n=3), confirming that changes in these responses are specific to Ang-II treatment and not due to injection alone. Importantly, as shown in Figure 4B, summary data of the peak changes in MAP and HR in saline- or AdsiGFP-treated mice confirm that Ang-II-induced cardiovascular responses were not affected by the viral vector (or control siRNA) itself (saline: 12±1 mmHg, −103±21 bpm vs. AdsiGFP: 13±1 mmHg, −99±12 bpm, p>0.05). In contrast, either AdsiNox2 or AdsiNox4 alone caused partial reductions in the peak pressor and bradycardic responses induced by Ang-II (AdsiNox2: 6±1 mmHg, −60±12 bpm; AdsiNox4: 7±1 mmHg, −47±18 bpm, p<0.05 vs. AdsiGFP and saline; Figure 4A and 4B). The inhibitory effects of AdsiNox2 and AdsiNox4 were further seen as a reduction in the duration of these responses as indicated by a lesser area under the pressure-time plot for 15 minutes following ICV Ang-II (saline: 5.8±1.1×105, AdsiGFP: 7.7±0.8 ×105 vs. AdsiNox2: 1.3±0.7×105, AdsiNox4: 2.8±0.8×105 mmHg*sec, p<0.05). Even more importantly, treatment with both AdsiNox2 and AdsiNox4 together abolished the pressor and bradycardic responses with respect to both the peak (AdsiNox2 + AdsiNox4: 1±1 mmHg, −18±8 bpm, p<0.05 vs. all other groups) and duration (AdsiNox2 + AdsiNox4: −0.6±0.6×105 mmHg*sec, p<0.05 vs. all other groups). Together, these results demonstrate that both Nox2- and Nox4-containing NADPH oxidases are required for the full cardiovascular response profile to central Ang-II.
Figure 4. Inhibiting both Nox2 and Nox4 expression in the SFO prevents the pressor and bradycardic responses to central Ang-II.
A) Representative recordings of the effects of ICV Ang-II (200ng, 200nl) on MAP and HR in mice that underwent SFO-targeted delivery of saline, AdsiGFP, AdsiNox2, AdsiNox4, or AdsiNox2 and AdsiNox4 concomitantly 7 days earlier. Arrows indicate Ang-II injection. B) Summary data of the peak changes in MAP and HR in response to ICV Ang-II in mice that received brain microinjections of saline (n=7), AdsiGFP (n=11), AdsiNox2 (n=12), AdsiNox4 (n=6), or AdsiNox2 and AdsiNox4 concomitantly (AdsiNox2 + AdsiNox4, n=6) 7 days earlier. Data are expressed as mean±SEM. *P<0.05 vs. saline and AdsiGFP; †P<0.05 vs. saline, AdsiGFP, AdsiNox2 and AdsiNox4.
A role for ROS in the regulation of baseline blood pressure and heart rate remains controversial7. Since both cytosolic and membrane-bound Nox enzyme subunits, including Nox2 and Nox4, are expressed at baseline conditions in central cardiovascular control regions7, 17, we examined the effects of silencing Nox2 and Nox4 in the SFO on resting cardiovascular function six days following brain gene transfer. As summarized in Table 1, average baseline MAP and HR were comparable in all groups, indicating that neither Nox2 nor Nox4 in the SFO are involved in the maintenance of basal cardiovascular parameters.
Table 1. Silencing Nox2 or Nox4 expression in the SFO does not affect baseline blood pressure and heart rate.
