Abstract
Docosahexaenoic acid (DHA, 22:6n-3), the major polyunsaturated fatty acid accumulated in the brain during development, has been implicated in learning and memory, but underlying cellular mechanisms are not clearly understood. Here, we demonstrate that DHA significantly affects hippocampal neuronal development and synaptic function in developing hippocampi. In embryonic neuronal cultures, DHA supplementation uniquely promoted neurite growth, synapsin puncta formation and synaptic protein expression, particularly synapsins and glutamate receptors. In DHA-supplemented neurons, spontaneous synaptic activity was significantly increased, mostly because of enhanced glutamatergic synaptic activity. Conversely, hippocampal neurons from DHA-depleted fetuses showed inhibited neurite growth and synaptogenesis. Furthermore, n-3 fatty acid deprivation during development resulted in marked decreases of synapsins and glutamate receptor subunits in the hippocampi of 18-day-old pups with concomitant impairment of long-term potentiation, a cellular mechanism underlying learning and memory. While levels of synapsins and NMDA receptor subunit NR2A were decreased in most hippocampal regions, NR2A expression was particularly reduced in CA3, suggesting possible role of DHA in CA3-NMDA receptor-dependent learning and memory processes. The DHA-induced neurite growth, synaptogenesis, synapsin, and glutamate receptor expression, and glutamatergic synaptic function may represent important cellular aspects supporting the hippocampus-related cognitive function improved by DHA.
Keywords: docosahexaenoic acid, hippocampal development, long-term potentiation, neurite growth, synaptic function, synaptogenesis
Docosahexaenoic acid (DHA, 22:6n-3), an n-3 polyunsaturated fatty acid highly enriched in the brain, has been recognized as an essential nutrient for proper brain development and function (Salem et al. 2001; Innis 2008). It has been reported that early dietary supply of DHA improves later cognitive development in human infants (Willatts et al. 1998; Birch et al. 2000), and memory-related learning ability in young rats (Gamoh et al. 1999). Conversely, n-3 fatty acid deficiency during development, which lowers DHA in the brain, has been shown to induce cognitive deficit in experimental animals (Moriguchi et al. 2000; Catalan et al. 2002). These findings suggested that DHA influences hippocampus-dependent function. An important role of DHA in hippocampal development is further indicated as depletion of DHA in rodent brains during development has been shown to decrease the soma size in the hippocampus (Ahmad et al. 2002) and the density of synaptic vesicles in the terminals of the CA1 region after learning tasks (Yoshida et al. 1997), and hampers neurite growth (Calderon and Kim 2004) and survival (Akbar et al. 2005) in embryonic hippocampal cultures. DHA administration during gestation and nursing has also been reported to increase hippocampal dendritic spine density and some synaptic proteins in the brains of weanling rat pups (Cansev et al. 2009). Recently, a role of DHA in cell migration in the developing rat brain has been demonstrated (Yavin et al. 2009). Despite these extensive studies, the cellular basis for DHA-promoted hippocampus-related cognitive functions is not clearly understood. Using in vitro DHA supplementation and in vivo depletion models, we demonstrate in this study that DHA influences not only neurite growth and synaptogenesis, but also synapsin and glutamate receptor subunit (NR and GluR) levels and synaptic activity in hippocampal neurons in culture. Moreover, developmental depletion of DHA led to reduced expression of synapsins and two types of glutamate receptors, N-methyl-D-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methylisoxazole (AMPA) receptors, in the hippocampi of postnatal 18-day-old (P-18) mice. Immunohistochemical analysis indicated that synapsin expression was decreased in CA1, CA2, and CA3 as well as dentate gyrus (DG) while the reduction of NR2A was particularly prominent in CA3. In these DHA-depleted hippocampi, we observed significantly impaired long-term potentiation (LTP), a well-characterized form of synaptic plasticity similar to that involved in hippocampus-based learning and memory.
Materials and methods
Animals and diets
Timed pregnant C57/BL6 mice were obtained from Charles River Laboratories (Portage, MI, USA). For the experiments with fatty acid supplementation in culture, mice at 16 days of pregnancy were purchased and fed with NIH-31 diet for 2 days before collecting the embryonic day 18 (E18) fetuses. For experiments with in vivo alteration of the fatty acid composition, mice at 2 days of pregnancy were obtained and fed with either an n-3 fatty acid adequate or deficient diet for 16 days for E18 culture, or throughout the pregnancy and lactation period for the analysis at P-18. Both semisynthetic pelleted diets were based on the AIN-93G formula (Reeves et al. 1993) varied only in fat composition (Dyets, Bethlehem, PA, USA). The adequate diet consisted of 7.45, 1.77, 0.48, and 0.3 g of hydrogenated coconut, safflower, flaxseed, and DHASCO® oil (Martek, Columbia, MD, USA) in 100 g diets, respectively. The deficient diet contained 8.1 and 1.9 g of hydrogenated coconut and safflower oil per 100 g, respectively. The resulting n-3 fatty acid content was 2.5 wt% linolenic acid (LNA) plus 0.9 wt% DHA in the adequate diet, and only 0.09 wt% of LNA in the deficient diet. The procedures employed in this study were approved by the National Institute on Alcohol Abuse and Alcoholism (LMS-HK21).
Hippocampal primary culture and fatty acid supplementation
Pregnant females were killed by cervical dislocation after exposure to CO2 inhalation. Embryonic neurons were prepared from E18 mouse hippocampi and cultured as described previously (Calderon and Kim 2004) with slight modification. Briefly, hippocampi were dissected and treated with 0.25% trypsin (Gibco Invitrogen Corporation, Grand Island, NY, USA) in Hank's balanced salt solution at 37°C for 15 min. After the trypsin solution was aspirated off, cells were washed with same volume of balanced salt solution, suspended in neurobasal medium (Gibco) containing 2% B27 supplement (Gibco), 0.5 mM glutamine (Gibco), 100 U/ml penicillin (Gibco) and 100 μg/ml streptomycin (Gibco), and seeded on poly-d-lysine chambered slides (Lab-Tek, Naperville, IL, USA) at a density of 30 000 cells/cm2. On the second day after seeding, the cells were treated with fatty acids. Fatty acids (Nu-Chek Prep, Elysian, MN, USA), such as DHA, oleic acid (OLA), arachidonic acid (ARA), or DPA (22:5n-6), were complexed with fatty acid-free bovine serum albumin (BSA; Sigma, St Louis, MO, USA) in the presence of α-tocopherol (Sigma). An aliquot of DHA, OLA, ARA, or DPAn-6, which had been dissolved in methanol under an argon atmosphere, aliquoted and stored at –70°C, was mixed with α-tocopherol under an argon atmosphere and darkness to prevent oxidation of the fatty acids. The mixture was dried under argon and then complexed with BSA, vortexed and kept on dry ice until it was dissolved with fresh Neurobasal medium containing B27 supplement, 1 mM glutamine and the above antibiotics. Final concentrations of fatty acids, α-tocopherol, and BSA in the culture medium were 1 μM, 40 μM, and 0.01%, respectively. Thereafter, one-half of the growth medium was replaced every 3 days with the above Neurobasal medium with or without fatty acids. For western blot (WB) analysis, E18 hippocampal cells were plated in 100 mm plates at 50 000 cells/cm2 density and supplemented with 1 μM fatty acids.
