Abstract
Purpose
Concurrent temozolomide (TMZ) and radiation therapy (RT) followed by adjuvant TMZ is standard treatment for patients with GBM, although the relative contribution of concurrent versus adjuvant TMZ is unknown. In this study, the efficacy of TMZ/RT was tested in a panel of 20 primary GBM xenografts.
Methods and Materials
Mice with intracranial xenografts were treated with TMZ, RT, TMZ/RT, or placebo. Survival ratio for a given treatment/line was defined as the ratio of median survival for treatment vs. placebo.
Results
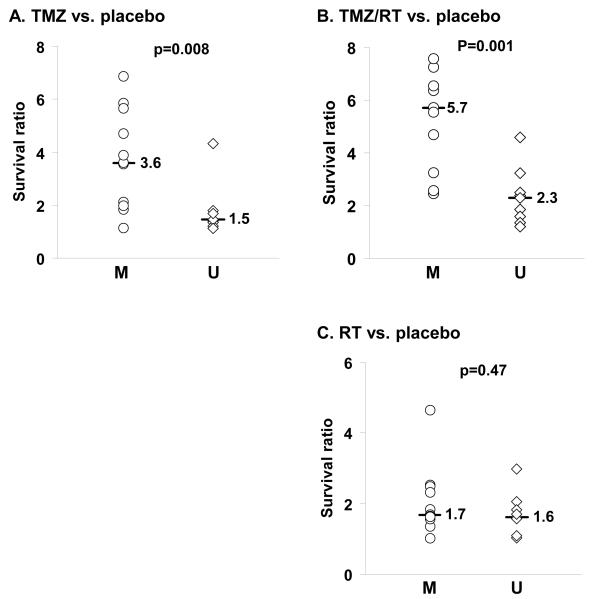
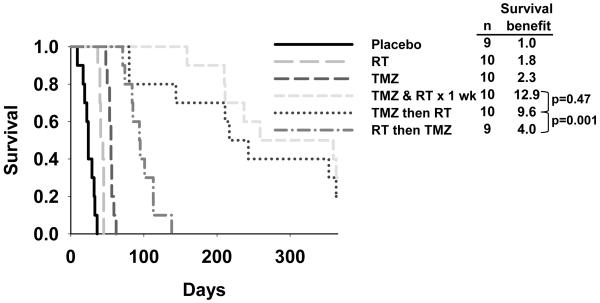
The median survival ratio was significantly higher for MGMT methylated tumors versus unmethylated tumors following treatment with TMZ (median survival ratio 3.6 vs. 1.5, respectively; p=0.008) or TMZ/RT (5.7 vs. 2.3, respectively; p=0.001), but not RT alone (1.7 vs. 1.6; p=0.47). In an ANOVA analysis, MGMT methylation status and p53 mutation status were significantly associated with treatment response. In analyzing the additional survival benefit conferred specifically by combined therapy, only a subset (5 of 11) MGMT methylated tumors derived substantial additional benefit from combined therapy, while none of the MGMT unmethylated tumors did. Consistent with a true radiosensitizing effect of TMZ, sequential treatment, in which RT (week 1) was followed by TMZ (week 2), proved significantly less effective than TMZ followed by RT or concurrent TMZ / RT (survival ratios of 4.0, 9.6, and 12.9, respectively; p<0.0001).
Conclusions
Concurrent treatment with TMZ and RT provides significant survival benefit only in a subset of MGMT methylated tumors, and provides superior anti-tumor activity relative to sequential administration of RT and TMZ.