Baseline blood pressure and heart rate were recorded in mice 6 days following SFO-targeted delivery of saline (n=12), AdsiGFP (n=15), AdsiNox2 (n=14), AdsiNox4 (n=8), AdsiNox2/4 (n=7). Data is expressed as mean±SEM. P>0.05 between all groups/
| Saline | AdsiGFP | AdsiNox2 | AdsiNox4 | AdsiNox2/4 | |
|---|---|---|---|---|---|
| MAP (mmHg) | 100±2 | 102±3 | 100±2 | 100±3 | 103±5 |
| HR (bpm) | 537±12 | 576±15 | 574±12 | 553±31 | 594±19 |
Silencing Nox2 expression alone in the SFO is sufficient to prevent the dipsogenic effect of central Ang-II
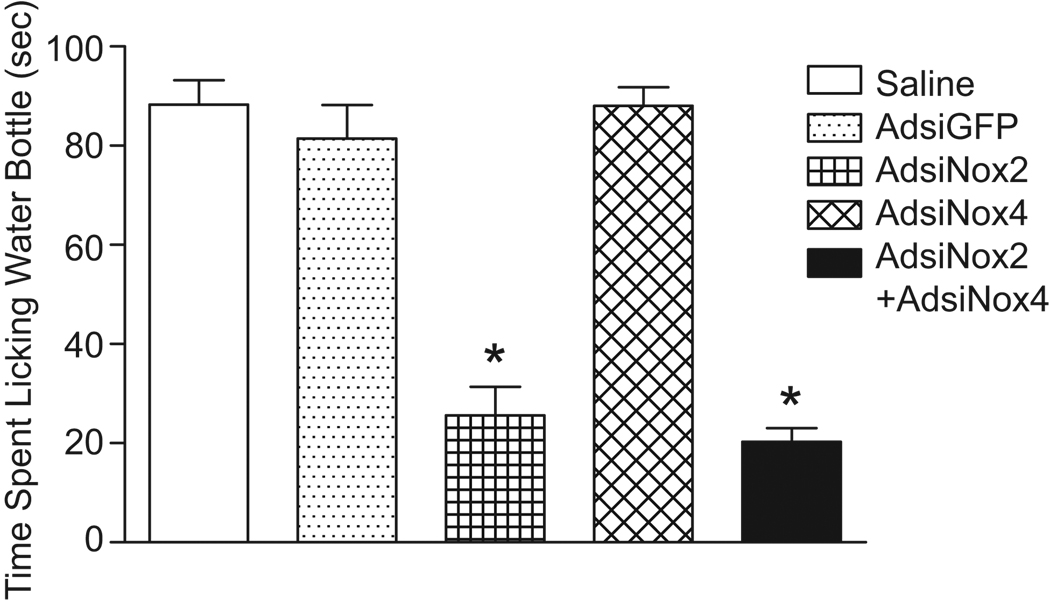
The cardiovascular responses to central Ang-II are accompanied by a brisk and pronounced water drinking response28 that is prevented by genetic inhibition of NADPH oxidase-dependent O2•− generation in the SFO12. To examine the role of Nox2 and Nox4 in the dipsogenic response elicited by central Ang-II, time spent licking the water bottle for 15 minutes following ICV Ang-II was measured in the same groups of mice as the cardiovascular studies. It should be noted that all experiments were conducted during daylight hours when baseline drinking responses are negligible. As summarized in Figure 5, ICV Ang-II induced similar potent dipsogenic responses in saline- and AdsiGFP-treated mice (saline: 88±5 sec vs. AdsiGFP: 81±7 sec, p>0.05). This drinking response was significantly attenuated in mice treated with AdsiNox2 alone (AdsiNox2: 26±6 sec, p<0.05 vs. saline and AdsiGFP), however AdsiNox4 alone had no effect on dipsogenesis (AdsiNox4: 88±4 sec, p>0.05 vs. saline and AdsiGFP). Treatment with AdsiNox2 and AdsiNox4 concomitantly inhibited the drinking response to a similar extent as did treatment with AdsiNox2 alone (AdsiNox2 + AdsiNox4: 20±3 sec, p<0.05 vs. saline, AdsiGFP, or AdsiNox4). These results demonstrate a crucial role for Nox2 in the central effects of Ang-II on water intake, and suggest that Nox4-containing NADPH oxidase is not involved in mediating this response.
Figure 5. Inhibiting Nox2 expression in the SFO prevents the dipsogenic response to central Ang-II.
Summary of the drinking response to ICV AngII (200ng, 200nl) in mice administered saline (n=11), AdsiGFP (n=17), AdsiNox2 (n=16), AdsiNox4 (n=13), or AdsiNox2 and AdsiNox4 concomitantly (AdsiNox2 + AdsiNox4, n=12) in the brain 7 days earlier. Data are expressed as the total time spent licking the water bottle (seconds) for 15 minutes after ICV injection of Ang-II. *P<0.05 vs. saline, AdsiGFP, and AdsiNox4.
