Pigmentation defects in mice and humans result from mutations in any of over 120 genes, many of which also influence additional physiological functions (Bennett and Lamoreux, 2003). Identification of genes mutated in diseases such as Hermansky-Pudlak and Chediak-Higashi syndromes and their corresponding mouse models have unveiled components of the intracellular trafficking machinery required for the formation of melanosomes and other tissue-specific lysosome-related organelles (Raposo et al., 2007). In a recent report, Chow and colleagues (Chow et al., 2007) identify another likely component of this machinery by mapping the gene that is defective in both the “pale tremor” mouse and a form of Charcot-Marie-Tooth (CMT) disease in humans. The gene, Fig4 (in the mouse; FIG4 in humans), encodes an inositol phospholipid phosphatase that likely regulates the biogenesis of melanosomes and neurosecretory granules. Its identification provides a new twist that will likely enhance our emerging understanding of melanosome protein trafficking.
In addition to dilute pigmentation, pale tremor mice suffer from severe tremor, an abnormal gait, and reduced body weight. Neuronal pathologies include extensive cell loss in the central and peripheral nervous systems and reduced peripheral nerve activity; cell loss is also observed in the spleen. Neuronal cell death is preceded by an accumulation of intracellular vacuoles that are also apparent in primary cultures of fibroblasts and hippocampal neurons - implying a primary defect in intracellular membrane dynamics. Most pups die by six weeks of age (Chow et al., 2007). A similar progressive motor neuropathy and associated sensory defects are also observed in patients with CMT disease, a heterogeneous set of disorders characterized by muscle atrophy, bone malformation, and other variable symptoms (Niemann et al., 2006). Defects in a number of genes have been identified in CMT patients, but the genes affected in others remain uncharacterized.
Chow et al used standard mapping techniques to localize the defective gene in pale tremor mice to a 2 megabase region on mouse chromosome 10, and then sequenced RT-PCR products for candidate genes within this region to identify mRNAs that are altered in affected brain tissue. They found that pale tremor mice harbor a retrotransposon insertion within an intron of the Fig4 gene (Chow et al., 2007). Because the pathologies observed in pale tremor mice are similar to those of CMT, Chow et al. then sequenced the human FIG4 gene in CMT patients who lacked mutations in other characterized genes. They identified FIG4 mutations in four patients with a severe form of the disease. Each patient carried one FIG4 allele predicted to encode a truncated product and a second allele – identical in all patients - in which a missense mutation converts a conserved isoleucine at position 41 to a threonine (I41T) (Chow et al., 2007). This missense mutation likely confers a loss of function based on its inability to functionally rescue a deletion of the homologous gene in the yeast, Saccharomyces cerevisiae (see below).
The sequence of the predicted product of the mouse Fig4 gene is 35% identical and 66% similar to the S. cerevisiae Fig4 protein, a phosphatase for which the substrate is a specific phosphorylated isomer of phosphatidylinositol (PtdIns) bisphosphate. PtdIns is an unique phospholipid in eukaryotes because it can be phosphorylated at the 3, 4 and/or 5 positions of its inositol ring head group. The differentially phosphorylated forms of PtdIns (known as phosphoinositides) have distinct functional properties by virtue of their ability to bind specific target proteins, and thereby regulate either their activity or their localization (Lemmon, 2003).