Immunocytochemistry and evaluation of neurite outgrowth and synapsin puncta formation
Cells were fixed with 0.4% p-formaldehyde (Sigma) in phosphate-buffered saline (PBS), pH 7.4, for 30 min. After permeabilization with 0.1% Triton X-100 (Sigma), cells were blocked for 1 h at 37°C with PBS containing 10% goat serum (Gibco), and then incubated with the primary antibody against microtubule-associated protein (mouse monoclonal 1 : 250; Sigma) and synapsin I (rabbit polyclonal 1 : 1000; Sigma). After washing with PBS, cells were incubated with the corresponding Cy2-, Cy3-conjugated secondary antibody (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA). For the nuclear staining, 0.06% 4, 6-diamidino-2-phenylindole (Sigma) was used. After immunostaining, images were collected using an inverted motorized IX81 Olympus (Melville, NY, USA) and the neurite length and synapsin puncta were analyzed with metamorph software (Molecular Devices Corporation, Downingtown, PA, USA). To minimize bias, neurons were evaluated blindly without the knowledge of sample identity. Five to six fields per well were chosen at random and only non-clustered neurons were evaluated to ensure the precision of the measurements. From each well, 120 neurons were evaluated at 20× magnification for total neurite length/neuron, number of branches and the number of synapsin puncta/neuron. Twenty neurons were analyzed from each well for the number of synapsin puncta/10 μm neurite length at 60× magnification. The data were obtained from triplicate wells and the experiments were repeated at least three times.
Western blot analysis
Twenty-five micrograms of protein were loaded in each lane. After sodium dodecyl sulfate–polyacrylamide gel electrophoresis, proteins were blotted onto poly vinylidene difluoride membranes (Amersham Pharmacia Biotech., Buckinghamshire, UK). The membranes were blocked for 15 min in Tris-buffered saline (TBS) containing 5% skim milk and 0.1% Tween 20 (Bio-Rad, Hercules, CA, USA). Incubation with primary and secondary antibodies was performed in TBS containing 5% skim milk for overnight and 2 h, respectively. Subsequently, membranes were washed in TBS containing 0.1% Tween 20. The primary antibodies used were mouse monoclonal GABAAR (Millipore, Billerica, MA, USA), rabbit monoclonal GluR1 (Abcam, Cambridge, MA, USA), rabbit monoclonal GluR2 (Abcam), rabbit monoclonal synapsin1 (Sigma), goat monoclonal β-actin (β-actin; Santa Cruz Biotech., San Francisco, CA, USA), rabbit monoclonal NR1 (Sigma), rabbit polyclonal NR2A (ProSci Inc, Canton, MA, USA) and mouse polyclonal NR2B (Neuromap Facility, Davis, CA, USA). Secondary antibodies were horseradish peroxidase-conjugated anti-mouse, anti-goat, and anti-rabbit antibodies (Santa Cruz Biotech). Enhanced chemiluminescence kit (Thermo Fisher Scientific, Rockford, IL, USA) was used for detection. Blots were imaged using Kodak Gel Logic 440 Imaging System and quantified using Kodak Scientific Imaging Software (Kodak Eastman, New Haven, CT, USA).
Immunohistochemistry
Brains from offspring mice at P-18 from dams fed with the n-3 fatty acid adequate or deficient diet were sliced in the sagittal or coronal plane (30 μm), ~3.8 mm posterior to Bregma, and processed for synapsin1 immunohistochemistry as described previously (Vaynman et al. 2004). The images were collected using an inverted motorized IX81 Olympus microscope.
Electrophysiology
For evaluating synaptic function of the cultured neurons, synaptic currents were measured in the conventional whole-cell voltage-clamp mode using a glass pipette electrode (3–5 MΩ) at a holding potential of –60 mV (Sheinin et al. 2008). The intracellular whole-cell patch pipette solution contained the following (in mM): 150 CsCl, 2 MgCl2, 0.3 Na-GTP, 3 Mg-ATP, 0.2 BAPTA-4K (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrapotassium), and 10 HEPES, pH adjusted to 7.23 with CsOH, and osmolarity adjusted to 298 mOsm with sucrose. The composition of extracellular buffer was (in mM) 150 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 d-glucose, pH adjusted to 7.4 with NaOH, osmolarity adjusted to 320 mOsm with sucrose. To isolate GABAergic spontaneous post-synaptic currents (GABA-sPSCs), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium (NBQX; 5 μM), and d-2-amino-5-phosphonopentanoic acid (AP5; 50 μM) were locally applied to the target neurons using a multibarrelled array of square glass applicators on a Perfusion Fast-Step system (Warner Instruments, Hamden, CT, USA). For measurement of glutamatergic sPSC (Glu-sPSCs), bicuculline (20 μM) was superfused onto the neuron using the same application system. Tetrodotoxin (1 μM) superfused to block action potentials in the pre-synaptic neurons did not alter the amplitude and frequency of PSCs indicating that detected sPSCs were miniature PSCs. The spontaneous synaptic currents were detected using minianalysis Software (Synaptosoft, Decatur, GA, USA) with a threshold detection of 20 pA and manual detection for smaller events (down to 10 pA). Field excitatory post-synaptic potentials (fEPSPs) were recorded from hippocampal slices (400 μm thickness) using a glass micropipette filled with NaCl (1 N), with a pipette resistance of 2–5 MΩ as described earlier (Zhu and Lovinger 2007). Slices were maintained in oxygenated artificial CSF (124 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, 10 glucose, and 2 CaCl2 (in mM), osmolarity adjusted to 320 mOsm with sucrose) at 25°C for at least 1 h after a 30 min incubation at 33°C. During the recording, the chamber was constantly superfused with artificial CSF at 31–33°C(~1.5 mL/min). The electrode was placed in the CA1 apical dendritic layer (stratum radiatum) and electrical stimulation (40 μs duration current pulses) was delivered at the Schaffer collaterals within CA2 via a bipolar electrode made of twisted tungsten wires (teflon coated, 175 μm diameter; AM-systems, Carlsborg, WA, USA). LTP was induced by two successive high-frequency stimulus (HFS) trains (100 Hz, 100 pulses for each burst, 10 s intertrain interval) using an S48 stimulator (Astro-Med, Inc., West Warwick, RI, USA).