Discussion
We and others have previously established the importance of NADPH oxidase-derived ROS in the brain as a key feature of Ang-II-mediated physiologic responses and in the pathogenesis of neurogenic hypertension10, 12, 14, 15, 26. Although a wealth of evidence has demonstrated the importance and unique roles of the various Nox enzymes in the actions of Ang-II in the periphery, the function of the Nox homologues involved in brain Ang-II signaling are not clear. Here, through the use of Ad-mediated delivery of siRNA to the SFO of mouse brain, we have identified Nox2 and Nox4 as key elements of Ang-II-mediated ROS production and actions in the CNS. Our data demonstrate that Nox2 and Nox4 each contribute to the vasopressor effects of central Ang-II, however neither one alone can account for the entire response. In contrast, Nox2 alone is selectively linked to the water intake effects of ICV Ang-II. These results demonstrate for the first time a functional divergence between Nox2- and Nox4-containing NADPH oxidases in central Ang-II signaling.
A key feature of our studies involved the use of Ad-mediated delivery of siRNA for selective silencing of Nox homologues within targeted brain regions. Though this approach has just recently emerged24, 25, it has already proven to be a powerful strategy for unraveling complex molecular mechanisms in the CNS, including central Ang-II signaling29. While previous studies of NADPH oxidase in the CNS established the importance of this enzyme within discrete brain regions12, 14, 15, these experiments were not able to address the contributions of specific Nox homologues. Furthermore, recent studies have questioned the specificity of currently available pharmacologic inhibitors of NADPH oxidase30. Genetic ablation of specific Nox homologues in knockout animals overcomes such limitations, and these models have served as valuable tools for studying the pathophysiological roles of specific Nox homologues in cardiovascular disease7, 17. However, global deletions of specific Nox enzymes make it difficult to interpret their role(s) within distinct brain regions.
The use of Ad-mediated delivery of siNox2 or siNox4 in this study enabled the selective silencing of distinct Nox homologues in discrete CNS nuclei. It should be noted that while our cell culture studies show ~80–90% silencing of Nox222 and Nox4 using these reagents in vitro, western blot analysis shows significant but incomplete silencing of Nox2 or Nox4 within brain tissue. We believe that this result is most likely related to the technical challenge of selectively harvesting the virally transduced SFO, which is only 0.1×0.2 mm in the mouse. As such, the western data probably reflect Nox2 and Nox4 levels in areas immediately surrounding the SFO that were not transduced by our targeted delivery of AdsiNox2 or AdsiNox4. Related to this, we confirmed that ICV delivery of viral vectors is an efficient and reproducible method for targeting the SFO. However, as demonstrated in previous studies 27, this strategy also results in transduction of the ventricle lining, as well as occasional sparse transgene expression in the OVLT. While it is highly unlikely that silencing Nox2 and/or Nox4 in the ependymal layer could impact the physiological responses to central Ang-II, we cannot entirely rule out the possibility that occasional targeting of a few cells the OVLT might play some role in changes in cardiovascular parameters seen in our studies, especially given the pivotal role of this region in regulating water intake31, 32.
Our previous studies have demonstrated that the pressor, bradycardic, and dipsogenic responses to central Ang-II are almost entirely attenuated either by scavenging O2•− or by treatment with a dominant negative mutant of Rac1, a major regulatory component of NADPH oxidase10, 12. Placed in the context of these findings, the present study suggests that both Nox2- and Nox4-containing NADPH oxidases are key sources of Ang-II-generated O2•− production in the SFO. Furthermore, our data suggest that Nox4, like Nox2, may be regulated either directly or indirectly by Rac1. While the mechanisms of Nox2 activation have been studied extensively16, 33, the molecular events involved in the activation of Nox4 remain unresolved, including the specific role of Rac-GTPase. In studies involving heterologous Nox4 expression, activation of Nox4 may be independent of cytosolic subunits required for Nox2 activation, including p47, p67, and Rac33, 34. In addition, Nox4 lacks the specific residues present in Nox2 that are required for Rac-dependent regulation35. It has also been suggested that Nox4 activity is regulated primarily at the transcriptional level36. However, the kinetics of Nox4 activation observed in systems that express this homologue endogenously have suggested a requirement for regulatory subunits33, 34, and a recent study has provided indirect evidence for Nox4 regulation by the Rho GTPases Rac1 and RhoA37.
