Abstract
Although it is known that (i) O6-alkylguanine—DNA alkyltransferase (AGT) confers tumor cell resistance to guanine O6-targeting drugs such as cloretazine, carmustine, and temozolomide and that (ii) AGT levels in tumors are highly variable, measurement of AGT activity in tumors before treatment is not a routine clinical practice. This derives in part from the lack of a reliable clinical AGT assay; therefore, a simple AGT assay was devised based on transfer of radioactive benzyl residues from [benzene-3H]O6-benzylguanine ([3H]BG) to AGT. The assay involves incubation of intact cells or cell homogenates with [3H]BG and measurement of radioactivity in a 70% methanol precipitable fraction. Approximately 85% of AGT in intact cells was recovered in cell homogenates. Accuracy of the AGT assay was confirmed by examination of AGT levels by Western blot analysis with the exception of false-positive results in melanin-containing cells due to [3H]BG binding to melanin. Second-order kinetic constants for human and murine AGT were 1100 and 380 M-1 s-1, respectively. AGT levels in various human cell lines ranged from less than 500 molecules/cell (detection limit) to 45,000 molecules/cell. Rodent cell lines frequently lacked AGT expression, and AGT levels in rodent cells were much lower than in human cells.
Keywords: O6-alkylguanine—DNA alkyltransferase, assay, [Benzene-3H]O6-benzylguanine, Cloretazine, Carmustine, Temozolomide, AGT-positive and -negative cells, B16F10 melanoma, Drug binding to melanin
The cytotoxic effects of antitumor agents such as cloretazine [1], carmustine [2], and temozolomide [3] are primarily a consequence of their ability to alkylate DNA at the O6 position of guanine. The O6-methylguanine lesion generated by temozolomide [3] and the O6-chloroethylguanine and subsequently rearranged N1,O6-ethan-oguanine lesions generated by carmustine [4] and cloretazine [5] are subject to repair by O6-alkylguanine—DNA alkyltransferase (AGT).1 By covalently transferring the alkyl moieties to the active site cysteine, AGT restores the O6 position of guanine to its native state and attenuates the cytotoxicity of guanine O6-targeting agents [6]. The reaction is irreversible; thus, the AGT protein is inactivated during the repair process [6]. A number of in vitro and in vivo studies have established an inverse relationship between the AGT concentration and the sensitivity to guanine O6-targeting drugs [7–9].
The AGT content of tumor and normal tissues is highly variable [10]. In humans, liver contains the highest level of AGT, followed by small intestine and lung [11]. Histochemical studies have shown that human tumor tissues often express more AGT than adjacent normal tissues [10], whereas 22–27% of human brain tumors completely lack AGT activity [12,13]. The lack of AGT expression in tumors is due to silencing of the AGT gene through hypermethylation [14,15] possibly caused by epigenetic malfunction during tumorigenesis [16].
Because AGT protects normal host tissues from the deleterious effects of guanine O6-targeting drugs, unlike most antineoplastic agents whose mechanisms of tumor selectivity are not well defined, a lower AGT content in tumors than in normal tissues constitutes a basis of tumor selectivity for guanine O6-targeting drugs. Clear tumor selectivity is manifested by cloretazine being curative in AGT-negative tumors in preclinical mouse models [1,17]. In phase II clinical trials, cloretazine produced a 28% complete response rate in elderly patients with acute myeloid leukemia or high-risk myelodysplastic syndromes with modest extramedullary toxicity [18]. These results collectively point to the potential importance of AGT measurements in tumor and normal tissues before treatment with O6-targeting drugs.
The known AGT assays use DNA reacted with N-methyl-N-nitrosourea (MNU) [11,19,20] or 32P- or fluorescein-labeled double-stranded oligonucleotides containing O6-methylguanine in a restriction endonuclease site [21,22] as substrates for AGT. These assays require multistep substrate preparation, DNA and protein hydrolysis in some cases, and analytical procedures such as HPLC and gel electrophoresis. Therefore, these assays are complex, laborious, cumbersome, and time-consuming.
In this article, we present a simple AGT assay using O6-benzylguanine (O6-BG), a small chemical inhibitor of AGT [23], labeled with 3H in the benzyl moiety. Although 3H labeling of the benzyl portion of the O6-BG analog O6-(p-hydroxy[3H]methylbenzyl)guanine has been reported, an AGT assay employing this labeled compound has not been established [24]. The assay takes advantage of the transfer of a radioactive residue from a methanol-soluble small chemical substrate to a methanol-insoluble high-molecular-weight protein.
Materials and methods
Synthesis of 3H-labeled O6-BG
[Benzene-3H]O6-benzylguanine ([3H]BG) was prepared with a specific activity of 46.2 Ci/mmol and with radiopurity of more than 98% by Moravek Biochemicals (Brea, CA, USA) by reaction of 2-amino-6-chloropurine with the sodium salt of [ring-3H]benzyl alcohol followed by repeated purification using HPLC. The specific activity was determined by quantifying [3H]BG by mass spectrometry on the final product, which was supplied in methanol at a concentration of 1 mCi/ml (21.6 μM).