The abundance of a given phosphoinositide is regulated by the activities of both lipid kinases (for example, PtdIns3P is generated from PtdIns by PtdIns 3-kinase) and phosphoinositide phosphatases [for example, PtdIns(4,5)P2 can be generated from PtdIns(3,4,5)P3 by a 3-phosphatase]. Yeast Fig4p is a highly specific PtdIns(3,5)P2 5-phosphatase, meaning that it removes the phosphate from the 5 position of the inositol ring in PtdIns(3,5)P2 (Rudge et al., 2004)(Figure 1a), converting it to PtdIns3P; human FIG4 has similar activity in vitro (although its substrate specificity in vitro appears to be more promiscuous; Sbrissa et al., 2007). In yeast, Fig4p is also required to activate Fab1p - the PtdIns3P 5-kinase that generates PtdIns(3,5)P2 - in response to osmotic stress; thus, yeast fig4 mutants have a muted osmotic stress response because of a paradoxically impaired ability to synthesize, rather than dephosphorylate, PtdIns(3,5)P2 (Duex et al., 2006). As might be predicted from these yeast studies, mammalian FIG4 physically associates with the human homologue of Fab1p (Sbrissa et al., 2007), and cultured fibroblasts derived from the mouse Fig4 mutant show a marked reduction in PtdIns(3,5)P2 levels (Chow et al., 2007). These results imply that mammalian FIG4 functions similarly to the yeast protein. Interestingly, other variants of CMT carry mutations in MTMR2 – encoding a 3-phosphatase active on PtdIns3P and PtdIns(3,5)P2 – or in a related gene, MTMR13/SBF2 (Niemann et al., 2006). By extension, it is reasonable to hypothesize that the pathologic effects of the Fig4 mutants in pale tremor mice and of several types of CMT patients are due to alterations in PtdIns(3,5)P2 metabolism.
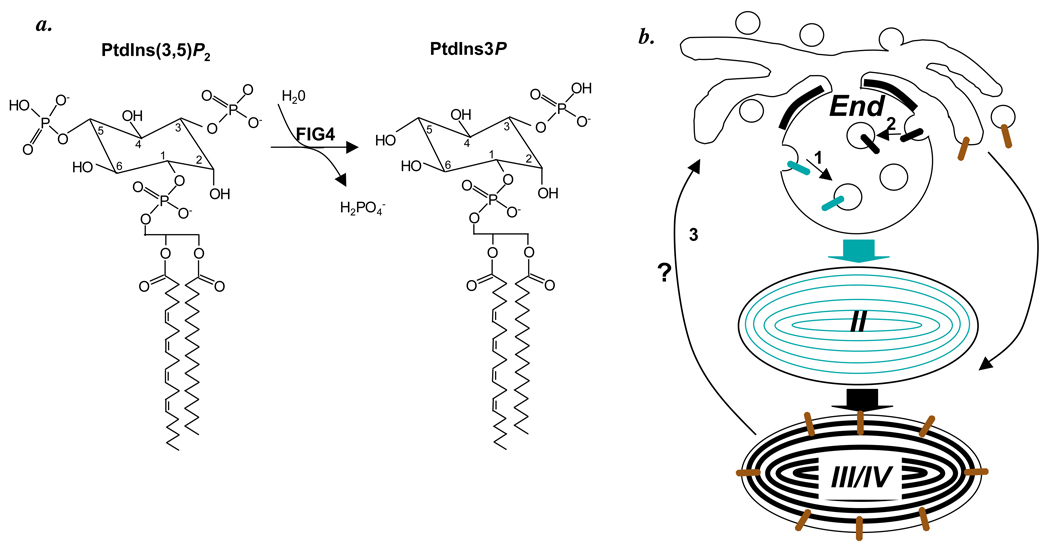
Figure 1.
a. FIG4 catalyzes the hydrolysis of PtdIns(3,5)P2 to PtdIns3P as shown, with the structure of the phosphoinositol headgroups of a typical phosphatidyl phosphoinositide emphasized. b. Model of steps in melanosome biogenesis from early endosomes (End) to early stage II melanosomes (II) and mature stages III and IV melanosomes (III/IV) that might require FIG4 activity. 1, invagination of Pmel17 (green). 2, invagination of MART-1 (black). 3, retrograde transport from melanosomes to endosomes. Also shown is anterograde transport of tyrosinase and tyrosinase related protein-1 (brown) from endosomes to mature melanosomes.