Fatty acid analysis
Lipids from cells in culture or tissue homogenates were extracted and fatty acid analysis was performed using gas chromatography after transmethylation using boron trifluoride-methanol as described earlier (Wen and Kim 2004).
Statistical analysis
All data were presented as mean ± SD unless specified. Statistical analysis was performed using unpaired student t-test unless specified; *p < 0.05, **p < 0.01, and ***p < 0.001. Significant differences between groups were determined by post hoc Tukey's Honestly Significant Differences (HSD) test. Different alphabetical letters indicate significant differences at p < 0.05, unless specified.
Results
DHA supplementation uniquely promotes neurite growth and synaptogenesis
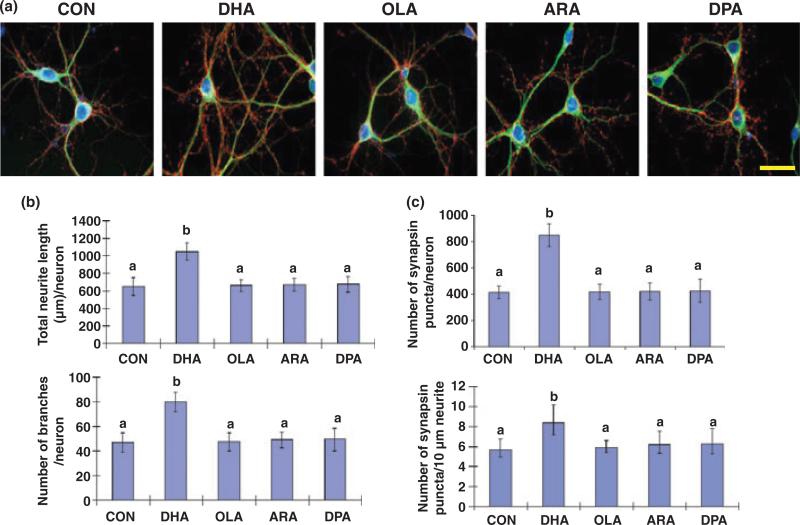
To evaluate the effect of DHA on hippocampal development, we examined neurite growth and synapsin puncta formation in E18 hippocampal neuronal cultures. The hippocampal cultures in B27 supplemented medium contained more than 90% neurons (data not shown) as reported earlier (Brewer et al. 1993), unlike the neuron/astroglia (~1/1) co-culture obtained with N2 supplement (Calderon and Kim 2004). As was the case for rat hippocampal neurons grown with N2 supplement (Calderon and Kim 2004), DHA uniquely promoted neurite growth and branching in E18 mouse hippocampal cultures grown with B27 supplement (Fig. 1a and b). After 10 days in vitro supplementation with DHA at 1 μM, the neurite length and number of branches increased significantly in comparison to those of unsupplemented control or neurons treated with other fatty acids (Fig. 1b). Synapsins, a family of neuron-specific phosphoproteins associated with the membranes of synaptic vesicles (Südhof et al. 1989), have been identified as a molecular component involved in synaptogenesis (Chin et al. 1995) and functional maturation of synapses (Lu et al. 1992). With DHA-treatment, hippocampal neurons showed significantly increased number of synapsin puncta (synapsins associated with synaptic vesicles) normalized per neuron or a certain neurite length, the later of which is indicative of improved synaptogenesis (Fig. 1c). This effect on synaptogenesis was specific to DHA as ARA, OLA, or docosapentaenoic acid (DPAn-6) showed no effects.
Fig. 1.
Hippocampal neurite growth and synaptogenesis promoted uniquely by DHA supplementation. (a) Representative photomicrographs of E18 mouse hippocampal neurons after culturing 10 days with various fatty acids: microtubule-associated protein 2 (MAP2, a neuron-marker protein; green), synapsin1 (red), and DAPI for nuclei (blue). Scale bar, 30 μm. (b and c) Quantitative changes in neurite growth (b) and synapsin-positive puncta formation (c) after fatty acid treatments. Statistical analysis was performed by post hoc Tukey's HSD test at the significance level of p < 0.01. Different alphabetical letters indicate statistically significant differences.
Glutamatergic synaptic activity is enhanced in DHA-supplemented hippocampal neurons
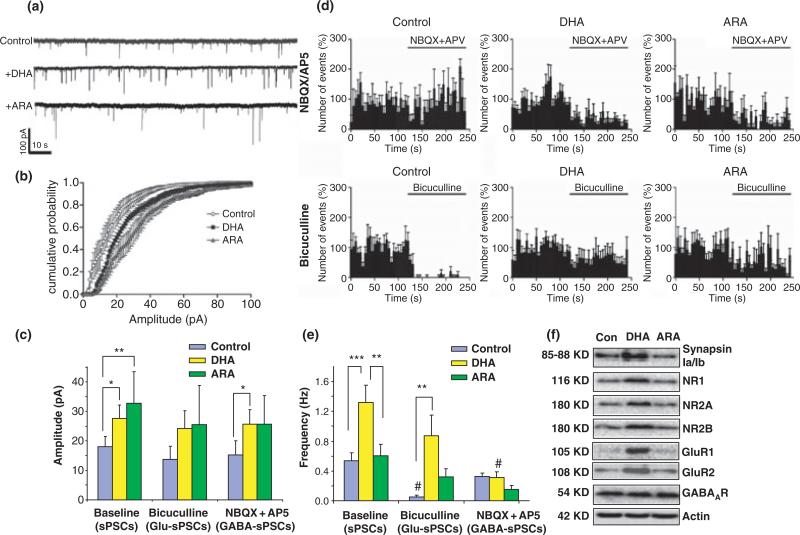
The functional implications of the longer neurites, more branches and more synapsin-positive puncta formation in the synaptic function was evaluated by means of electrophysiology. The DHA-supplemented neurons exhibited enhanced spontaneous synaptic activity evaluated by measuring amplitude and frequency of sPSCs using the whole-cell voltage-clamp technique (Fig. 2). ARA was chosen as a control for fatty acid supplementation as DHA and ARA were the most representative n-3 and n-6 polyunsaturated fatty acids in the hippocampus, respectively. Successful incorporation of DHA and ARA was apparent, as the cellular content of DHA and docosatetraenoic acid (22:4n-6), the elongated product of ARA, increased significantly after supplementation with DHA or ARA, respectively (DHA, from 2.4 ± 0.1 to 8.8 ± 0.1% with DHA supplementation; 22:4n-6, 4.5 ± 0.4 to 8.5 ± 0.2% with ARA supplementation) (Table S1). The average amplitudes of sPSCs recorded from both DHA- and ARA-treated cultures were higher than those from control neurons (Fig. 2a–c). The amplitudes averaged 18.03 ± 3.44 pA (n = 5), 27.64 ± 4.59 pA (n = 5), and 32.75 ± 10.70 pA (n = 5) for control, DHA-, and ARA-treated neurons, respectively (p < 0.05 for both DHA- and ARA-treated conditions, paired t-test against control condition) (Fig. 2c). More importantly, the sPSC frequency, which reflects the number of active synapses and relative levels of pre-synaptic release, was greater particularly in the DHA-treated neurons in comparison to the other two groups (0.54 ± 0.11, 1.32 ± 0.23, and 0.60 ± 0.16 Hz for control, DHA-, and ARA-treated neurons, respectively) (Fig. 2a, d, and e). To isolate glutamatergic and GABAergic sPSCs, bicuculline (GABAA receptor antagonist) or NBQX/AP5 (antagonists of ionotropic glutamate receptors) was applied, respectively, and the relative contributions of these two types of synaptic inputs were determined. Acute application of either bicuculline or NBQX/AP5 affected the frequency but not the amplitude of sPSCs (Fig. 2c–e). The frequency of sPSCs in control neurons was decreased significantly in the presence of bicuculline (Fig. 2d and e), indicating that the observed activity was mainly GABAergic as expected for developing neurons at an early stage (Tyzio et al. 1999). In contrast, the sPSC frequency in DHA-treated neurons decreased more prominently when they were treated with NBQX/AP5 in comparison to bicuculline (Fig. 2d and e), indicating a greater relative contribution of glutamatergic synapses to the increased synaptic activity observed from the DHA-treated neurons. WB data indicated that the protein level of glutamate receptor subunits, GluR1, GluR2, NR1, NR2A, and NR2B, was significantly higher in the DHA-treated cultures while GABA receptor or β-actin expression was not altered (Fig. 2f). Synapsin 1 expression in DHA-treated cultures was also increased in comparison to control or ARA-supplemented cultures in agreement with the increased synapsin puncta formation observed with DHA supplementation (Fig. 1c). These data indicated that DHA has a unique capability for promoting neurite growth, synaptogenesis, and synaptic protein expression, particularly glutamate receptors and synapsins, and for improving glutamatergic synaptic activity.
Fig. 2.
Hippocampal neuronal synaptic activity enhanced by DHA supplementation. (a) Representative spontaneous synaptic current (sPSC) traces from hippocampal neurons supplemented with fatty acids for 10 days in culture. (b and c). Cumulative probability curves of the sPSC amplitude (b) for each condition, showing that sPSC amplitude is increased by the treatment with DHA and ARA (c). The acute application of bicuculline or NBQX/AP5 did not affect sPSC amplitude. The resting membrane current levels were similar among groups (–69.0 ± 9.27, –60 ± 4.59, and –67.8 ± 1.16 pA for control, DHA-, and ARA-treated neurons, respectively). (d) Frequency histograms of sPSCs, GABA-sPSCs (NBQX/AP5-treated condition), and Glu-sPSCs (bicuculline-treated condition) in cultured neurons of unsupplemented control, DHA-, and ARA-treated groups expressed as percentages of average frequency of initial condition. Bin size in the histogram is 5 s. (e) The frequency of sPSCs, Glu-sPSCs, and GABA-sPSCs in control, DHA-, and ARA-supplemented hippocampal neurons. DHA-treatment increased the frequency of sPSCs. The frequency of sPSCs from unsupplemented control neurons was significantly decreased by bicuculline while NBQX/AP5 decreased the frequency in the neurons supplemented with DHA or ARA. (f) Western blot analysis for synaptic protein expression. The expression of synapsins and subunits of glutamate receptors were elevated in the DHA-treated neuronal culture. Error bars represent SE (n = 5 for each condition). Paired t-test were performed against the baseline value of each group (#p < 0.05) or between indicated groups (*p < 0.05, **p < 0.01, and ***p < 0.001).
In vivo DHA-depletion in fetal hippocampi results in inhibited hippocampal neuronal development in culture
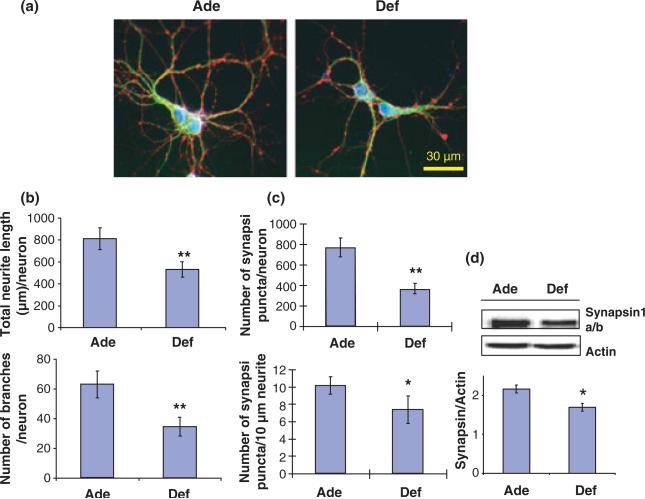
As DHA supplementation of neuronal cells in culture increased the neurite growth and synaptogenesis, the opposite effect is expected when DHA is lowered in hippocampal neurons. To test this possibility, we first depleted DHA in fetal hippocampi by maternal dietary deprivation of n-3 fatty acids from days 2 to 18 of pregnancy. The deficient diet contained 0.09 wt% of LNA, the precursor for DHA synthesis, as the only source of n-3 fatty acids. Control animals (n-3 fatty acid adequate group) received a diet containing 2.5 wt% LNA plus 0.9 wt% DHA. In control E18 mouse hippocampi, DHA was the predominant n-3 fatty acid while other n-3 fatty acids were less than 0.5 wt% of the total fatty acid (Table S2). Depriving dietary n-3 fatty acids lowered almost exclusively DHA (deficient vs. adequate group; 0.55 ± 0.08 vs. 7.80 ± 0.76 wt%) which was substituted mainly by DPAn-6 (deficient vs. adequate group; 5.55 ± 1.24 vs. 0.43 ± 0.06 wt%) with minimal changes in other fatty acids (Table S2). In contrast to the case of DHA supplementation, depletion of DHA from fetal hippocampi inhibited neuronal development in culture despite the compensatory increase of DPAn-6. The DHA-deficient hippocampal neuronal culture exhibited significantly shorter neurites, less branches (Fig. 3a and b) and less synapsin-positive puncta (Fig. 3c) in comparison to the adequate control culture. The decreased expression of synapsins in deficient neurons was also confirmed by the WB data (Fig. 3d).