Keywords: radiation, temozolomide, glioblastoma, MGMT, xenografts
INTRODUCTION
The integration of temozolomide (TMZ) into upfront therapy for newly diagnosed patients with glioblastoma multiforme (GBM) has significantly extended the survival for this uniformly fatal disease. In the landmark EORTC 22891 trial, patients randomized to TMZ and radiation therapy (RT) had a significantly longer survival as compared to patients randomized to RT alone, and these data established concurrent TMZ/RT followed by adjuvant TMZ (Stupp regimen) as the standard of care for GBM 1. Companion studies in this and other clinical trials also suggest that the benefit of TMZ therapy is greatest for patients in which expression of the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) is suppressed by promoter hypermethylation 2, 3. On the basis of these data, there is significant interest not only in using MGMT promoter hypermethylation as a prognostic assay, but also as a predictive assay to identify the subset of patients who will benefit from TMZ-based therapy.
The current oncologic paradigm of concurrent chemo-radiotherapy is based on the observation that chemotherapy administered during radiation can potentiate the cell killing effects of radiation. For most epithelial tumor types, the use of concurrent chemotherapy in conjunction with RT is routine and is supported by robust radiosensitizing effects in pre-clinical studies 4-6. In comparison, the in vitro radiosensitizing effects demonstrated with TMZ are minimal at clinically relevant radiation doses 7-11. Given the significant efficacy of TMZ monotherapy in pre-clinical models and the documented clinical benefit of mono-therapy in patients with recurrent GBM 12-15, these data raise the possibility that the dominant survival benefit from TMZ in the Stupp regimen is unrelated to a radiosensitizing effect and simply reflects the additive cytotoxicities of RT and TMZ monotherapies. However, the relative importance of concomitant TMZ versus adjuvant TMZ cannot be addressed in clinical trials because of the ethical considerations of potentially withholding an important component of what is now standard GBM therapy. Thus, in the current study, the importance of concomitant TMZ during RT was evaluated in a panel of 20 GBM xenograft lines initially derived from patient tumor specimens, and these results were correlated with tumor MGMT methylation status and other key genetic features potentially associated with resistance to therapy.
METHODS and MATERIALS
Xenograft information
Each of the 20 serially passaged xenografts used in this study were derived from unique tumors derived from different patients. Molecular genetic alterations and corresponding patient tumor histopathologic classifications for 17 of xenografts have been previously described 16-18. Three additional xenografts not previously reported, GBM5, GBM58 and GBM59, all diagnosed as GBM, have been included in the current investigation. Prior Mayo Institutional Review Board authorization was obtained for the use of human tissue to establish the xenograft lines, and all patients consented to participation in research at Mayo Clinic.
Orthotopic xenograft model and therapy response experiments
All xenograft therapy evaluations were conducted using an orthotopic tumor model and a protocol approved by the Mayo Institutional Animal Care and Use Committee. The procedure for establishing intracranial tumors has been described in detail previously 16, 17. Mice with established orthotopic tumors were randomized to therapy with placebo/sham RT, TMZ alone (66 mg/kg daily × 5 days), RT alone (2 Gy twice daily × 5 days - 20 Gy total), and concomitant TMZ and RT. TMZ was purchased from the Mayo Clinic pharmacy, suspended in Ora-Plus (Paddock Laboratories, Minneapolis, MN), and dosed by oral gavage 1 hour prior to the morning dose of radiation. Radiation was delivered to the entire head of unanaesthetized mice, immobilized in a plastic restraint, through a single right lateral beam from a 137Cs source. The remainder of the body was shielded with a lead block. A minimum of 6 hours was maintained between each radiation fraction. All mice were observed daily by an experienced technician blinded to the treatment received, and mice were euthanized upon reaching a moribund condition, which typically is characterized by a hunched posture, lethargy, inability to maintain an upright position, spasticity, seizures, circling, paresis, or paralysis.
Evaluation of MGMT methylation by MS-PCR
DNA was extracted from flank xenograft samples using the Gentra DNA extraction kit (Puregene, Minneapolis, MN). DNA was extracted from paraffin-embedded patient samples using the Masterpure Complete DNA and RNA Purification kit (EpiCentre Biotechnologies, Madison, WI). Isolated tumor DNA was bisulfite-treated using the EZ DNA methylation kit (Zymo Research, Orange, CA). The modified DNA was amplified using primers specific for either methylated or unmethylated MGMT promoter sequences as described previously 19, 20.
