Abstract
Emotion and motivation have crucial roles in determining human behavior. Yet, how they interact with cognitive control functions is less understood. Here, the basic elements of a conceptual framework for understanding how they interact are introduced. More broadly, the `dual competition' framework proposes that emotion and motivation affect both perceptual and executive competition. In particular, the anterior cingulate cortex is hypothesized to be engaged in attentional/effortful control mechanisms and to interact with several other brain structures, including the amygdala and nucleus accumbens, in integrating affectively significant signals with control signals in prefrontal cortex. An implication of the proposal is that emotion and motivation can either enhance or impair behavioral performance depending on how they interact with control functions.
Perceptual and executive competition
Although the impact of affective significance (see Glossary) on behavioral performance is well documented [1], in general, the mechanisms by which this impact is manifested remain poorly understood. And, whereas some progress has been made concerning the interactions between emotion and specific cognitive processes [2,3], important gaps still remain. Crucially, relatively little is known about the role of affective significance in `executive control' functions. Here, our goal is to propose a conceptual framework that describes how affective significance impacts the flow of information processing in the brain, with a particular aim at understating how it can either enhance or impair behavioral performance according to the situation at hand. Items laden with affective significance include those that involve threat (e.g. via pairing with mild shock) and reward (e.g. via pairing with cash). The framework thus attempts to describe how both emotion and motivation interact with executive control to determine behavioral outcome – in contrast with proposals that focus on either threat or reward processing. Here, it is suggested that both emotion and motivation signals are integrated with executive functions so as to effectively incorporate value into the unfolding of behavior. The proposed framework is referred to as the `dual competition' model to reflect the suggestion that affective significance influences competition at both the perceptual and executive levels – and because the impact is caused by both emotion and motivation.
According to many proposals of attention, objects compete for limited perceptual processing capacity and control of behavior [4,5]. To understand the flow of information processing more widely, in addition to the role of perceptual competition, it is crucial to understand the impact of executive control functions on item processing. Executive control involves a host of `adjustment processes', including perceptual selection, detection and resolution of conflict, and maintenance of contextual information. Executive control is not unitary, and different mechanisms can have their own limited processing capacities or resources [6,7]. Neuropsychological research also supports the dissociation of executive functions, consistent with the fractionation of the central executive [8–10]. The exact fractionation of executive functions is subject to debate, but probably involves at least three functions [11,12]: inhibition, shifting and updating. Crucially, ample evidence indicates some unity of executive functions as well, consistent with the notion that mechanisms are shared across functions [12,13]. This `capacity sharing' has important implications for the understanding of human information processing – because it leads to `executive competition'. Here, it is proposed that subcomponents of executive control are mutually interacting, such that resources devoted to one component will detract from those available to other components. For instance, if resources required to carry out behavioral inhibition are partly shared with those needed during shifting, an individual needing to withhold responding in a trial might exhibit an increased switching cost if she is asked in close temporal succession to switch between tasks (e.g. Ref. [14]).
It is hypothesized that affective significance determines the flow of information processing in at least two general ways: in a `stimulus-driven' and a `state-dependent' manner.
Stimulus-driven effects
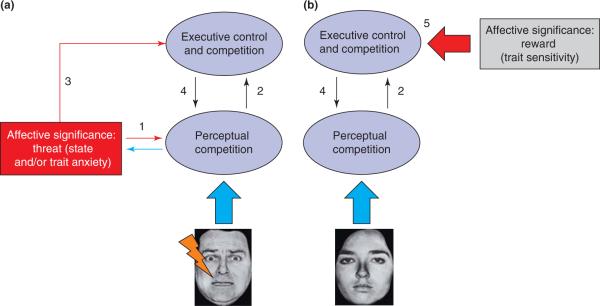
Emotion-laden stimuli include those involving emotional expressions or affective scenes, in addition to originally neutral items that might have acquired affective significance by previous pairing with aversive events (e.g. pairing with mild shock). It is hypothesized that affective significance impacts both perceptual competition and executive control (Figure 1a). Perceptual competition, which takes place in visual cortex, is affected because emotional content enhances sensory representations of emotional items (Figure 1a, arrow 1), which is well documented in human visual cortex [1]. Such enhancement depends, at least in part, on output connections from the amygdala, which is known to project to multiple levels of visual cortex, including the primary visual cortex [15].