Fig. 3.
Neurite growth and synaptogenesis in cultured hippocampal neurons inhibited by prenatal depletion of DHA. (a) Representative photomicrographs of hippocampal neurons at 10 days in vitro obtained from E18 fetuses of pregnant mice fed with an n-3 fatty acid adequate (Ade) or deficient (Def) diet: MAP2 (green), synapsin 1 (red), and nucleus (blue). (b and c) Inhibition of hippocampal neurite growth and branching (b) as well as synapsin-positive puncta formation (c) by prenatal n-3 fatty acid deficiency. (d) Western blot data showing that synapsin expression was inhibited by prenatal n-3 fatty acid deprivation (n =3; *p < 0.05, **p < 0.01).
In vitro DHA supplementation reverses the inhibited hippocampal neuronal development caused by prenatal depletion of DHA
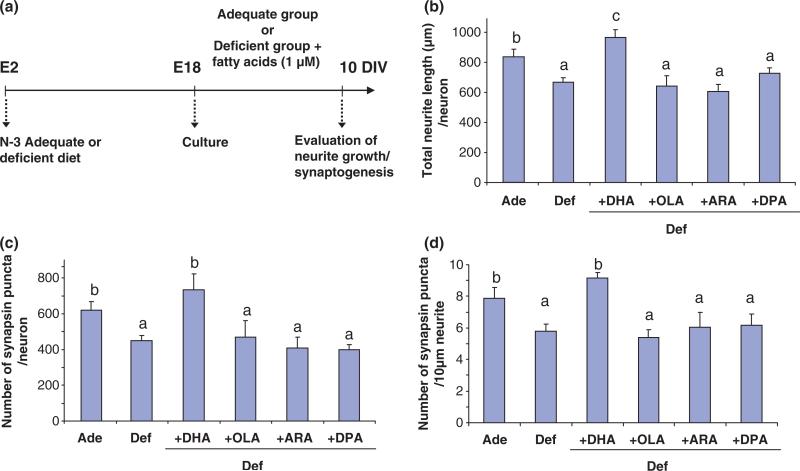
As we found that gestational DHA-deprivation in fetal hippocampi inhibited neurite outgrowth and synaptogenesis in cultured hippocampal neurons, we questioned whether the observed developmental deficit can be overcome by subsequent supplementation of DHA to the DHA-depleted neurons in culture. As shown in Fig. 4, in vitro DHA supplementation restored the neurite growth and synaptogenesis inhibited by embryonic DHA-deprivation in vivo, further supporting the unique role of DHA in promoting hippocampal development indicated in Fig. 1.
Fig. 4.
Restoration of hippocampal neuronal development inhibited by in vivo DHA-depletion by subsequent DHA supplementation in vitro. (a) Time line of the experimental design. E18 hippocampal neuronal cultures were obtained from pregnant mice fed adequate (Ade) or deficient (Def) diets for 16 days during pregnancy (E2–E18). The culture was grown in chemically defined medium in the presence or absence of 1 μM fatty acids for 10 days. (b–d). Total neurite length/neuron (b), number of synapsin-positive puncta/neuron (c), and number of synapsin-positive puncta/10 μm neurite (d) which were reduced by in vivo DHA-depletion but restored by subsequent DHA supplementation in vitro. Statistical analysis was performed by post hoc Tukey's HSD test at the significance level of p < 0.05. Different alphabetical letters indicate statistically significant differences.
Developmental depletion of DHA impairs LTP with concomitant reduction of synaptic proteins involved in LTP
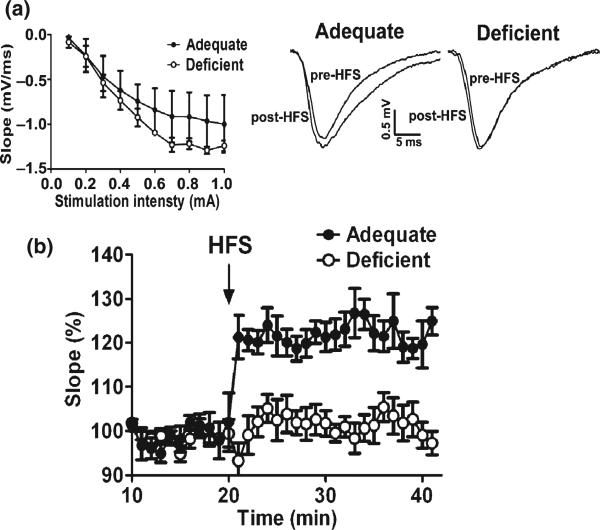
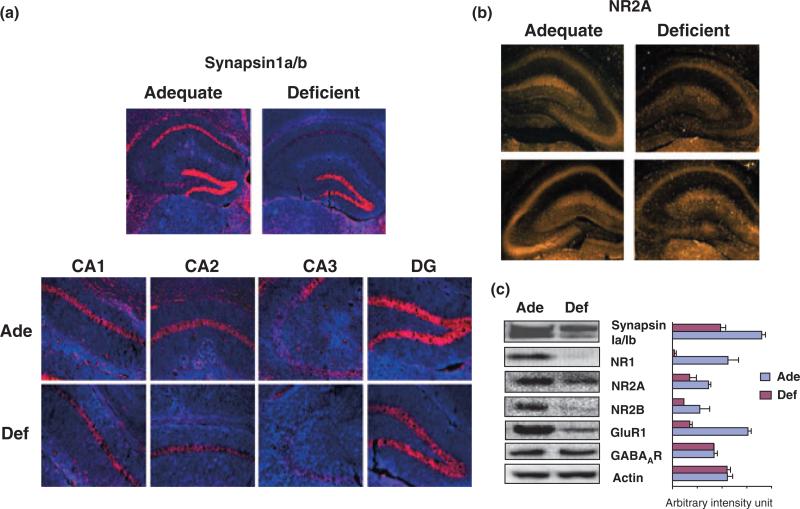
Inadequate neurite development and synaptogenesis observed in the hippocampal neuronal cultures from DHA-depleted E18 hippocampi may contribute to the cognitive impairment associated with n-3 fatty acid deficiency in vivo. To test this possibility, we examined LTP of hippocampal synaptic transmission, a form of activity-dependent synaptic plasticity considered as a cellular mechanism underlying learning and memory (Davis et al. 1992; Martinez and Derrick 1996). Pregnant mice were fed with an n-3 fatty acid adequate (control) or deficient diet throughout pregnancy and the lactation period, and hippocampal slices were obtained from offspring mice at the P-18 for electrophysiological evaluation. The DHA content in P-18 hippocampi decreased significantly (from 12.39 ± 0.99 to 3.33 ± 1.50 wt%) with compensatory increase of mostly DPAn-6 (from 0.30 ± 0.03 to 8.55 ± 1.24 wt%) and to a much lesser extent docosatetraenoic acid (22:4n-6) (from 1.98 ± 0.17 to 3.28 ± 0.25 wt%) (Table S3). The fEPSP recorded from the CA1 stratum radiatum after stimulation of the Schaffer collaterals clearly indicated that DHA-deficiency inhibited the induction of LTP (Fig. 5). The ‘input–output’ curves of the fEPSP slope as a function of afferent stimulus intensity indicated no difference in this measure of synaptic efficacy between two groups (Fig. 5a). However, HFS produced an increase of the fEPSP slope (Fig. 5b) which persisted for 20 min in the DHA-adequate mouse hippocampal slices, indicating induction of LTP. Hippocampal slices from deficient mice showed only a transient increase of the fEPSP slope with the average increase at 20 min post-HFS (n = 6; 101.2 ± 9.2% of baseline) significantly lower than that from the adequate mice (n = 6; 121.9 ± 9.7% of baseline) (p < 0.001, unpaired t-test). Although the LTP was tested only for 20 min, LTP persisting for 20 min is at least early phase LTP, and DHA-depletion produced statistically significant impairments in this measure. In the hippocampi of the deficient mice where the retarded LTP were observed, the levels of synapsins and NMDA receptor subunits were significantly reduced while GABA receptor expression was not altered (Fig. 6). The observed significant inhibition of LTP in the deficient hippocampi is consistent with the substantial decrease in the expression of NMDA receptors, the activation of which is known to be crucial for the induction of LTP. The expression of synapsins and NMDA receptors probed by NR2A subunit antibody was detected in the CA1, CA2, CA3, and DG regions (Fig. 6a and b). The localized expression of other NMDA receptor subunits could not be visualized as specific antibodies for immunohistochemistry are not currently available. DHA-deficiency diminished NR2A expression in all hippocampal area with particular decrease in the CA3 region (Fig. 6b). Similarly, a pre-synaptic protein synapsin 1, that can influence LTP induction through glutamate release, was reduced in most hippocampal regions including CA1, CA2, CA3, and DG regions (Fig. 6a). Inadequate hippocampal neurite development along with decreased expression of both pre- and post-synaptic proteins critically involved in synaptic transmission and induction of LTP may have contributed to the observed impairment of LTP in the DHA-deficient hippocampi.
Fig. 5.
Impaired LTP in young mouse hippocampi deprived of DHA during development. (a and b) fEPSPs recorded from CA1 after stimulation of Schaffer Collaterals using hippocampal slices obtained from DHA-adequate (Ade) or DHA-deficient (Def) mice at P-18. (a) Left: Input–output curves showing no difference in synaptic efficacy between the two groups. Right: Representative fEPSPs before and after high-frequency stimulation (HFS) indicating distinctive differences in the initial falling phase of fEPSPs between the two groups. (b) Inhibition of LTP by DHA-deficiency shown by the relative slope of the initial falling phase of fEPSPs plotted for 20 min before and after HFS with an averaging bin size of 1 min.
Fig. 6.
Localized reduction of synapsins and NR2A in young mouse hippocampi depleted with DHA during development. (a) Immunohistochemical probing of synapsin1 and NR2A in P-18 hippocampal slices, showing dramatic decreases of synapsin1 expression in CA1, CA2, and CA3 regions from DHA-depleted (Def) mice in comparison to DHA-adequate (Ade) mice; synapsin1 in red and DAPI counterstaining in blue. (b) Immunohistochemical probing of NR2A in P-18 hippo-campal slices from DHA-adequate (Ade) or DHA-depleted (Def) mice, indicating particular decrease of NR2A in the CA3 region of DHA-depleted hippocampi. (c) Western blot analysis of synapsin1, NR, GluR and GABA receptor expression in hippocampi of DHA-adequate or deficient mice at P-18.
Discussion
Our study demonstrates that DHA supplementation significantly promotes neurite growth, synaptogenesis, and increases the levels of pre- and post-synaptic proteins involved in synaptic transmission and LTP, improving synaptic function. It is generally accepted that more synaptic connections can be made with longer neurites and a higher number of dendritic branches (Jan and Jan 2001). It has been also shown that synaptic activity in developing neurons further promotes dendritic arbor elaboration and stabilizes dendritic structure, which is critical for synaptic remodeling during memory (Cline 2001). As the synaptic activity was not altered by acute applications of DHA (data not shown), availability of DHA during development for neurite growth, synaptogenesis and synaptic protein expression may be an important aspect of ultimately enhancing hippocampus-related learning and memory function.
In the hippocampus, DHA is the major polyunsaturated fatty acid found while its precursor LNA is not detected (Tables S2 and S3). LNA at 1 μM which is far exceeding physiological concentrations of LNA expected as the free fatty acid did not affect the hippocampal neurite growth or synaptogenesis (data not shown). We observed that DHA at a concentration greater than 5 μM was toxic while the concentration below 0.5 μM did not exert measurable effects in our hippocampal neuronal cultures (data not shown). The free DHA concentration in the brain has been reported to be ~1.3 μM (Contreras et al. 2000), and therefore, 1 μM DHA employed in our study was in the physiological concentration range.
The enhanced synaptic activity in DHA-treated neurons suggested a unique role of DHA in promoting active synapse formation and/or pre-synaptic neurotransmitter release, which was supported by the DHA-induced increase of synaptogenesis and synapsin protein expression (Fig. 1). Moreover, the primarily glutamatergic nature of the enhanced synaptic activity was consistent with the observation that DHA increased expression of glutamate receptors (Figs 2f and 5c) without affecting GABA receptor levels. It has been demonstrated that GABAergic synapses are established before glutamatergic synapses during development (Tyzio et al. 1999). Consistently, the baseline synaptic activity observed in control embryonic hippocampal neurons in 10 days in vitro culture was mostly GABAergic (Fig 2e). The synergistic activation of GABA and NMDA receptors has been suggested to contribute to the maturation of glutamatergic synapses (Tyzio et al. 1999). In this regard, the observed DHA-induced increase in NR expression may have played a significant role in the maturation of glutamatergic synapses, enhancing excitatory synaptic activity.