The AGT assay
For assays using intact cells, cells in exponential growth were condensed to a density of 2 × 107 cells/ml in culture medium supplemented with 20 mM Hepes (pH 7.4), and 100-μl aliquots (2 × 106 cells) were dispensed into 1.5-ml microcentrifuge tubes. Assays were initiated by the addition of 1 μl(1 μCi) of [3H]BG in the absence or presence of excess unlabeled O6-BG. The addition of 0.5 μl of 50 mM unlabeled O6-BG in Me2SO was prior to that of [3H]BG. The concentrations of [3H]BG and unlabeled O6-BG were 0.21 and 250 μM, respectively. Incubations were conducted at 37 °C for the indicated periods in open air. At the end of incubations, 240 μl of cold 100% methanol was added to denature and precipitate cellular macromolecules, and then tubes were chilled at -70 °C for 30 min. Pellets were collected by centrifugation at 12,000g for 3 min in an Eppendorf microcentrifuge, and supernatants were aspirated using a blunt-ended, 20-gauge, 1.5-inch needle connected to a vacuum suction flask. Pellets were suspended in 1 ml of cold 70% methanol using 5.75-inch Pasteur pipettes followed by centrifugation and aspiration of supernatants, and this washing procedure was repeated four times. Tubes were kept on ice during the washing procedure. Pasteur pipettes were prewetted with cold 70% methanol to prevent precipitates from sticking onto inner walls. The use of a vortex mixer was avoided to suspend pellets to minimize radioactive spillage from tubes onto the walls of the microcentrifuge during centrifugation. Pellets were suspended in 100 μl of 0.5% Triton X-100 aqueous solution and transferred to plastic scintillation vials. After the addition of 5 ml of SafeScint Scintillation Cocktail (American Bioanalytical, Natick, MA, USA), radioactivity was determined using a Beckman LS 6500 Scintillation Spectrometer (Fullerton, CA, USA). The amount of [3H]benzyl residue transferred to AGT ([3H]B—AGT) was obtained by subtracting radioactivity in the presence of unlabeled O6-BG from that in the absence of unlabeled O6-BG. The counting efficiency (cpm/dpm) for 3H was determined by suspending nonradioactive pellets in 100 μl of 0.5% Triton X-100 containing 10,000 dpm of [3H]BG and measuring the radioactivity therein.
To prepare cell homogenates, cells were washed with Hanks’ balanced salt solution and suspended at a density of 5 × 107 cells/ml in a buffer containing 50 mM Tris—HCl (pH 7.5) and 1 mM dithiothreitol (DTT), and then cell suspensions were sonicated four times in short bursts on ice using a Branson Sonifier (Danbury, CT, USA). AGT assays were then conducted as described for intact cells using 100 μl of cell homogenate equivalent to 5 × 106 cells/assay.
Hypoxanthine—guanine phosphoribosyltransferase assay
Cytoplasmic extracts for hypoxanthine—guanine phosphoribosyltransferase (HGPRT) assays were prepared from Friend murine erythroleukemia (F-MEL) wild-type 745-PC-4 and HGPRT-deficient 745-TG-11 cells [25] as described previously [26] except that the concentration of Triton X-100 in the lysis buffer was 0.08%. Nucleotide formation from [8-14C]6-mercaptopurine (51 mCi/mmol, Moravek Biochemicals) and [3H]BG was determined based on the ability of nucleotides to bind to DE81 filter discs as described previously [26].
Cell culture
All suspension cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) in a humidified 5% CO2 incubator except that appropriate factors were supplied for factor-dependent cell lines: 10 ng/ml of recombinant human granulocyte-macrophage colony-stimulating factor (GM—CSF) for TF-1 cells and 10% conditioned medium from WxEHI-3B cells as a source of interleukin-3 (IL-3) for Ba/-F3, FDC-P1, and B6SUtA cells. Attached cell lines were maintained in McCoy’s 5A medium supplemented with 10% FBS except that Daoy, A427, and B16F10 cells were cultured in medium 199 supplemented with 10% FBS, 1 mM sodium pyruvate, 1% MEM nonessential amino acid solution, and 1% MEM vitamin solution. Human melanoma cell lines A2058 (CRL-11147), C32 (CRL-1585), and G-361 (CRL-1424) were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in McCoy’s 5A medium supplemented with 10% FBS, 1 mM sodium pyruvate, and 1% MEM nonessential amino acid solution.
Western blot analysis
Whole cell extracts were prepared by washing cells with Hanks’ balanced salt solution and solubilizing 5 × 106 cells in 0.25 ml of 2 × Laemmli’s sample buffer at 100 °C for 5 min. Then 20 μl/lane of the whole cell extract was subjected to SDS—10% PAGE. Anti-AGT antibody (MT 3.1) and anti-HSC 70 antibody (sc-1059) were obtained from NeoMarkers (Fremont, CA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. HSC 70, a heat shock protein whose expression was constant, was used as a loading control.
Statistical and mathematical analyses
Values are reported as triplicate averages with standard deviations unless otherwise stated, with each experiment repeated at least twice for reproducibility. IC50 values were derived from logistic three-parameter regression analyses using KaleidaGraph (Synergy Software, Reading, PA, USA).
Results
Rationale of AGT assay
O6-BG was discovered by Dolan and coworkers [23] as a relatively potent small chemical inhibitor of AGT. Subsequently, Pegg and coworkers [27] reported that the reaction of [purine-8-3H]O6-BG with recombinant human AGT resulted in the stoichiometric production of [3H]guanine, whereas the reaction of 35S-labeled recombinant AGT with O6-BG resulted in the formation of [35S]benzylcysteine. These results have demonstrated unequivocally that (i) O6-BG inactivates AGT by acting as a substrate to produce S-benzylcysteine at the active site and that (ii) AGT can act on a small chemical substrate without a DNA structure. These studies prompted us to develop an AGT assay based on the transfer of the radioactive benzyl residue from 3H-labeled O6-BG to AGT molecules. To this end, Moravek Biochemicals was commissioned to synthesize and purify [3H]BG (Fig. 1).
Fig. 1.
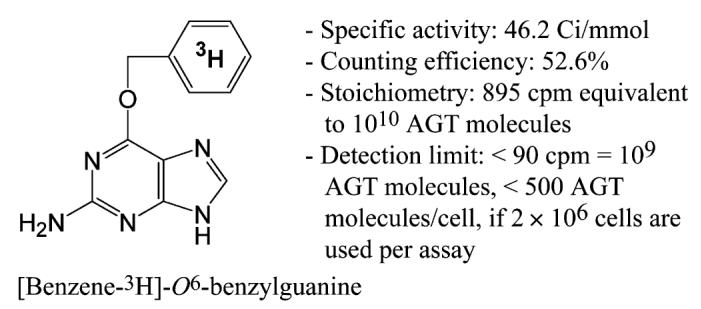
Structure of [3H]BG. Counting efficiency was determined as described in Materials and methods. Stoichiometry was derived from the counting efficiency and definitions; Avogadro’s number = 6.022 × 1023 mol-1 and 1 μCi = 2.22 × 106 dpm. Because mass spectrometry indicated that [3H]BG consisted of a mixture of unlabeled, 3H-mono-labeled, 3H-di-labeled, and 3H-tri-labeled materials, 3H was placed in the center of the benzene ring to indicate that 3H was located in the benzene ring of O6-BG.