Figure 1.
Dual competition model framework. Affective significance impacts the flow of information processing both in a (a) `stimulus-driven' (note fearful face paired with shock as input) and a (b) `state-dependent' fashion based on motivational manipulations (note neutral face input). In both cases competition is suggested to occur at the perceptual and executive levels. Arrows denote functional pathways that do not necessarily map one-to-one to specific anatomical connections. Individual differences in state and/or trait anxiety and sensitivity to reward are expected to modulate the impact of affective significance on information processing.
Executive control is affected by emotional content because; firstly, strengthened sensory representations will receive prioritized attention (Figure 1a, arrow 2). For example, items with increased visual responses can direct spatial attention towards those locations – this will occur as long as sufficient processing resources are available [16]. Secondly, executive control is modulated because affective information might be directly conveyed to control structures (Figure 1a, arrow 3). For instance, amygdala outputs might convey the significance of an item via connections with anterior cingulate cortex (ACC) territories, which might help direct attention towards the location of the emotional item via connections with the dorsolateral prefrontal cortex (PFC) (see later). In this manner, the modulation of executive control eventually affects visual processing [17] (Figure 1a, arrow 4).
The impact of an emotion-laden stimulus on behavior crucially depends on how it affects the flow of executive functions. It is hypothesized that this will depend on the level of threat, which, accordingly, will determine if emotional content enhances or impairs behavioral performance. When emotional content is low in threat, processing is biased in favor of the emotional item (Figure 2a) – this situation also extends to positive stimuli [18] (Box 1). In particular, the spatial locus of the emotional item is privileged, possibly because items that are low in threat are somewhat ambiguous and so might attract further attention as part of additional information gathering [19]. In this manner, emotional content enhances target processing with relatively minor effects on irrelevant stimuli and other executive functions that might be needed (e.g. if task switching is involved). Thus, in the low-threat case, although emotional items are prioritized, the impact on behavior is modest – in this sense, it can be said that a `soft' prioritization occurs. Because the effect on performance is relatively weak, behavioral findings can be difficult to replicate and might be observed only in high-anxious individuals (e.g. Ref. [20]). Furthermore, whereas low-threat emotional stimuli comprise a privileged stimulus category, their processing is highly dynamic and depends on the interplay of a host of factors that sculpt the associated neural responses, including attention, task context, awareness and perceptual interpretation [21].
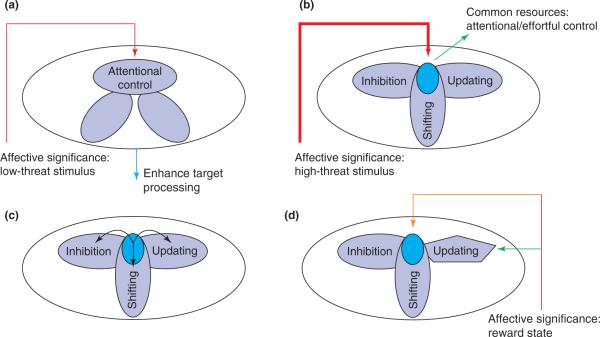
Figure 2.
Executive control and competition are viewed as involving multiple mechanisms, or resources. Larger unfilled ellipses represent executive control; smaller shapes represent processing resources. (a) When threat level is low, affective significance enhances the processing of the item. Other executive functions are not strongly impacted (smaller ellipses). (b-d) Processes are hypothesized to share resources, here called common-pool resources (smaller ellipses in bright blue), such that the engagement of one will detract from the processing of the other. Common-pool resources are proposed to be necessary for general functions of attentional/effortful control. (b) High-threat emotion-laden stimuli will typically recruit common-pool resources that allow their processing to be prioritized, which will detract from other mechanisms sharing those resources (see intersections indicated in bright blue). (c) High threat will also trigger specific executive functions to handle the challenges to the organism, as indicated by the arrows emanating from attentional/effortful control. For instance, `updating' might be needed to refresh the contents of working memory, `shifting' might be recruited to switch the current task set and `inhibition' could be called for to cancel previously planned actions. (d) State-dependent affective significance, such as reward, is hypothesized to have two main effects on executive function. Firstly, motivation fine-tunes executive functions that are important for the task at hand (represented by the change of shape of the updating function; see green arrow). Secondly, motivation can rearrange the allocation of common-pool resources (bright blue ellipse; see orange arrow), thereby affecting other executive resources.