A separate controversy exists over the mechanisms involved in the generation of ROS by Nox4. While initial characterization studies demonstrated that Nox4 constitutively generates hydrogen peroxide38, a more recent study utilizing nitro blue tetrazolium (NBT) staining suggests that Nox4, like Nox2, primarily generates O2•−36. The use of NBT in this study also underscores discrepancies regarding the measurement of Nox4-dependent ROS production using DHE. In the study by Serrander et al.36, NBT was utilized because of the suggestion that Nox4-dependent ROS production occurs within an intracellular compartment that is accessible to NBT, but not DHE. However, in the present study, we were indeed successful in utilizing DHE to monitor Nox4-dependent ROS production, with our data showing that Ang-II-induced increases in DHE-fluorescence in primary neurons were significantly blunted by inhibiting Nox4 expression. Our findings are supported by a study by Peshavarita et al. demonstrating that siRNA-mediated silencing of Nox4 expression inhibits DHE fluorescence in an endothelial cell line39. The discrepancies in studies of Nox4 regulation as well as inconsistencies in Nox4-dependent ROS measurements are likely due to differences in cell-type and systems utilized, as well as in variations in experimental procedure. Further studies of Nox enzyme regulation and activation patterns in neurons are needed, as a full understanding of the biology of Nox4 and other Nox homologues will yield important clues regarding their unique functional roles in central Ang-II and other types of signaling.
At the present time we can only speculate as to the precise mechanisms by which ROS production by different Nox enzymes mediates specific physiologic responses to central Ang-II. The SFO consists of an extremely diverse and uncharted population of cells40, and it has been suggested that separate populations of neurons in the SFO differentially regulate the blood pressure and drinking effects of Ang-II. In rats, Ang-II infusion at low doses selectively activates neurons at the core of the SFO (as indicated by c-Fos staining) and raises pressure, but does not induce a drinking response31. Higher doses of Ang-II induce dipsogenesis, and activate neurons both in the core as well as the periphery of the SFO31. In addition, other dipsogenic stimuli, including relaxin and hypertonic saline, activate neurons exclusively at the outer boundary of the SFO31. In addition, patterns of known efferent projections from the SFO seem to support a separation of function. Projections to the bed nucleus of the stria terminalis, a region known to be important in regulating autonomic tone41, 42, arise only from the core of the SFO41, while projections to the paraventricular nucleus originate from the outer regions43. In addition, it is thought that projections to the median preoptic nucleus originating in the periphery of the SFO are involved in dipsogenesis32. Further intrigue is added by the fact that the vasopressor and dipsogenic responses to central Ang-II are mediated by distinct isoforms of the Ang-II type 1 (AT1) receptor in the mouse; the AT1a receptor is linked to the pressor response, whereas the AT1b receptor is linked to the drinking response44. Interestingly, the AT1a receptor is expressed primarily in the core of the SFO45, supporting the notion that the location of this receptor is linked to its function. While a lack of appropriate antibodies has prevented comprehensive immunohistochemical localization of these receptor subtypes as well as the Nox homolgues in the SFO, it is tantalizing to consider the possibilities that the Ang-II receptor isoforms are differentially expressed in the core and peripheral regions of the SFO, and that Nox2 and Nox4 are uniquely coupled to these different AT1 receptor isoforms, whereby both Nox2 and Nox4 are linked to the AT1a receptor, while only Nox2 is linked to the AT1b receptor.