Standardization of AGT assay
AGT assays were standardized using HL-60 human leukemia cells expressing a relatively high level of AGT as determined by Western blot analysis. Standard AGT assays consisted of incubation of cells (2 × 106 cells/100 μl) with 1 μCi of [3H]BG in the absence (total binding) or presence (nonspecific binding) of more than 1000-fold excess of unlabeled O6-BG at 37 °C. The mean values ± standard errors (SE) for total and nonspecific binding in standard assays for 2 h in HL-60 cells were 2780 ± 380 and 300 ± 30 cpm, respectively (six independent experiments with each conducted in duplicate). The amount of [3H]benzyl residue transferred to AGT was obtained by subtracting nonspecific binding from total binding.
Radioactivity recovered in the reaction product was less than 1% of the total input of radioactivity (1 μCi = 2.22 × 106 dpm). Thus, efficient washing of the reaction product from unreacted [3H]BG was critical for accurate measurement. Centrifugation of pellets and aspiration of the supernatants using a blunt-ended, 20-gauge, 1.5-inch needle was the most effective washing method with minimum production of radioactive waste (4 × 1 ml of 70% methanol). The use of 5% trichloroacetic acid to precipitate and wash cellular macromolecules was inadequate due to a high background derived from the insolubility of O6-BG in acidic solution. The use of glass fiber filter GF/C or GF/A discs to collect and wash pellets was also inadequate due to a high background resulting from binding of [3H]BG to the filters.
Kinetics of [3H]benzyl transfer to AGT
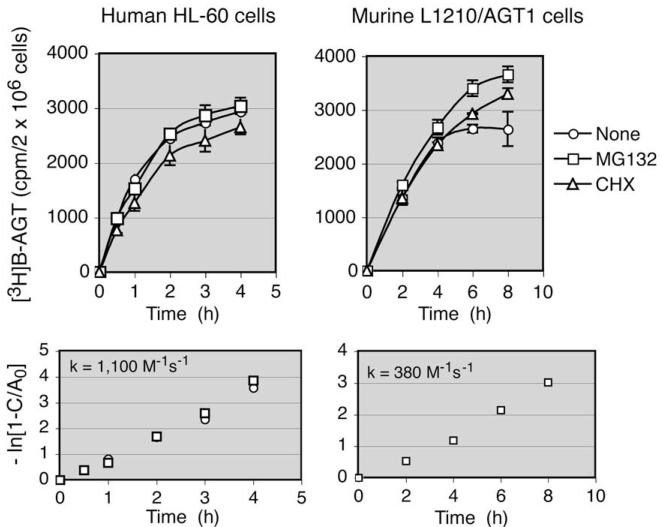
The rate of reaction of [3H]BG with AGT was measured in HL-60 human and L1210/AGT1 murine cells. AGT1 cells are a derivative of L1210 cells stably transfected with a murine AGT expression plasmid [8]. Because alkylated AGT is subject to ubiquitination-dependent degradation by proteasomes [28] to eventually become acid-soluble fragments [29], the proteasome inhibitor MG132 was included in the reaction mixture. To examine the effects of continued protein synthesis on AGT levels in intact cells, the protein synthesis inhibitor cycloheximide (CHX) was also included. Fig. 2 shows that reaction of [3H]BG with AGT reached plateaus at approximately 4 and 8 h in human and murine cells, respectively. The presence of MG132 produced little difference in the recovery of the radioactive product in HL-60 cells, whereas it caused a slight increase in AGT1 cells where prolonged incubation was needed to reach a plateau. The level of reaction product in the presence of CHX was approximately 10% less than that in the absence of CHX in HL-60 cells. The plateau levels, representing the amount of [3H]BG reacted with all of the AGT molecules present in cells, were 3100 and 3860 cpm/2 × 106 cells in HL-60 and AGT1 cells, respectively. These values were translated to 17,000 and 22,000 AGT molecules/cell, respectively, using the conversion factor 895 cpm/1010 AGT molecules (see Fig. 1).
Fig. 2.
Rate of [3H]benzyl transfer to AGT in intact HL-60 and L1210/AGT1 cells. Cells (2 × 106 cells/100 μl) were incubated with 1 μCi of [3H]BG in the absence or presence of 20 μM MG132 or 20 μM CHX for the indicated periods of time, and radioactivity in a 70% methanol-insoluble fraction was determined. Radioactivity in the presence of excess unlabeled O6-BG was subtracted from the total radioactivity. The bottom panels show the plots used to determine the second-order kinetic constant (k).
Determination of the second-order kinetic constant (k) enables extrapolation of the saturation value from a single time point. Because the reaction of O6-BG and AGT follows a bimolecular displacement (SN2-type) reaction (Eq. (1) below), the reaction rate is proportional to the product of the concentrations of AGT and [3H]BG, where A0 and B0 are the initial concentrations of AGT and [3H]BG, respectively, and B0 >> A0 (1 μCi of [3H]BG corresponds to 1.3 × 1013 AGT molecules) (Eq. (2) below). The plot of ln(1—C/A0) as a function of time (Eq. (3) below) gave second-order rate constants of 1100 and 380 M-1 s-1 for human and murine AGT, respectively (Fig. 2).
| (1) |
| (2) |
| (3) |
Effects of various purines on reaction of O6-BG and AGT
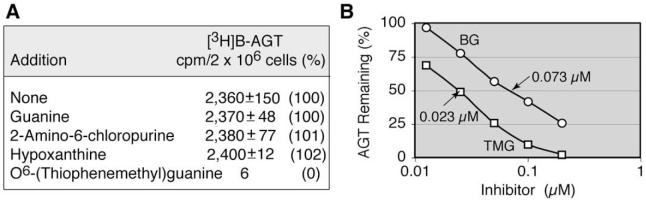
To examine the specificity of the reaction of O6-BG and AGT, the AGT assay was conducted in the presence of 150 μM various purines, including guanine (the leaving group of the O6-BG/AGT reaction), 2-amino-6-chloropurine (the starting material for the synthesis of O6-BG), hypoxanthine, and the AGT inhibitor O6-(thiophenemethyl)guanine (TMG) [30]. These purines, except for TMG, exerted no inhibitory effects on the reaction (Fig. 3A). To compare the potencies of TMG and O6-BG as inhibitors of AGT, HL-60 cells pretreated with these inhibitors for 1 h were subjected to AGT assays for 1 h to measure the remaining AGT activity. In keeping with the report of McElhinney and coworkers [30],TMG was three times more potent than O6-BG; their IC50 values were 0.023 and 0.073 μM, respectively (Fig. 3B).