When emotional content is high in threat, resources are diverted towards the processing of the item. The mobilization of the resources is more extreme, and the effects on behavior considerably more dramatic [22,23]. In this case, the main impact on behavior comes from the recruitment of attentional/effortful control that is required to prioritize the processing of high-threat information (Figure 2b) – thus, `hard' prioritization occurs. In particular, attentional/effortful control is envisaged as involving processing resources that are strongly shared by several executive functions (see also Refs. [24–26]). Because high-threat is expected to recruit such `common-pool resources', it will impair other executive functions that are reliant on them, including inhibition, shifting and updating. For instance, in a recent study, performance during response inhibition was compromised when participants viewed high-versus low-arousing pictures [27]. Specifically, emotional scenes preceding both go and stop stimuli increased the stop-signal reaction time, a measure of the temporal evolution of inhibitory processes (see also Ref. [28]). The processing of threat typically will require further actions and, in addition to the consumption of common resources, could involve the triggering of multiple mechanisms that are specific to the task at hand (Figure 2c).
Although the notion of resources has at times been viewed as vague [29] (but see Ref. [30]), one approach to understanding resource consumption could be to probe the correspondence of brain sites that are sensitive to specific experimental conditions. It is particularly instructive, for instance, to observe the overlap between attentional manipulations and those that are sensitive to higher levels of threat. The `attentional network' has been extensively researched and is believed to involve fronto-parietal regions, including the middle frontal gyrus (MFG), ACC, inferior frontal gyrus (IFG) and anterior insula [31,32]. To assess brain regions that are sensitive to high levels of threat, the activation sites of the contrast of CS+ (i.e. stimuli paired with an unconditioned stimulus) versus CS− (i.e. stimuli never followed by an unconditioned stimulus) of 34 aversive conditioning studies were reviewed here. In addition to the amygdala, several frontal activation sites were consistently reported, including MFG, ACC, IFG and anterior insula (Figure 3a,b). Thus, it seems that high-threat processing engages key nodes of the attentional network, consistent with the notion that it is linked to resource consumption.
Figure 3.
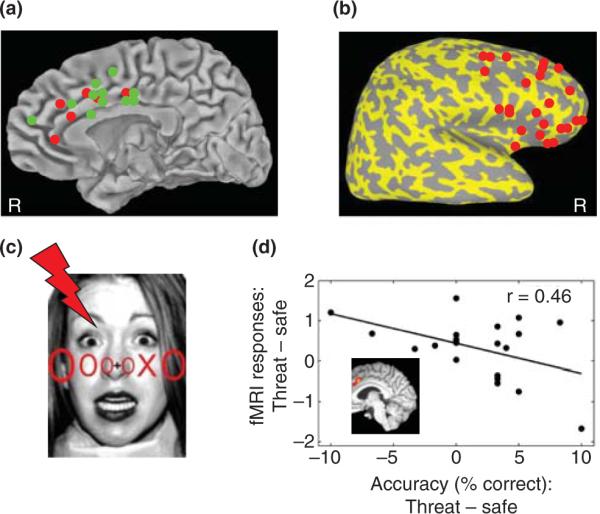
Processing resources and threat. Summary of results from 34 positron emission tomography (PET) and fMRI studies of conditioning from 1995 to 2008, illustrating the coordinates provided for the contrast of threat (CS+) versus safe (CS−). (a) Activation peaks that were observed in the ACC, or nearby cortex, are shown in green for right hemisphere results and red for left hemisphere results (all coordinates were projected onto a midline view for display purposes). (b) Results for the right lateral surface are shown on an inflated surface to reveal multiple PFC sites, including ones that are not on the surface. These included the middle frontal gyrus, inferior frontal gyrus and anterior insula (note that the surface inflation `pushed up' some of the activation sites relative to their standard anatomical positions). (c) Subjects viewed an array of letters superimposed on task-irrelevant faces and were asked to report whether or not the target letter X was present [33]. During the low attentional load condition shown here, the target appeared among a uniform array of distractors (`pop-out' condition). During the high attentional load condition (not shown), a non-uniform array of letters was employed (search condition). During the threat condition, faces were previously paired with mild electrical shock, whereas safe stimuli were never paired with shock. (d) Differential responses to task-irrelevant threat and safe faces were inversely correlated with behavioral performance, suggesting that the processing of threat captured processing resources needed for task execution as a function of threat-related responses. Results are shown for a region of interest in the ACC that was defined in terms of a separate contrast of high versus low attentional load (shown in the inset). Data reanalyzed from Ref. [33].