Within this framework, one possibility is that the Nox homologues are differentially expressed in the SFO, whereby both Nox2 and Nox4 are expressed at the core of the SFO, in circuitry that gives rise to the pressor response, while only Nox2 would be expressed in outer regions of the SFO, in circuitry that underlies the dipsogenic response. However, it is just as possible that Nox2 and Nox4 are co-expressed in cells throughout the SFO, but that Nox4-dependent ROS production is not a key feature of neurons involved in dipsogenesis. Such a scenario would involve compartmentalization of Nox-dependent redox signaling, whereby Nox2 or Nox4 may be utilized differentially by Ang-II within separate neuronal populations in the SFO. Interestingly, numerous examples of specialized roles for different Nox homologues within the same cell have been discovered in peripheral cardiovascular cells. Work from Griendling’s group has localized Nox1 and Nox4 to unique sub-cellular compartments in vascular smooth muscle cells, where these different homologues are thought to regulate unique temporal phases of Ang-II signaling46. In this same cell type, Miller and colleagues demonstrated that Nox1-dependent ROS production and subsequent transcription factor activation following cytokine stimulation occurs within tightly-regulated and compartmentalized signaling endosomes47. In addition, a recent study by Feng Wu et al.37 demonstrated that Nox2 and Nox4 are independently linked to the activation of separate MAP kinase pathways in endothelial cells by HIV Tat. In support of this, Shah’s group reported that overexpression of Nox2 or Nox4 in HEK293 cells triggers unique profiles of protein kinase activation48. Interestingly, in this same study, while both Nox2 and Nox4 were involved in intracellular responses to insulin, only Nox2 was found to be linked to Ang-II signaling48, suggesting that Nox4 is not a player in the actions of Ang-II in this cell type. However, a recent study by Block et al. demonstrated that, in glomerular mesangial cells, silencing of Nox4 prevents ROS formation, activation of protein kinases Src and PDK-1, and hypertrophy following Ang-II stimulation49. These findings, along with our studies demonstrating that Nox4 mediates Ang-II dependent ROS generation in neurons, indicate that the extent of Nox4 involvement in Ang-II signaling may vary by cell type and the stimulus used. While subcellular expression patterns of Nox homologues in neurons has yet to be established, accumulating evidence in peripheral cell types strongly suggests that compartmentalization of redox signaling is a prevailing feature of Nox enzyme biology. Importantly, in the present study we found that both Nox2 and Nox4 are key sources of Ang-II-induced ROS formation in primary lamina terminalis cultures from mouse pups. While it is possible that Nox expression patterns change during development and maturation, this finding suggests that at least a subset of neurons in the lamina terminalis do indeed co-express Nox2 and Nox4. Thus, it is likely that Nox2 and Nox4 are co-expressed in cells throughout the SFO, and that compartmentalization of Nox-dependent redox signaling exists such that Nox2 or Nox4 may be utilized differentially by Ang-II within separate neuronal populations in the SFO. As the molecular and biochemical tools become available, careful and meticulous localization of the Nox homologues at the regional, cellular, and sub-cellular levels will be a critical next step in determining the mechanisms linking Nox2 and Nox4 with central Ang-II-induced physiologic responses.
Perspectives
Our results demonstrate that both Nox2- and Nox4-containing NADPH oxidases are required for the pressor effects of Ang-II, whereas only Nox2 is coupled to Ang-II-induced drinking. This study is the first to demonstrate that these Nox enzymes are differentially involved in mediating the volume regulatory and blood pressure effects of brain Ang-II. We speculate that the dysregulation of these enzymes is a major contributor to the pathogenesis of neuro-cardiovascular disease, and that further characterization of the mechanisms underlying the differential roles of Nox homologues will lead to novel treatment strategies for a variety of cardiovascular disorders.
Supplementary Material
Acknowledgements
We thank Matthew Zimmerman, Ph.D. for expert advice, and Alyson Spealman, B.S. for technical assistance.
Sources of Funding
This work was supported by grants to R.L.D. from the the National Institutes of Health (HL 63887 and HL 84624) and American Heart Association (0540114N). J.R.P. is supported by NIH MSTP grant GM07739 and Ruth L. Kirschstein National Research Service Award (1F30NS060410-01A1).
Footnotes
Conflicts of Interest / Disclosures
None.