Fig. 3.
Effects of various purines on the reaction of O6-BG with AGT. (A) HL-60 cells were incubated with [3H]BG in the absence or presence of 150 μM various purines for 2 h. TMG was synthesized in our laboratory and dissolved in Me2SO at a concentration of 20 mM. (B) HL-60 cells were exposed to O6-BG or TMG for 1 h, followed by the AGT assay for 1 h, to measure remaining AGT activity.
Inability of O6-BG to serve as substrate for HGPRT
The possibility of conversion of O6-BG to the nucleotide level by HGPRT and subsequent incorporation of O6-BG into DNA and RNA was examined. Cytoplasmic extracts from wild-type and HGPRT– F-MEL cells were incubated with 6-mercaptopurine (6-MP) and [3H]BG as substrates. 6-MP was readily converted to 6-MP nucleotide by wild-type extracts but not by HGPRT– extracts, whereas neither extract produced O6-BG nucleotide (Fig. 4). AGT assays gave equal values in wild-type and HGPRT– F-MEL cells (Table 1), confirming that incorporation of [3H]BG into DNA and RNA did not occur.
Fig. 4.
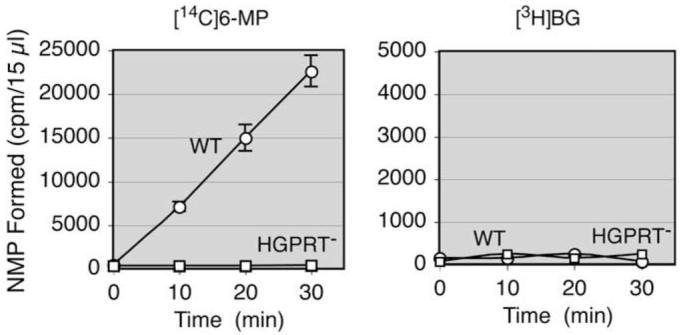
Inability of O6-BG to serve as a substrate for hypoxanthine—guanine phosphoribosyltransferase. Cytoplasmic extracts (120 μg of protein) from wild-type (WT) and HGPRT–F-MEL cells were incubated with 0.2 mM [14C]6-MP or [3H]BG at a radioactive concentration of 2 μCi/ml and 2 mM 5-phosphoribosyl-1-pyrophosphate in a volume of 200 μl. NMP, nucleoside monophosphate.
Table 1.
AGT levels in various human and rodent cell lines
| Human AGT k = 1100 M-1 s-1 A0 = 1.2 C (2 h incubation) |
Murine AGT k = 380 M-1 s-1 A0 = 2.2 C (2 h incubation) |
||
|---|---|---|---|
| Human leukemia cell lines | Murine suspension cell lines | ||
| U-937 | <500 | L1210 | <500 |
| TF-1 | <500 | P388 | <500 |
| K-562 | <500 | F-MEL | <500 |
| NB4 | 8500 | HGPRT– F-MEL | <500 |
| CCRF-CEM | 14,000 | FDC-P1 | <500 |
| Jurkat | 15,000 | B6SUtA | 1400 |
| Raji | 17,000 | Ba/F3 | 3200 |
| HL-60 | 17,000 | WEHI-3B D+ | 3400 |
| Human carcinoma cell lines | Murine attached cell lines | ||
| U251 | <500 | EMT6 | <500 |
| HCT 116 | 1500 | NIH/3T3 | 2600 |
| A427 | 1700 | B16F10 | N.M. |
| A549 | 6600 | ||
| Daoy | 8800 | Hamster attached cell line | |
| HeLa S3 | 22,000 | CHO | <500 |
| MCF7 | 42,000 | ||
| DU145 | 42,000 | Rat attached cell line | |
| LNCaP | 45,000 | NRK | 2900 |
Note. Values are AGT molecules/cell. AGT assays using the standard conditions were conducted for 2 h. The saturation levels (A0) were calculated by multiplication of the experimental values (C) and the constant determined by k. N.M., not measurable due to erroneous incorporation of [3H]BG.
Nature of nonspecific binding
The [3H]BG preparation contained less than 2% radioimpurities. Standard AGT assays using HL-60 cells performed at different temperatures for 30 min revealed that AGT functioned optimally between 37 and 45 °C, whereas nonspecific binding increased in a temperature-dependent manner (Fig. 5).
Fig. 5.
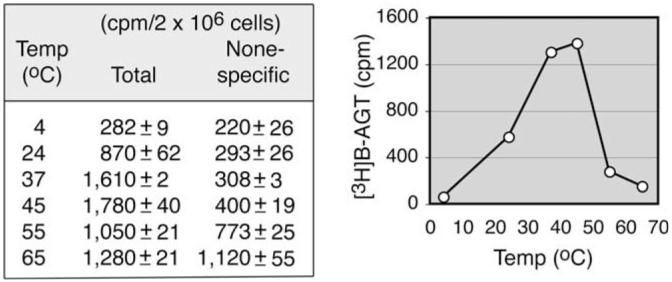
Optimal temperature for AGT activity and temperature-dependent increase in nonspecific binding. Standard AGT assays using intact HL-60 cells were conducted at various temperatures (Temp) for 30 min.