It is possible to further operationalize resource consumption by linking observed evoked functional magnetic resonance imaging (fMRI) responses and behavioral performance. For instance, in a recent experiment [33], subjects performed a search task under low and high attentional demands (Figure 3c), which were contrasted to determine brain sites sensitive to the availability of processing resources. Differential responses (high versus low) were observed in several fronto-parietal regions commonly associated with the attentional network, including the ones previously listed. In the same study, subjects were also shown task-irrelevant threat and safe faces (Figure 3c). Interestingly, increased responses to threat versus safe faces were observed in several of the same fronto-parietal regions. To further test the idea that additional processing resources were recruited during the viewing of threatening stimuli (relative to safe), in a new analysis, we correlated evoked fMRI responses in the regions modulated by attentional load with behavioral accuracy during the task. As illustrated in Figure 3d, the higher the ACC recruitment during the threat condition, the worse the behavioral performance (relative to the safe condition; p<0.05). Interestingly, a similar pattern of results was observed in multiple regions, including MFG, IFG and anterior insula, in addition to superior parietal lobule (although the exact spatial overlap between attentional load and threat effects varied slightly for these regions). Consistent with the increased processing of shock-paired stimuli, such stimuli exhibited increased behavioral priming and fMRI repetition effects relative to unpaired faces during a subsequent implicit-memory task [33]. These findings indicate that consumption of processing resources engaged by task-irrelevant threat faces (as indicated via, e.g. ACC responses) impaired performance on the main task.
Overall, interactions between high threat processing and executive functions are proposed to take place via at least three types of neural mechanisms (Figure 4). Firstly, it is hypothesized that threat processing engages attentional/effortful control mechanisms in the ACC and, in particular, the dorsal site observed in the previous analysis (see inset in Figure 3d) – in contrast to more rostral sites [34]. The ACC is important for integrating inputs from multiple sources, including affective and motivational inputs [35,36] – and in this respect works in close cooperation with the anterior insula and OFC [37]. The ACC has also been suggested to be involved in conflict detection, error likelihood processing and error monitoring, and helps determine the benefits and costs of acting. It is suggested here that ACC engagement during threat will impair executive function because common-pool resources that are required to prioritize threat processing are taken up. Secondly, threat also recruits multiple PFC sites that are involved in specific executive functions (Figure 4, green arrows). This recruitment is suggested to depend, at least in part, on the ACC, whose signals are known to influence activity in other brain regions and to modulate cognitive, motor and visceral responses [35]. For instance, the ACC might engage the MFG, which is important in the manipulation of information, among other important functions. In this manner, additional specific processing resources are diverted to the processing of threat information (Figure 4, orange regions). Thirdly, threat affects executive functions by inducing state changes that are implemented via ascending systems [38,39] (Figure 4, red arrows).
Figure 4.
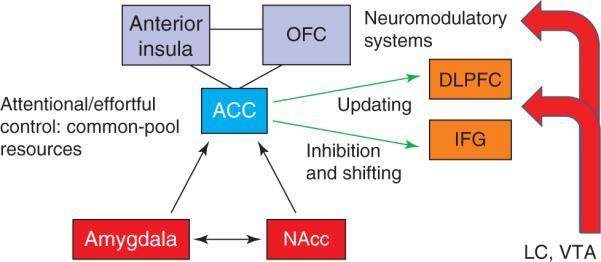
Effects of threat and motivation on executive function. Key brain regions mediating the interactions between emotion and/or motivation with executive control function. Both types of interaction are hypothesized to depend on the anterior cingulate cortex (ACC). Abbreviations: DLPFC, dorsolateral PFC; IFG, inferior frontal gyrus; LC, locus coeruleus; Nacc, nucleus accumbens; OFC, orbitofrontal cortex; VTA, ventral tegmental area.