PtdIns(3,5)P2 is an extremely rare phospholipid, comprising less than 0.1% of total phosphoinositides in a typical cell (Michell et al., 2006). Alterations in PtdIns(3,5)P2 metabolism in yeast result specifically in abnormalities in membrane trafficking into and out of the vacuole - the yeast organelle most like the mammalian lysosome. In particular, mutants lacking Fab1p have grossly enlarged vacuoles resulting in part from a defect in retrograde membrane trafficking from the vacuole toward the Golgi (Dove et al., 2004) and in part to defective invagination of the vacuole limiting membrane to form intralumenal membranes (Odorizzi et al., 1998). Intralumenal membrane vesicles accumulate in obligate endosomal intermediates called multivesicular bodies (MVBs) (Piper and Katzmann, 2007). In most cells, MVBs largely serve to deliver the internal membranes and associated transmembrane proteins to lysosomes for degradation, but they have additional functions in certain cell types. Defects in either retrograde transport from lysosomes or MVB formation in the neurons of pale tremor mice and CMT patients would explain the accumulation of intracellular vacuoles and consequent cell death resulting from altered endosomal or lysosomal function.
How might Fig4 mutations alter pigmentation? Chow et al provide few clues – they show only that hair follicles are largely devoid of pigment, and that the few remaining pigment granules in hair shafts are present in small clumps (Chow et al., 2007). However, the role of PtdIns(3,5)P2 in vacuole dynamics in yeast suggests that FIG4 might be required in melanocytes for processes involved in the formation of melanosomes or in subsequent melanosome uptake by keratinocytes. Characterization of these processes in pale tremor mice will likely provide valuable insight into the molecular mechanisms regulating membrane transport required for melanosome biogenesis and perhaps of melanosome transfer.
One process that may require FIG4 is the formation of early stage melanosomes. Stage I melanosomes are morphologically identical to multivesicular early endosomes and function within both the endocytic and melanogenic pathways (Raposo et al., 2001). Within these compartments, Pmel17 – the pigment cell-specific protein that forms the core of melanosome fibrils (Theos et al., 2005b) – becomes incorporated onto the internal membranes. Indeed, Pmel17 sorting to the internal membranes is required for subsequent fibril formation (Hoashi et al., 2006; Theos et al., 2006). Genetic deficiencies of Pmel17 cause hypopigmentation in animal models - at least in part due to melanocyte death (Theos et al., 2005b). Thus, one potential mechanism behind the hypopigmentation in Fig4 deficient mice might be a failure to properly sort Pmel17 (Figure 1b, step 1). Similarly, MART-1, another melanocyte-specific protein that regulates Pmel17 fibril formation (Hoashi et al., 2005), is also incorporated onto intralumenal membranes of multivesicular endosomes, but by a different mechanism reliant on ubiquitylation of the MART-1 cytoplasmic domain and consequent recognition by a conserved ESCRT (endosomal sorting complex required for transport) machinery (Lévy et al., 2005). This ESCRT-dependent mechanism for MVB formation is conserved in yeast and appears to be defective in fig4 and fab1 mutants (Odorizzi et al., 1998), suggesting that it requires PtdIns(3,5)P2. The ESCRT pathway (Tsujita et al., 2004) and MVB formation (Sbrissa et al., 2007) in mammals are likely to be similarly dependent on PtdIns(3,5)P2, suggesting that Fig4 mutations might affect melanosome architecture indirectly by altering MART-1 sorting to intralumenal membranes (Figure 1b, step 2).