AGT assay using cell homogenates
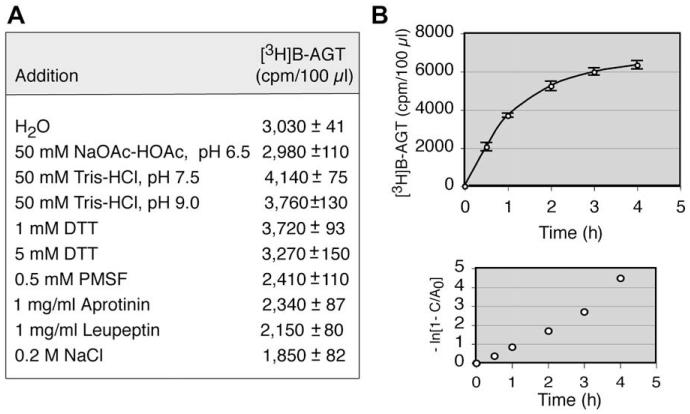
To obtain optimal conditions, various ingredients were tested individually using HL-60 cell homogenates prepared in water. Consistent with previous findings [31], AGT activity was optimal in alkaline conditions and in the presence of 1 mM DTT, whereas the additions of high salt and protease inhibitors were detrimental (Fig. 6A). Thus, cell homogenates were prepared in buffer (pH 7.5) containing 1 mM DTT. The rate of [3H]benzyl transfer in cell homogenates was indistinguishable from that in intact cells, with a k value of approximately 1100 M-1 s-1 (Fig. 6B). The recovery of AGT activity in cell homogenates was 83% (6420 cpm/5 × 106 cells for cell homogenates vs. 3100 cpm/2 × 106 cells for intact cells).
Fig. 6.
AGT assay using cell homogenates. (A) Effects of various ingredients on AGT activity were measured using HL-60 cell homogenates prepared in H2O(5 × 106 cells/ 100 μl). PMSF, phenylmethylsulfonylfluoride. (B) Kinetics of [3H]benzyl transfer using HL-60 cell homogenates (5 × 106 cells/100 μl) prepared in a buffer (pH 7.5) containing 1 mM DTT and plots to determine the second-order kinetic constant (k).
AGT content of human and rodent cell lines
The values obtained from standard AGT assays for 2 h were used to calculate saturation values (A0). The multiplication factors defined by second-order kinetic constants were 1.2 and 2.2 for human and murine cells, respectively. The AGT content of human cells varied from less than 500 to 45,000 molecules/cell (Table 1). AGT levels were below the detection limit in three of eight leukemia cell lines. Because most human carcinoma cells are polyploid, the AGT levels in LNCaP, DU145, and MCF7 cells, if expressed based on the DNA content, were similar to the level in HL-60 cells (17,000 AGT molecules/cell).
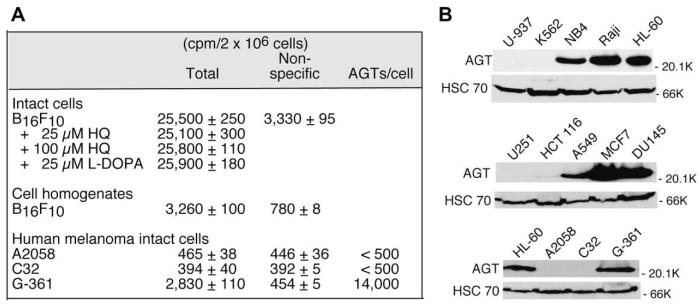
A number of murine cell lines lacked AGT expression (Table 1). Consistent with the previous observations that AGT levels in normal mouse tissues are generally approximately 10% of those in corresponding human tissues [11], AGT-positive murine cell lines contained much lower AGT activities than human cell lines. Unexpectedly, the AGT assay produced erroneous results in B16F10 melanoma cells; the experimental values for total and nonspecific binding were 59,300 and 7910 cpm/2 × 106 cells, respectively. B16F10 cells were assessed as AGT negative by the lack of sensitization to cloretazine on pretreatment with 20 μM O6-BG for 2 h in growth inhibition assays (data not shown).
Correlation between [3H]BG incorporation into methanol-insoluble fraction and melanin content
Although B16F10 murine melanoma cells are highly melanotic as judged by dark brown colorization of cells, these cells gradually lose the capacity to produce melanin in serial cell passages. The amount of [3H]BG incorporation into the methanol-insoluble fraction was inversely related to the passage number (data not shown). [3H]BG incorporation conducted for intact B16F10 cells was unaffected by hydroquinone (HQ), an inhibitor of tyrosinase, or L-3,4-dihydroxyphenylalanine (L-DOPA), a substrate for tyrosinase, which catalyzes the production of melanin [32] (Fig. 7A). For the same passage of B16F10 cells, [3 H]BG incorporation conducted for cell homogenates, in which melanin polymer was dispersed by repeated sonication, was less than 15% of that in intact cells (Fig. 7A).
Fig. 7.
AGT assays conducted for various melanoma cell lines and the correlation between AGT levels measured by the AGT assay and Western blot analysis. (A) Standard AGT assays were performed for various human melanoma cell lines and for intact B16F10 cells in the presence of HQ or L-DOPA. HQ was dissolved in Me2SO at a concentration of 20 mM. L-DOPA was dissolved in 0.001 N HCl at a concentration of 5 mM. (B) Whole cell extracts were prepared from various human cell lines and subjected to Western blot analysis for AGT using HSC 70 as a loading control. K, thousands (000) (far right of panel B).
AGT assays were performed for three human melanoma cell lines (A2058, C32, and G-361), all of which visually lacked dark brown deposits in cells, with parallel measurement of AGT levels by Western blot analysis (Fig. 7A and B). Both A2058 and C32 cells in which specific [3H]BG binding was less than 90 cpm/2 × 106 cells were negative in AGT expression by Western blot analysis, whereas G-361 cells in which 14,000 AGT molecules/cell were estimated in the AGT assay showed an AGT level similar to that in HL-60 cells (17,000 AGT molecules/cell) by Western blot analysis. These results imply that [3H]BG incorporation correlates with the melanin (polymer) content of cells. [3H]BG incorporation in B16F10 cells is reminiscent of the phenomenon that a variety of drugs and xenobiotics are selectively concentrated in pigmented tissues such as the eye, inner ear, skin, and melanoma [33].