The observed increases in synaptic proteins may involve activation of transcriptional factors during development. DHA has been shown to be an endogenous ligand for RXR (de Urquiza et al. 2000). RXR forms heterodimers with nuclear receptors such as retinoic acid receptor (RAR), peroxisome proliferator-activated receptors, liver X receptor, constitutive androstane receptor, farnesoid X receptor, pregnane X receptor, thyroid hormone receptor, vitamin D receptor, or Nurr1 for transcriptional regulation of target genes (Aarnisalo et al. 2002; Lane and Bailey 2005). It is well established that retinoic acid signaling plays an important role in neurodevelopment by regulating genes involved in the control of synaptic plasticity, cytoskeleton, and membrane assembly, as well as signal transduction and ion channel formation (Maden 2002; Lane and Bailey 2005). Although RAR plays the dominant role in the RAR–RXR dimer function (Kurokawa et al. 1994), it is possible that DHA binding to RXR within the functional dimer enhances its transcriptional activity, as has been reported earlier for retinoid-dependent gene expression (Minucci et al. 1997). It is also possible that Nurr1–RXR signaling played a role in preventing the loss of synaptic proteins as DHA binding to RXR in Nurr1–RXR heterodimers in embryonic CNS has been shown to support neuronal survival, particularly dopaminergic neurons (Wallen-Mackenzie et al. 2003).
Under neurodegenerative conditions, such as in aging, reduction of GluR expression (Dyall et al. 2007) and impairment of LTP in the DG has been observed (McGahon et al. 1999), which was reversed by n-3 fatty acid supplementation. Long-term dietary n-3 fatty acid depletion in aging brains has been shown to decrease NMDA receptor subunits in the cortex and hippocampus, which were further decreased in transgenic mice with the human Alzheimer's disease gene APPswe (Calon et al. 2005). Our data indicated that DHA-depletion during development also leads to decreased expression of GluR and NR glutamate receptor subunits and retarded LTP in the young mouse hippocampus, suggesting that developmental DHA inadequacy can be similarly deleterious in young brains as in aged brains with neurodegenerative conditions.
It is clear from our data that the maternal intake of DHA at an early stage of development greatly influences the synaptic plasticity in the CA1 region of the offspring brain. Significant influence of prenatal availability of DHA on neuro-development in an early stage was apparent as hippocampal neuronal cultures obtained from DHA-deficient E18 embryos develop shorter neurites, less branches and reduced number of synapsin puncta in comparison to DHA-adequate neurons (Fig. 3). Our data also indicated that the developmental deficit, at least for neurite growth and synaptogenesis, because of prenatal DHA-deficiency can be reversed by DHA supplementation at an early stage (Fig. 4), supporting importance of postnatal DHA provision in neurodevelopment. DHA-dependent expression of synapsins and subunits of glutamate receptors, NR and GluR, was also evident from DHA-supplemented in vitro embryonic cultures (Fig. 2f) as well as DHA-depleted hippocampal tissues at P-18 (Fig. 6c). Although expression of synapsins and NR2A decreased in all regions of DHA-depleted hippocampus, it is interesting to note that the decrease of NR2A was particularly prominent in the CA3 region (Fig. 6b). It has been reported that NMDA receptors in the CA3 region play a crucial role in fast learning of one-time experience (Nakazawa et al. 2003) and associative memory recall (Nakazawa et al. 2002), implying learning and memory deficit as a consequence of developmental DHA-deficiency.
Underlying mechanisms facilitating synaptic plasticity include the increased release of neurotransmitters and increased synaptic expression of glutamate receptors. Although several forms of LTP exist in the brain, the most prevalent form found in the hippocampal CA1 region involves enhanced sensitivity of post-synaptic neurons to glutamate (Nicoll and Malenka 1995; Kerchner and Nicoll 2008). This enhancement can be achieved by the increased activity of existing receptors and/or by increasing the number of receptors at the functional synapses. As LTP in Schaffer collateral-CA1 synapses is known to be mediated by two types of glutamate receptors, NMDA and AMPA receptors (Hayashi et al. 2000; Grosshans et al. 2002), the decreased expression of these receptors in DHA-depleted mouse hippocampi potentially contributed to the inhibited LTP. In addition, reduced synapsin1 expression may have played a role as synapsins have been shown to be involved in the formation of a readily releasable pool of vesicles for neurotransmitter release during action potential (Hvalby et al. 2006; Baldelli et al. 2007). The implication of the retarded LTP observed with reduced synapsin1 expression under the DHA-deficient condition is particularly noteworthy in light of the previous finding that lack of the synapsin1 gene is associated with human phenotypes displaying learning difficulties (Garcia et al. 2004).
Phospholipase activity leading to ARA release has been implicated in neurite growth, particularly in retinoic acid-mediated signaling (Smalheiser et al. 1996; Farooqui et al. 2004). In addition, involvement of ARA metabolism by cyclooxygenase-2 in hippocampal long-term synaptic plasticity has been demonstrated (Chen et al. 2002). The fact that hippocampal development does occur even under DHA-depleted conditions clearly indicates that other mechanisms are also in operation to support the basic neurite growth and synaptogenesis. Unlike the DHA supplementation which significantly increased the DHA level in hippocampal neuronal culture, ARA supplementation increased only its elongation product 22:4n-6, suggesting that ARA levels are fairly well regulated (Table S1). Similarly, the ARA level was maintained upon in vivo depletion of DHA, and the reduction of DHA was compensated by the increases of 22:4n-6 and DPAn-6, elongation products of ARA (Tables S2 and S3), suggesting a need to maintain ARA at relatively constant levels. Although our study addressed the impact of rather unexplored n-3 fatty acids on hippocampal development and function, the important role of ARA or its metabolites in neurodevelopment and LTP may not be overlooked. Unlike ARAs involvement in LTP where cyclooxygenase-2-generated prostaglandin E2 regulates membrane excitability and long-term synaptic plasticity (Chen et al. 2002), the synaptic activity enhanced by DHA supplementation most probably resulted from the DHA-promoted neurite growth and synaptogenesis, as acute application of DHA to the target neurons did not affect synaptic activity.
Our study demonstrates that DHA can promote neurite development, synaptogenesis and expression of synapsins and glutamate receptors, improving glutamatergic synaptic transmission. Inadequate expression of synapsins because of DHA-deficiency during development by maternal n-3 fatty acid deprivation may hamper synaptogenesis and reduce the pre-synaptic pool of synaptic vesicles for neurotransmitter release during the action potential, similar to the case demonstrated for the hippocampal neurons lacking synapsin 1 (Baldelli et al. 2007). Concomitantly, reduced expression of glutamate receptors may cause inadequate support for proper glutamatergic synaptic transmission and synaptic plasticity, contributing to impaired learning and memory. In conclusion, our data provide evidence that developmental DHA availability influences hippocampal neurite growth, synaptogenesis and synaptic protein expression critically involved in glutamatergic synaptic transmission and LTP. Compromised DHA-dependent neurodevelopment and synaptic function may be an important mechanism for the learning disability and memory deficit associated with dietary deficiencies of n-3 fatty acids.