The neural interactions described previously indicate that the effects of affective significance on behavioral performance will typically depend on multiple factors. For example, emotional content will enhance stimulus-driven processing in a way that could enhance or impair task performance. An important dimension in determining the impact of affective significance on information processing is task relevance. Specifically, an emotion-laden item that is task relevant will often improve behavioral performance because additional processing resources will typically be devoted to it (relative to neutral). At the same time, a task irrelevant emotional item will usually impair performance because resources will be taken away from the main task. As described, another important dimension of emotional information corresponds to the level of threat. On the one hand, emotional items that are relatively low in threat will benefit from sensory enhancement, which might improve, for instance, reaction time when the item is task relevant. On the other hand, emotional items that are relatively high in threat will lead to enhanced sensory enhancement but, crucially, will also divert processing resources away from other mechanisms. Thus, in many tasks, items that are high in threat will impair behavioral performance even though sensory processing is enhanced. The impairment will be typically observed when the item is task irrelevant, especially in high-anxious individuals [26].
State-dependent effects
State-dependent effects on executive control depend on general factors such as mood and anxiety [40]. This section focuses instead on a less explored source of state-dependent effects on executive function involving reward-related manipulations of motivation.
A wealth of non-human and human studies has described brain regions that are involved in the representation of reward [41]. However, how motivation impacts other brain regions that contribute to improving behavioral performance has received less attention. In humans, important steps in attempting to fill in this gap have been taken in recent years. For instance, Locke and Braver [42] showed that incentives modulated task performance, potentially by altering the control strategy employed by participants. Neuroimaging data indicated that the reward condition was associated with a sustained increase in parietal and PFC regions of the right hemisphere (see also Refs [43–46]).
It is hypothesized that motivation, like threat, impacts both perceptual competition and executive control (Figure 1b). Furthermore, motivation has two main effects on executive function. Firstly, reward will lead to the sharpening of executive functions. Crucially, reward is suggested to have specific influences on cognitive function (Figure 2d, green arrow) – as opposed to general effects, such as arousal. Recent behavioral results are consistent with this notion. For instance, motivation affected both the orienting and reorienting of attention, and the impact was evident during both exogenous [47] and endogenous tasks (J. B. Engelmann et al., unpublished*). Observed effects were specific, such that detection sensitivity (i.e. dprime) increased as a function of absolute incentive level. In parallel with improvements in behavioral performance, fMRI responses in visual cortex also increased as a function of absolute incentive level. Therefore, the findings indicate that elevated motivation leads to improved efficiency in orienting and reorienting of attention and that one mechanism by which attention and motivation interact involves the enhancement of attention during motivationally salient conditions – resulting in the boosting of responses in occipitotemporal visual regions engaged by the task at hand (Figure 1b, arrow 4) (see also Refs [45,46,48,49]).
Secondly, motivation is proposed to recalibrate the allocation of processing resources available to executive functions, to maximize potential reward. Because of capacity sharing, such reallocation is suggested to impact not only target functions directly associated with rewarded behaviors but also other processes that share some of the same processing resources (Figure 2d, orange arrow). In this manner, motivation could affect executive function in a way that is actually deleterious to behavioral performance. For instance, in a recent study, participants who were rewarded for accurate and fast performance on go trials of a stop-signal task exhibited impaired inhibitory performance as evidenced by prolonged stop-signal reaction time (S. Padmala and L. Pessoa, unpublished†). One possibility is that, to maximize reward, participants enhanced attention to the go stimulus, leaving fewer resources to process the stop stimulus (see Ref. [50]). In this sense, incentives can be viewed as reallocating resources to prioritize the processing of the rewarded function in a way that is similar to that discussed for threat processing.