Determination of the AGT level in B16F10 murine melanoma cells by Western blot analysis was not possible due to the inability of the anti-human AGT antibody (MT 3.1) to cross-react with murine AGT. AGT levels in human leukemia and carcinoma cell lines examined by Western blot analysis (Fig. 7B) and by the AGT assay (Table 1) were strictly correlated, confirming the accuracy of the AGT assay using [3H]BG in nonmelanotic cells.
Discussion
A variety of methods exist to measure AGT activity; difficulty in quantitation of AGT levels derives in part from the fact that AGT is not an enzyme. Because a single AGT molecule produces a single reaction product, a highly sensitive method is needed to detect minute amounts of AGT present in cells. The classical AGT assay employing [3H]MNU-reacted DNA lacks sensitivity because of the predominant reactivity of MNU at sites other than the O6 position of guanine coupled with the relatively low specific activity of [3H]MNU, whereas the assay employing 32P-labeled oligonucleo-tides containing O6-methylguanine is inappropriate as a clinical assay largely because of the use of 32P. A nonradioactive enzyme-linked immunosorbent assay (ELISA) employing oligonucleotides containing biotinylated O6-BG as a substrate for AGT has been reported [34]; however, the presence of a bulky molecule such as biotin at the benzyl moiety diminishes affinity for AGT [35] and quantitation falls in a narrow range (0.1–2.0) of optical density measurement. Other methods such as Western blotting and histo-chemical analysis are time-consuming and not quantitative. Methylation-specific PCR (MSP) that examines the methylation status of the AGT gene promoter is currently used to correlate AGT expression with clinical efficacy of temozolomide in glioblastoma [36]. Although MSP reveals a therapeutically critical aspect of AGT expression (i.e., silencing of the AGT gene by hypermethylation), it does not determine actual AGT activity.
In this article, we have devised a simple assay for AGT using [3H]BG as a substrate. This assay relies on the incorporation of the labeled benzyl residue from a methanol-soluble small chemical substrate into the methanol precipitable protein product AGT. Time-consuming substrate preparation and analytical procedures such as HPLC and gel electrophoresis are eliminated in this assay. The assay is superior to existing methods in many ways; it is sensitive due to the high specific activity of [3H]BG (41.3 Ci/mmol), quantitative, accurate, simple, applicable to both intact cells and cell homogenates, and (importantly) suitable for routine clinical assay.
The reaction of O6-BG and AGT follows second-order kinetics. The second-order kinetic constant (k) was 1100 M-1 s-1 for human AGT in our assay. Pegg and coworkers [27] reported a k value of 600 M-1 s-1 in their system using [purine-8-3H]O6-BG and recombinant human AGT. Because they estimated the specific activity of recombinant AGT by assuming that the protein is 100% active, the discrepancy in the k values may be the result of an overestimation of the AGT concentration. The lower k value (380 M-1 s-1) for murine AGT in our study is consistent with the observation that murine AGT is 2- to 3-fold more resistant to inhibition by O6-BG than human AGT [23]. These k values for O6-BG are approximately 3000-fold lower than the reported k values for various oligonucleotide substrates containing O6-methylguanine [37], indicating that AGT reacts with the O6-methylguanine lesion in DNA with enormous effectiveness. As such, the reaction of [3H]BG with human AGT in intact cells and homogenates took nearly 4 h to reach a plateau. The saturation level representing the total amount of AGT in intact cells or homogenates was mathematically extrapolative by simple multiplication of an experimental value from a single incubation time and a factor defined by the reaction constant. Inclusion of the proteasome inhibitor MG132 to prevent degradation of alkylated AGT was unnecessary for incubations up to 2 h. In addition, we have ruled out the possibility of [3H]BG being a substrate for HGPRT and incorporation of [3H]BG into DNA and RNA.
The shortcomings of this new AGT assay are as follows. First, the assay is not applicable to pigmented cells or tissues such as B16F10 melanoma in which it produces false-positive results. Natural melanins, especially eumelanins, have been shown to act as weak cation exchange polymers with the capacity to bind to metal ions and a variety of chemicals, including 2-thiouracil [38], chlorpromazine, chloroquine, haloperidol, cocaine, and polycyclic aromatic hydro-carbons [33]. Interaction of electron-rich chemicals with melanin can also be nonelectrostatic whereby the chemicals act as electron donors with melanin as the acceptor [33]. Considering the capacity of [3H]BG to bind to filters such as GF/C and GF/A, it is plausible that [3H]BG exhibits high affinity toward melanin. Second, a number of natural variant forms of the AGT gene that encode an altered protein have been reported in humans [39]. The AGT assay produces false-negative results for the polymorphism variant G160 R, which imparts high resistance to O6-BG, although occur-rence of this variant is rare [39]. Third, and obviously, this assay is not applicable to variant forms of AGT selected for resistance to inactivation by O6-BG [40].
McElhinney and coworkers [30] reported two O6-(hetarylmethyl)guanines that are more potent than O6-BG as inhibitors of AGT. Consistently, O6-(thiophenemethyl)guanine was approximately three times more potent than O6-BG in our assays. These compounds labeled with 3H at the guanine O6-alkyl residue are attractive candidates as substrates for AGT, producing faster reactions and reducing incubation times to reach a saturation level. However, these compounds exhibit the unfavorable chemical feature that their half-lives in an alkaline buffer (pH 8.3) are short; for example, the half-life of O6-(thiophenemethyl)guanine is 40 min compared with more than 48 h for O6-BG [30].
In this article, we have described the development of a simple AGT assay applicable to clinical measurements. The availability of this assay should allow a quantification of the AGT content of tumor and normal tissues before treatment and the selection of patients with a high probability of responding to guanine O6-targeting drugs with the least number of side effects to normal host tissues, thereby making cancer therapy by these agents as targeted and personalized as possible.