Retrograde transport also likely plays an important role in melanosome biogenesis. Analyses of melanocytes from models of Hermansky-Pudlak syndrome suggest that melanogenic enzymes such as tyrosinase and tyrosinase related protein-1 are transported biosynthetically from early endosomes to melanosomes by anterograde (forward – i.e. endosome to melanosome) membrane trafficking (see Figure 1b; Setty et al., 2007; Theos et al., 2005a). Such trafficking must be balanced by retrograde (backward – i.e., melanosome to endosome) trafficking (Bonifacino and Rojas, 2006) in order to maintain nascent melanosome size. Failure to recycle membrane out of nascent melanosomes results in melanosome enlargement with either a failure to mature or to be transferred to keratinocytes, as seen in Chediak-Higashi syndrome (Shiflett et al., 2002) and Hermansky-Pudlak syndrome types 1 and 4 (Wei, 2006). Such a defect might be another potential mechanism underlying hypopigmentation in Fig4 mutants (Figure 1b, step 3). Indeed, the absence of hair follicle pigment observed in the pale tremor mouse might also reflect a defect in uptake of melanin by keratinocytes, a poorly understood process (Van Den Bossche et al., 2006) that may require phagocytosis or autophagy within the keratinocyte, both of which are affected by altering PtdIns(3,5)P2 metabolism (Michell et al., 2006).
The hypopigmentation of Fig4 mutants (Chow et al., 2007) and the known role of PtdIns(3,5)P2 in endosomal membrane dynamics underscores the keen relationship between melanosomes and endosomes. While the precise mechanisms underlying hypopigmentation in the pale tremor mouse remain to be discovered, future studies to decipher them will likely provide great insight into the membrane dynamics underlying melanosome formation.
Acknowledgements
I thank Mark Lemmon and Leslie King for critical review of the manuscript and extremely helpful comments, and the National Institutes of Health (grant # R01 EY015625) for funding.
References
- Bennett DC, Lamoreux ML. The color loci of mice - a genetic century. Pigment Cell Res. 2003;16:333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, Weisman LS, Meisler MH. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SK, Piper RC, McEwen RK, Yu JW, King MC, Hughe sDC, Thuring J, Holmes AB, Cooke FT, Michell RH, Parker PJ, Lemmon MA. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23:1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duex JE, Tang F, Weisman LS. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J. Cell Biol. 2006;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoashi T, Muller J, Vieira WD, Rouzaud F, Kikuchi K, Tamaki K, Hearing VJ. The repeat domain of the melanosomal matrix protein Pmel17/gp100 is required for the formation of organellar fibers. J. Biol. Chem. 2006;281:21198–22208. doi: 10.1074/jbc.M601643200. [DOI] [PubMed] [Google Scholar]
- Hoashi T, Watabe H, Muller J, Yamaguchi Y, Vieira WD, Hearing VJ. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem. 2005;280:14006–14016. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Lévy F, Muehlethaler K, Salvi S, Peitrequin A-L, Lindholm CK, Cerottini J-C, Rimoldi D. Ubiquitylation of a melanosomal protein by HECT-E3 ligases serves as sorting signal for lysosomal degradation. Mol. Biol. Cell. 2005;16:1777–1787. doi: 10.1091/mbc.E04-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell RH, Heath VL, Lemmon MA, Dove SK. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem. Sci. 2006;31:52–63. doi: 10.1016/j.tibs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 2007;23 doi: 10.1146/annurev.cellbio.23.090506.123319. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001;152:809–823. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge SA, Anderson DM, Emr SD. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol. Biol. Cell. 2004;15:24–36. doi: 10.1091/mbc.E03-05-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport: novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J. Biol. Chem. 2007;282:23878–23891. doi: 10.1074/jbc.M611678200. [DOI] [PubMed] [Google Scholar]
- Setty SRG, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, Dell'Angelica EC, Raposo G, Marks MS. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15:251–257. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005a;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Raposo G, Marks MS. The Silver locus product Pmel17/ gp100/ Silv/ ME20: Controversial in name and in function. Pigment Cell Res. 2005b;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita K, Itoh T, Ijuin T, Yamamoto A, Shisheva A, Laporte J, Takenawa T. Myotubularin regulates the function of the late endosome through the GRAM domain-phosphatidylinositol 3,5-bisphosphate interaction. J. Biol. Chem. 2004;279:13817–13824. doi: 10.1074/jbc.M312294200. [DOI] [PubMed] [Google Scholar]
- Van Den Bossche K, Naeyaert J-M, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7:769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]