As in the case of threat processing, it is hypothesized that motivation influences executive function by engaging the ACC, partly via influences from the ventral striatum and OFC (Figure 4). As before, it is hypothesized that motivation-related ACC recruitment has an important role in controlling the operations of other brain regions, so as to maximize utility (i.e. maximize reward or minimize punishment); see Figure 4, green arrows. For instance, the dorsolateral PFC might be recruited to resolve conflict in a way that increases utility. Motivation also affects executive functions by inducing state changes that are implemented via ascending systems – again, in a way that is parallel to threat (Figure 4, red arrows). For instance, incentives affect responses in the locus coeruleus (LC), which regulates norepinephrine function, and also modulate dopaminergic function. LC phasic firing often tracks task-relevant processes, such as target stimuli, but not task-irrelevant items, such as distractors (see Ref. [51]). These and other findings have led to the suggestion that the LC is responsive to ongoing evaluations of task utility provided by input from frontal structures [51].
The notion that motivation interacts with executive functions to meet current behavioral demands and opportunities is well supported by animal studies of reward, too. For instance, Redgrave and colleagues [52,53] have proposed that the dopamine response, and consequently the related striatal function, might be viewed as providing a signal that facilitates the reallocation of limited behavioral and cognitive processing capacity towards unexpected events of behavioral significance, including rewarding ones. These and other results (e.g. Refs [54,55]) are consistent with the notion that striatal activation drives the reallocation of available resources to process salient events whose processing is then prioritized – instead of simply providing a `reward signal'.
Above, interactions between emotion and motivation with executive functions were discussed in terms of stimulus-driven and state-dependent effects, respectively. However, these two types of effects were not meant to diagnostically capture the differences between emotion and motivation. For instance, when one is hungry (i.e. in a motivational state of hunger), food items are salient and can direct attention in a stimulus-related fashion [46]. Conversely, anxiety might be thought of as an emotional state of sustained threat, and leads to state-dependent effects [56], too. Finally, in general, emotion and motivation are broad constructs and here, for brevity, we focus on threat and reward processing only (Box 1).
Conclusions
The proposed framework of how emotion and motivation interact with executive functions draws upon several ideas in the literature, including biased competition [4] and resource theory [6,7,30,57] – see also Braver et al. [58] and Robbins et al. [59] for complementary proposals. A considerable body of work has investigated how emotional stimuli are prioritized in terms of attentional processes [1]. However, less research has been devoted to understanding the integration of emotional information and executive control. Likewise, a large amount of literature has considered the neural substrates of reward [41]. Again, less research has attempted to investigate how motivation directly interacts with executive function. The goal of the dual competition framework is to propose basic elements of a conceptual framework with which to understand how both emotion and motivation are integrated with executive control. An important implication of the proposal is that emotion and motivation can either enhance or impair behavioral performance depending on how they interact with key control functions. Carefully characterizing how emotion and motivation can be beneficial or deleterious to behavior (e.g. as in drug addiction) constitutes a great challenge for future research (Box 2).
Box 1 Positive and other stimuli.
In the main text, the effects of emotional content on information processing are discussed in terms of threat level because they provide the clearest example of cognitive-emotional interactions in which both perceptual and executive competition are needed to explain how affectively potent information impacts behavior (e.g. see Figure 3c,d in main text). However, the framework should be generalized to consider other stimulus classes, including high-intensity perceptual stimuli (including items high in arousal but of neutral valence [60]), novel and erotic stimuli. In these cases, stimulus-driven effects might function as in the low threat situation, although stronger effects might be generated by items of sufficiently high arousal (e.g. Ref. [61]). In other words, high-arousal items can command a form of `hard prioritization' (see main text) such that processing resources needed by executive functions are devoted to their processing, thereby impairing task performance in a manner analogous to that shown in Figure 2b (in the main text). In particular, exposure to erotica enhances visual responses [62] and can interfere with ongoing tasks [63,64]. Interestingly, during the viewing of these items, both the amygdala and the ventral striatum are strongly recruited, in addition to the ACC [65]. Because these regions are key nodes of the scheme proposed in Figure 4 (in the main text), the interaction between the processing of erotic stimuli and cognitive function might function in a manner closely related to that proposed here for threat-related information.