Acknowledgments
This work was supported by U.S. Public Health Service (USPHS) grants CA-090671, CA-122112, and CA-129186 from the National Cancer Institute and a grant from the National Foundation for Cancer Research. We express our gratitude to Bret L. Halpern for his helpful discussion on the second-order reaction kinetics, to Kevin P. Rice for his help in using KaleidaGraph software to obtain IC50 values, and to David C. Labree for his helpful advice on radiolabeling of O6-benzylguanine.
Footnotes
- AGT
- O6-alkylguanine—DNA alkyltransferase
- MNU
- N-methyl-N-nitrosourea
- O6-BG
- O6-benzylguanine
- [3H]BG
- [benzene-3H]O6-benzylguanine
- [3H]B–AGT
- [3H]benzyl residue transferred to AGT
- DTT
- dithiothreitol
- HGPRT
- hypoxanthine—guanine phosphoribosyltransferase
- F-MEL
- Friend murine erythroleukemia
- FBS
- fetal bovine serum
- GM—CSF
- granulocyte-macrophage colony-stimulating factor
- IL-3
- interleukin-3
- CHX
- cycloheximide
- TMG
- O6-(thiophenemethyl)guanine
- 6-MP
- 6-mercaptopurine
- HQ
- hydroquinone
- L-DOPA
- L-3,4-dihydroxyphenylalanine
- MSP
- methylation-specific PCR
References
- 1.Shyam K, Penketh PG, Loomis RH, Rose WC, Sartorelli AC. Antitumor 2-(aminocarbonyl)-1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazines. J. Med. Chem. 1996;39:796–801. doi: 10.1021/jm9505021. [DOI] [PubMed] [Google Scholar]
- 2.Ludlum DB. The chloroethylnitrosoureas: sensitivity and resistance to cancer chemotherapy at the molecular level. Cancer Invest. 1997;15:588–598. doi: 10.3109/07357909709047601. [DOI] [PubMed] [Google Scholar]
- 3.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide:a review of its discovery, chemical properties, pre-clinical development, and clinical trials. Cancer Treat. Rev. 1997;23:35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 4.Ludlum DB. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat. Res. 1990;233:117–126. doi: 10.1016/0027-5107(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 5.Penketh PG, Shyam K, Sartorelli AC. Comparison of DNA lesions produced by tumor-inhibitory 1,2-bis(sulfonyl)hydrazines and chloroethylnitrosoureas. Biochem. Pharmacol. 2000;59:283–291. doi: 10.1016/s0006-2952(99)00328-7. [DOI] [PubMed] [Google Scholar]
- 6.Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutat. Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 7.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J. Clin. Oncol. 2002;20:2388–2399. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 8.Ishiguro K, Shyam K, Penketh PG, Sartorelli AC. Role of O6-alkylguanine—DNA alkyltransferase in the cytotoxic activity of cloretazine. Mol. Cancer Ther. 2005;4:1755–1763. doi: 10.1158/1535-7163.MCT-05-0169. [DOI] [PubMed] [Google Scholar]
- 9.Verbeek B, Southgate TD, Gilham DE, Margison GP. O6-methylguanine—DNA methyltransferase inactivation and chemotherapy. Br. Med. Bull. 2008;85:17–33. doi: 10.1093/bmb/ldm036. [DOI] [PubMed] [Google Scholar]
- 10.Margison GP, Povey AC, Kaina B, Koref M.F. Santibanez. Variability and regulation of O6-alkylguanine—DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 11.Gerson SL, Trey JE, Miller K, Berger NA. Comparison of O6-alkylguanine—DNA alkyltransferase activity based on cellular DNA content in human, rat, and mouse tissues. Carcinogenesis. 1986;7:745–749. doi: 10.1093/carcin/7.5.745. [DOI] [PubMed] [Google Scholar]
- 12.Citron M, Decker R, Chen S, Schneider S, Graver M, Kleynerman L, Kahn LB, White A, Schoenhaus M, Yarosh D. O6-Methylguanine—DNA methyltransferase in human normal and tumor tissue from brain, lung, and ovary. Cancer Res. 1991;51:4131–4134. [PubMed] [Google Scholar]
- 13.Silber JR, Mueller BA, Ewers TG, Berger MS. Comparison of O6-methylguanine—DNA methyltransferase activity in brain tumors and adjacent normal brain. Cancer Res. 1993;53:3416–3420. [PubMed] [Google Scholar]
- 14.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine—DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- 15.Qian XC, Brent TP. Methylation hot spots in the 5′ flanking region denote silencing of the O6-methylguanine—DNA methyltransferase gene. Cancer Res. 1997;57:3672–3677. [PubMed] [Google Scholar]
- 16.Harris LC, von Wronski MA, Venable CC, Remack JS, Howell SR, Brent TP. Changes in O6-methylguanine—DNA methyltransferase expression during immortalization of cloned human fibroblasts. Carcinogenesis. 1996;17:219– 224. doi: 10.1093/carcin/17.2.219. [DOI] [PubMed] [Google Scholar]
- 17.Finch RA, Shyam K, Penketh PG, Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylamino)carbonylhydrazine (101M): A novel sulfonylhydrazine prodrug with broad-spectrum antineoplastic activity. Cancer Res. 2001;61:3033–3038. [PubMed] [Google Scholar]
- 18.Giles F, Rizzieri D, Karp J, Vey N, Ravandi F, Faderl S, Khan KD, Verhoef G, Wijermans P, Advani A, Roboz G, Kantarjian H, Bilgrami SF, Ferrant A, Daenen SM, Karsten V, Cahill A, Albitar M, Mufti G, O’Brien S. Cloretazine (VNP40101M), a novel sulfonylhydrazine alkylating agent, in patients age 60 years or older with previously untreated acute myeloid leukemia. J. Clin. Oncol. 2007;25:25–31. doi: 10.1200/JCO.2006.07.0961. [DOI] [PubMed] [Google Scholar]