References
- 1.Takei Y. Comparative physiology of body fluid regulation in vertebrates with special reference to thirst regulation. Jpn J Physiol. 2000;50:171–186. doi: 10.2170/jjphysiol.50.171. [DOI] [PubMed] [Google Scholar]
- 2.Salzet M, Deloffre L, Breton C, Vieau D, Schoofs L. The angiotensin system elements in invertebrates. Brain Res Brain Res Rev. 2001;36:35–45. doi: 10.1016/s0165-0173(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Parsons KK, Coffman TM. The renin-angiotensin system: It's all in your head. J Clin Invest. 2007;117:873–876. doi: 10.1172/JCI31856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: Converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 5.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinnayah P, Lazartigues E, Sakai K, Sharma RV, Sigmund CD, Davisson RL. Genetic ablation of angiotensinogen in the subfornical organ of the brain prevents the central angiotensinergic pressor response. Circ Res. 2006;99:1125–1131. doi: 10.1161/01.RES.0000250259.66683.f5. [DOI] [PubMed] [Google Scholar]
- 7.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 9.Mangiapane ML, Simpson JB. Subfornical organ: Forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol. 1980;239:R382–R389. doi: 10.1152/ajpregu.1980.239.5.R382. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 11.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 13.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 14.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–666. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- 15.Chan SHH, Hsu K, Huang C, Wang L, Ou C, Chan JYH. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 16.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 17.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: Distribution, regulation and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Wang W, Li Y, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 21.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: Comparison of two viral vector systems. Hypertension. 2002;39:603–608. doi: 10.1161/hy0202.103295. [DOI] [PubMed] [Google Scholar]
- 22.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 23.Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics. 2004;18:25–32. doi: 10.1152/physiolgenomics.00048.2004. [DOI] [PubMed] [Google Scholar]
- 24.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 25.Burmeister M, Lazartigues E, Tian X, Kutschke W, Sharma RV, Kapusta DR, Davisson RL. Virally expressed siRNA mediates localized gene silencing in specific cardiovascular (CV) regulatory nuclei of mouse brain. FASEB J. 2006;20:A364-a. [Google Scholar]
- 26.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 27.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res. 2004;94:402–409. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- 28.Fitzsimons J. Angiotensin stimulation of the central nervous system. Rev Physiol Biochem Pharmacol. 1980;87:117–167. doi: 10.1007/BFb0030897. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Chen H, Hoffmann A, Cool DR, Diz DI, Chappell MC, Chen AF, Morris M. Adenovirus-mediated small-interference RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension. 2006;47:230–237. doi: 10.1161/01.HYP.0000200259.01947.bb. [DOI] [PubMed] [Google Scholar]
- 30.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular reduced nicotinamide-adenine dinucleotide phosphate oxidases but an antioxidant. Hypertension. 2007;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 31.McKinley MJ, Cairns MJ, Denton DA, Egan G, Mathai ML, Uschakov A, Wade JD, Weisinger RS, Oldfield BJ. Physiological and pathophysiological influences on thirst. Physiol Behav. 2004;81:795–803. doi: 10.1016/j.physbeh.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 32.Hollis JH, McKinley MJ, D'Souza M, Kampe J, Oldfield BJ. The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex; a neuroanatomical framework for the generation of thirst. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00869.2007. [DOI] [PubMed] [Google Scholar]
- 33.Lambeth JD, Kawahara T, Diebold B. Regulation of nox and duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hordijk PL. Regulation of NADPH oxidases: The role of rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 35.Kao YY, Gianni D, Bohl B, Taylor RM, Bokoch GM. Identification of a conserved rac-binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J Biol Chem. 2008;283:12736–12746. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 tat activates dual nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 38.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 40.Dellmann HD. Structure of the subfornical organ: A review. [review] [85 refs] Microsc Res Tech. 1998;41:85–97. doi: 10.1002/(SICI)1097-0029(19980415)41:2<85::AID-JEMT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 41.Sunn N, McKinley MJ, Oldfield BJ. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J Neuroendocrinol. 2003;15:725–731. doi: 10.1046/j.1365-2826.2003.00969.x. [DOI] [PubMed] [Google Scholar]
- 42.Dunn JD, Williams TJ. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. J Comp Neurol. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- 43.Duan PG, Kawano H, Masuko S. Collateral projections from the subfornical organ to the median preoptic nucleus and paraventricular hypothalamic nucleus in the rat. Brain Res. 2008;1198:68–72. doi: 10.1016/j.brainres.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 44.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenkei Z, Corvol P, Llorens-Cortes C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res. 1995;30:53–60. doi: 10.1016/0169-328x(94)00272-g. [DOI] [PubMed] [Google Scholar]
- 46.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 47.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 48.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 49.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: ROLE IN MESANGIAL CELL HYPERTROPHY AND FIBRONECTIN EXPRESSION. J Biol Chem. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.