Box 2 Questions for future research.
Can the dual competition framework be extended to other sensory modalities? The answer seems to be in the affirmative, at least for auditory stimuli [66].
Can positive stimuli ever lead to a `hard' prioritization of processing that is comparable to that of threat? In at least one case, we failed to observe comparable effects [61].
Is the impact of higher levels of threat the same across distinct executive functions? For instance, does an affectively potent item impair conflict processing and behavioral inhibition to a similar extent?
Can the model be extended to a broader range of motivational states, including thirst, hunger and sexual drive?
Should executive functions be viewed as tied to specific regions or are they better conceptualized as engaging specific networks of brain regions [67]?
Acknowledgements
I would like to thank Todd Braver, Josh Brown, Greg Hajcak, Seung-Lark Lim, Leticia Oliveira, Srikanth Padmala, Mirtes Pereira and the anonymous reviewers for their insightful feedback on earlier versions of the manuscript. I am indebted to Seung-Lark Lim for the review of activation sites shown in Figures 3a,b and for the data analysis shown in Figure 3d. Finally, I thank Andrew Bauer and Srikanth Padmala for assistance with figures. The author's work is partly funded by the National Institute of Mental Health (R01 MH071589).
Glossary
- Affective significance
affectively significant items are those that have either negative or positive value to the organism
- Dprime
perceptual sensitivity measure that takes into account both `hits' (e.g. correct, target-present trials) and `false alarms' (incorrect, target-absent trials). Dprime scores effectively discount for elevated numbers of false alarms and are independent of response criterion
- Emotion
emotion and motivation (see later) are closely linked concepts as both depend on the relationship between the organism and its environment (e.g. positive-negative, approach-withdrawal)
- Executive control
set of functions, typically believed to depend on the frontal cortex (and probably the parietal cortex), which are needed when non-routine behaviors are called for - namely, when `control' is required. These functions are thought to confer behavioral flexibility and context-dependency to complex behaviors
- Motivation
commonly defined as what makes one work to obtain a reward or to avoid punishment. In the case of emotion (see earlier), the emphasis might be on the evaluative aspect of the organism-environment relationship, whereas in the case of motivation it might be on how the organism acts in a given situation
- Resources
specific information processing mechanisms (e.g. inhibition) have their own limited processing capacities or resources. Given the limited capacity of mental resources, performance is impaired if demands are greater than available capacity. In addition to having their own specific resources, executive functions are proposed to share a common resource pool
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
J. B. Engelmann et al. (2009). Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience (submitted)
S. Padmala and L. Pessoa. Motivation and inhibitory control: reward delays response inhibition and decreases responses in inferior frontal cortex. Paper/poster presentation at the 38th Annual Meeting of the Society for Neuroscience, Washington, D.C., 2008.
References
- 1.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 3.Pessoa L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 4.Desimone R, Duncan J. Neural mechanisms of selective attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 5.Pashler H. The Psychology of Attention. MIT Press: 1998. [Google Scholar]
- 6.Kahneman D. Attention and Effort. Prentice-Hall: 1973. [Google Scholar]
- 7.Norman DA. On data-limited and resource-limited processes. Cognit. Psychol. 1975;7:44–64. [Google Scholar]
- 8.Norman DA, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson RJ, et al., editors. Consciousness and Self-Regulation. Plenum: 1986. pp. 1–18. [Google Scholar]
- 9.Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 10.Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; 2002. [Google Scholar]
- 11.Smith EE, Jonides JJ. Neurosicence – storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 12.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex `Frontal Lobe' tasks: a latent variable analysis. Cognit. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 13.Duncan J, et al. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cognit. Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- 14.Verbruggen F, et al. The interaction between stop signal inhibition and distractor interference in the flanker and Stroop task. Acta Psychol. (Amst) 2004;116:21–37. doi: 10.1016/j.actpsy.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Amaral DG, et al. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- 16.Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr. Opin. Neurobiol. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 18.Brosch T, et al. Beyond fear: rapid spatial orienting toward positive emotional stimuli. Psychol. Sci. 2008;19:362–370. doi: 10.1111/j.1467-9280.2008.02094.x. [DOI] [PubMed] [Google Scholar]
- 19.Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Dir. Psychol. Sci. 1998;7:177–188. [Google Scholar]