- 19.Ro JY, Jensen DE, Kim S. Quantitation of S-methylcysteine formed in O6-methylguanine—DNA: methyltransferase. Cancer Lett. 1984;23:213–221. doi: 10.1016/0304-3835(84)90156-3. [DOI] [PubMed] [Google Scholar]
- 20.Watson AJ, Margison GP. O6-alkylguanine—DNA alkyltransferase assay. Methods Mol. Biol. 2000;152:49–61. doi: 10.1385/1-59259-068-3:49. [DOI] [PubMed] [Google Scholar]
- 21.Kreklau EL, Liu N, Li Z, Cornetta K, Erickson LC. Comparison of single-versus double-bolus treatments of O6-benzylguanine for depletion of O6-methylguanine—DNA methyltransferase (MGMT) activity in vivo: development of a novel fluorometric oligonucleotide assay for measurement of MGMT activity. J. Pharmacol. Exp. Ther. 2001;297:524–530. [PubMed] [Google Scholar]
- 22.Wu RS, Hurst-Calderone S, Kohn KW. Measurement of O6-alkylguanine—DNA alkyltransferase activity in human cells and tumor tissues by restriction endonuclease inhibition. Cancer Res. 1987;47:6229–6235. [PubMed] [Google Scholar]
- 23.Dolan ME, Moschel RC, Pegg AE. Depletion of mammalian O6-alkylguanine—DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc. Natl. Acad. Sci. USA. 1990;87:5368– 5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciocco GM, Moschel RC, Chae MY, McLaughlin PJ, Zagon IS, Pegg AE. Specific labeling of O6-alkylguanine—DNA alkyltransferase by reaction with O6-(p-hydroxy[3H]methylbenzyl)guanine. Cancer Res. 1995;55:4085–4091. [PubMed] [Google Scholar]
- 25.Gusella JF, Housman D. induction of erythroid differentiation in vitro by purines and purine analogues. Cell. 1976;8:263–269. doi: 10.1016/0092-8674(76)90010-6. [DOI] [PubMed] [Google Scholar]
- 26.Ishiguro K, Schwartz EL, Sartorelli AC. Characterization of the metabolic forms of 6-thioguanine responsible for cytotoxicity and induction of differentiation of HL-60 acute promyelocytic leukemia cells. J. Cell. Physiol. 1984;121:383–390. doi: 10.1002/jcp.1041210216. [DOI] [PubMed] [Google Scholar]
- 27.Pegg AE, Boosalis M, Samson L, Moschel RC, Byers TL, Swenn K, Dolan ME. Mechanism of inactivation of human O6-alkylguanine—DNA alkyltransferase by O6-benzylguanine. Biochemistry. 1993;32:11998–12006. doi: 10.1021/bi00096a009. [DOI] [PubMed] [Google Scholar]
- 28.Srivenugopal KS, Yuan XH, Friedman HS, Ali-Osman F. Ubiquitination-dependent proteolysis of O6-methylguanine—DNA methyltransferase in human and murine tumor cells following inactivation with O6-benzylguanine or 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochemistry. 1996;35:1328–1334. doi: 10.1021/bi9518205. [DOI] [PubMed] [Google Scholar]
- 29.Xu-Welliver M, Pegg AE. Degradation of the alkylated form of the DNA repair protein, O6-alkylguanine—DNA alkyltransferase. Carcinogenesis. 2002;23:823–830. doi: 10.1093/carcin/23.5.823. [DOI] [PubMed] [Google Scholar]
- 30.McElhinney RS, Donnelly DJ, McCormick JE, Kelly J, Watson AJ, Rafferty JA, Elder RH, Middleton MR, Willington MA, McMurry TB, Margison GP. Inactivation of O6-alkylguanine—DNA alkyltransferase: I. Novel O6-(hetarylmethyl)guanines having basic rings in the side chain. J. Med. Chem. 1998;41:5265–5271. doi: 10.1021/jm9708644. [DOI] [PubMed] [Google Scholar]
- 31.Pegg AE, Wiest L, Foote RS, Mitra S, Perry W. Purification and properties of O6-methylguanine—DNA transmethylase from rat liver. J. Biol. Chem. 1983;258:2327–2333. [PubMed] [Google Scholar]
- 32.Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007;127:751–761. doi: 10.1038/sj.jid.5700683. [DOI] [PubMed] [Google Scholar]
- 33.Prota G. Melanins and Melanogenesis. Academic Press; San Diego: 1992. [Google Scholar]
- 34.Nagel G, Brenner W, Johnsson K, Kaina B. DNA repair protein O6-methylguanine—DNA methyltransferase in testis and testicular tumors as determined by a novel nonradioactive assay. Anal. Biochem. 2003;321:38–43. doi: 10.1016/s0003-2697(03)00432-9. [DOI] [PubMed] [Google Scholar]
- 35.Damoiseaux R, Keppler A, Johnsson K. Synthesis and applications of chemical probes for human O6-alkylguanine—DNA alkyltransferase. ChemBioChem. 2001;2:285–287. doi: 10.1002/1439-7633(20010401)2:4<285::AID-CBIC285>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352:997– 1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 37.Liem LK, Wong CW, Lim A, Li BF. Factors influencing the repair of the mutagenic lesion O6-methylguanine in DNA by human O6-methylguanine—DNA methyltransferase. J. Mol. Biol. 1993;231:950–959. doi: 10.1006/jmbi.1993.1344. [DOI] [PubMed] [Google Scholar]
- 38.Napolitano A, Palumbo A, d’Ischia M, Prota G. Mechanism of selective incorporation of the melanoma seeker 2-thiouracil into growing melanin. J. Med. Chem. 1996;39:5192–5201. doi: 10.1021/jm9605243. [DOI] [PubMed] [Google Scholar]
- 39.Pegg AE, Fang Q, Loktionova NA. Human variants of O6-alkylguanine—DNA alkyltransferase. DNA Repair (Amst) 2007;6:1071–1078. doi: 10.1016/j.dnarep.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crone TM, Goodtzova K, Edara S, Pegg AE. Mutations in human O6 -alkylguanine—DNA alkyltransferase imparting resistance to O6 -benzylguanine. Cancer Res. 1994;54:6221–6227. [PubMed] [Google Scholar]