Acknowledgement
This research was supported by the Intramural Research Program of the NIAAA, NIH.
Abbreviations used
- AMPA
α-amino-3-hydroxy-5-methylisoxazole
- AP5
d-2-amino-5-phosphonopentanoic acid
- ARA
arachidonic acid
- BSA
bovine serum albumin
- DG
dentate gyrus
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- E18
embryonic day 18
- fEPSP
field excitatory post-synaptic potential
- GluR
AMPA receptor subunits
- HFS
high-frequency stimulation
- LNA
linolenic acid
- LTP
long-term potentiation
- MAP2
microtubule-associated protein 2
- NBQX
2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium
- NMDA
N-methyl-D-aspartic acid
- NR
NMDA receptor subunits
- OLA
oleic acid
- P-18
postnatal day 18
- PBS
phosphate-buffered saline
- RAR
retinoic acid receptor
- sPSCs
spontaneous post-synaptic currents
- TBS
Tris-buffered saline
- WB
western blot
Footnotes
Supporting information
Additional Supporting information may be found in the online version of this article:
Table S1 Fatty acid composition in E18 neuronal cultures supplemented with ARA or DHA
Table S2 Effect of prenatal dietary depletion of n-3 fatty acids on fetal hippocampal fatty acid composition at E18
Table S3 Effect of dietary depletion of n-3 fatty acids on hippocampal fatty acid composition at P-18
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Aarnisalo P, Kim CH, Lee JW, Perlmann T. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J. Biol. Chem. 2002;277:35118–35123. doi: 10.1074/jbc.M201707200. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Moriguchi T, Salem N., Jr Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr. Neurol. 2002;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of akt signaling in neuronal survival. Proc. Natl Acad. Sci. USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli P, Fassio A, Valtorta F, Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J. Neurosci. 2007;27:13520–13531. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Garfield S, Hoffman DR, Uauy RD, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B-27 supplemented Neurobasal™. A new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Role of RXR in neurite outgrowth induced by docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:227–232. doi: 10.1016/j.plefa.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur. J. Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- Cansev M, Marzloff G, Sakamoto T, Ulus IH, Wurtman RJ. Giving uridine and/or docosahexaenoic acid orally to rat dams during gestation and nursing increases synaptic elements in brains of weanling pups. Dev. Neurosci. 2009;31:181–192. doi: 10.1159/000193394. [DOI] [PubMed] [Google Scholar]
- Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N., Jr Cognitive deficits in docosahexaenoic acid-deficient rats. Behav. Neurosci. 2002;116:1022–1031. doi: 10.1037//0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc. Natl Acad. Sci. USA. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr. Opin. Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Contreras MA, Greiner RS, Chang MC, Myers CS, Salem N, Jr, Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J. Neurochem. 2000;75:2392–2400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J. Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol. Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Antony P, Ong WY, Horrocks LA, Freysz L. Retinoic acid-mediated phospholipase A2 signaling in the nucleus. Brain Res. Brain Res. Rev. 2004;45:179–195. doi: 10.1016/j.brainresrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gamoh S, Hashimoto M, Sugioka K, Hossain MS, Hata N, Misawa Y, Masumura S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship JA. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J. Med. Genet. 2004;41:183–187. doi: 10.1136/jmg.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat. Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hvalby O, Fassio A, Valtorta F, Benfenati F. Synapsin-regulated synaptic transmission from readily releasable synaptic vesicles in excitatory hippocampal synapses in mice. J. Physiol. 2006;571:75–82. doi: 10.1113/jphysiol.2005.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis SM. Dietary (n-3) fatty acids and brain development. J. Nutr. 2008;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Dendrites. Genes Dev. 2001;15:2627–2641. doi: 10.1101/gad.916501. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. Review. Erratum in: Nat. Rev. Neurosci. 2009 10:242.
- Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld MG, Heyman RA, Glass CK. Regulation of retinoid signaling by receptor polarity and allosteric control ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- Lane MA, Bailey SJ. Role of retinoid signaling in the adult brain. Prog. Neurobiol. 2005;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lu B, Greengard P, Poo MM. Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron. 1992;8:521–529. doi: 10.1016/0896-6273(92)90280-q. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signaling in the development of the central nervous system. Nat. Rev. Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Jr, Derrick BE. Long-term potentiation and learning. Annu. Rev. Psychol. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- McGahon BM, Martin DS, Horrobin DF, Lynch MA. Age-related changes in synaptic function: analysis of the effect of dietary supplementation with omega-3 fatty acids. Neuroscience. 1999;94:305–314. doi: 10.1016/s0306-4522(99)00219-5. [DOI] [PubMed] [Google Scholar]
- Minucci S, Leid M, Toyama R, et al. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol. Cell. Biol. 1997;17:644–655. doi: 10.1128/mcb.17.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Sheinin A, Talani G, Davis MI, Lovinger DM. Endocannabinoid- and mGluR5-dependent short-term synaptic depression in an isolated neuron/bouton preparation from the hippocampal CA1 region. J. Neurophysiol. 2008;100:1041–1052. doi: 10.1152/jn.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Dissanayake S, Kapil A. Rapid regulation of neurite outgrowth and retraction by phospholipase A2-derived arachidonic acid and its metabolites. Brain Res. 1996;721:39–48. doi: 10.1016/0006-8993(96)00134-5. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Czernik AJ, Kao HT, et al. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989;245:1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and gutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J. Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Urquiza AM, Liu S, Sjöberg M, Zetterström RH, Griffiths W, Sjövall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gómez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J. Neurosci. Res. 2004;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Mata de Urquiza A, Petersson S, et al. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes Dev. 2003;17:3036–3047. doi: 10.1101/gad.276003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J. Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- Willatts P, Forsyth JS, Di Modugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352:688–691. doi: 10.1016/s0140-6736(97)11374-5. [DOI] [PubMed] [Google Scholar]
- Yavin E, Himovichi E, Eilam R. Delayed cell migration in the developing rat brain following maternal Omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162:1011–1022. doi: 10.1016/j.neuroscience.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yasuda A, Kawazato H, Sakai K, Shimada T, Takeshita M, Yuasa S, Kobayashi T, Watanabe S, Okuyama H. Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of alpha-linolenate deficiency and a learning task. J. Neurochem. 1997;68:1261–1268. doi: 10.1046/j.1471-4159.1997.68031261.x. [DOI] [PubMed] [Google Scholar]
- Zhu P, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J. Neurophysiol. 2007;97:4386–4389. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]