- 20.Fox E, et al. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J. Exp. Psychol. Gen. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- 21.Ohman A. Automaticity and the amygdala: nonconscious responses to emotional faces. Curr. Dir. Psychol. Sci. 2002;11:62–66. [Google Scholar]
- 22.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford University Press; 1998. [Google Scholar]
- 23.Lang PJ, et al. Fear and anxiety: animal models and human cognitive psychophysiology. J. Affect. Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 24.Mathews A, Mackinstosh B. A cognitive model of selective processing in anxiety. Cognit. Ther. Res. 1998;22:539–560. [Google Scholar]
- 25.Eysenck MW, et al. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 26.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn. Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Verbruggen F, De Houwer J. Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cogn. Emotion. 2007;21:391–403. [Google Scholar]
- 28.Blair KS, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navon D. Resources-A theoretical soup stone? Psychol. Rev. 1984;91:216–234. [Google Scholar]
- 30.Park S, et al. Concurrent working memory load can facilitate selective attention: evidence for specialized load. J. Exp. Psychol. Hum. Percept. Perform. 2007;33:1062–1075. doi: 10.1037/0096-1523.33.5.1062. [DOI] [PubMed] [Google Scholar]
- 31.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 32.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 33.Lim SL, et al. Affective learning modulates spatial competition during low-load attentional conditions. Neuropsychologia. 2008;46:1267–1278. doi: 10.1016/j.neuropsychologia.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush G, et al. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 35.Devinsky O, et al. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 36.Rushworth MF, et al. Functional organization of the medial frontal cortex. Curr. Opin. Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci. Biobehav. Rev. 1995;19:449–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 38.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- 40.Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cogn. Emotion. 1992;6:409–434. [Google Scholar]
- 41.Schultz W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 42.Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- 43.Pochon JB, et al. The neural system that bridges reward and cognition in humans: an fMRI study. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert AM, Fiez JA. Integrating rewards and cognition in the frontal cortex. Cogn. Affect. Behav. Neurosci. 2004;4:540–552. doi: 10.3758/cabn.4.4.540. [DOI] [PubMed] [Google Scholar]
- 45.Small DM, et al. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb. Cortex. 2005;15:1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- 46.Mohanty A, et al. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb. Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelmann JB, Pessoa L. Motivation sharpens exogenous spatial attention. Emotion. 2007;7:668–674. doi: 10.1037/1528-3542.7.3.668. [DOI] [PubMed] [Google Scholar]
- 48.Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Della Libera C, Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychol. Sci. 2006;17:222–227. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 50.Boehler CN, et al. Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cereb. Cortex. 2008;19:134–145. doi: 10.1093/cercor/bhn063. [DOI] [PubMed] [Google Scholar]
- 51.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 52.Redgrave P, et al. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- 53.Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat. Rev. Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- 54.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 55.Zink CF, et al. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- 56.Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lavie N, et al. Load theory of selective attention and cognitive control. J. Exp. Psychol. Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- 58.Braver TS, et al. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway ARA, et al., editors. Variation in Working Memory. Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- 59.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos. Trans. R. Soc. Lond. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mourão-Miranda J, et al. Contributions of emotional valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20:1950–1963. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Pereira MG, et al. Sustained and transient modulation of performance induced by emotional picture viewing. Emotion. 2006;6:622–634. doi: 10.1037/1528-3542.6.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley MM, et al. Activation of the visual cortex in motivated attention. Behav. Neurosci. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- 63.Schupp HT, et al. Selective visual attention to emotion. J. Neurosci. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Most SB, et al. The naked truth: positive, arousing distractors impair rapid target perception. Cogn. Emotion. 2007;21:964–981. [Google Scholar]
- 65.Karama S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum. Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brosch T, et al. Behold the voice of wrath: cross-modal modulation of visual attention by anger prosody. Cognition. 2008;106:1497–1503. doi: 10.1016/j.cognition.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Dosenbach NU, et al. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]