Molecular evaluation of p53, PTEN and EGFR
The mutation status of p53 was determined by Sanger sequencing as described previously 21, 22. Similarly, PTEN mutations were identified by direct sequencing and homozygous deletion was demonstrated by PCR 18, 21, 23, 24. EGFR amplification was determined by fluorescence in situ hybridization and sequencing within the extracellular domain 23.
Statistical Analysis
Cumulative survival probabilities were estimated using the Kaplan-Meier method. The log rank test was used to compare survival of groups. To evaluate the correlation between treatment group and the status of 4 biomarkers (MGMT methylation, p53 mutation, PTEN mutation/deletion and EGFR amplification status) two analysis methods were considered. First, to facilitate the interpretation of treatment response in each line, the survival ratio for each treatment is defined as the ratio of the median survival for mice receiving the treatment versus placebo. The specific benefit of combined TMZ/RT compared to monotherapy was quantified by calculating a survival difference: (difference in the median survival for TMZ/RT versus most efficacious monotherapy) divided by placebo survival. Comparisons of survival ratio by treatment group for different biomarker status were evaluated using the two-sample rank sum test. Second, all of the individual xenograft experiments were pooled and a repeated measure analysis of variance (ANOVA) was used. A general linear model was used since the majority of the mice die. Specifically, survival time was the dependent variable, treatment group, marker (e.g. MGMT), and the treatment group by marker interaction were the independent variables. Tumor line was included as a repeated factor to account for potential correlation within tumor lines. All tests were two-sided and a p-value < 0.05 was considered to be statistically significant.
RESULTS
MGMT methylation status
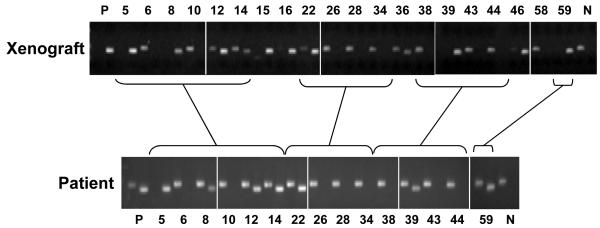
A total of 20 primary GBM xenograft lines were available for this study. As an initial assessment of the potential utility of this xenograft model for testing TMZ responsiveness, tumor samples from all 20 xenograft lines and 15 of the original patient tumor samples, used to establish these lines, were evaluated for MGMT promoter methylation using a standard MS-PCR assay. Similar to the incidence of MGMT promoter methylation in clinical samples, 11 of 20 primary xenograft lines (55%) were found to have appreciable MGMT methylation by this assay (Figure 1). More importantly, there was a close correlation between the MGMT promoter methylation results for the xenograft tumor samples and the derivative patient tumor samples. The single, methylated, PCR product for the xenograft samples GBM8, 39 and 59 and the dual, unmethylated and methylated, PCR products observed for corresponding patient tumor samples both would be considered methylated tumor samples according to the methods published by Hegi et. al. 2, 25. The unmethylated PCR product in these patient samples likely reflects a PCR signal from associated normal tissues within the tumor sample. This preservation of MGMT methylation status between tumor and xenograft samples supports the idea that evaluation of TMZ response in our xenograft lines will provide translationally relevant sensitivity data relating to MGMT status.
Figure 1.
MGMT methylation assessment of tumors. Methylation-specific PCR (MS-PCR) was performed on 20 primary GBM xenografts (upper panel) and 15 corresponding patient samples from which these xenografts were derived. For each tumor sample, 2 PCR reaction products are resolved side-by-side from reactions using primers specific for unmethylated (left lane) or methylated (right lane) MGMT promoter. P: positive methylation control. N: negative methylation control.
Intracranial survival studies
The combination of TMZ and RT was evaluated in 20 xenograft lines using an intracranial therapy evaluation model. For each tumor line, mice with established orthotopic tumors were randomized to therapy with TMZ alone, radiation alone, or the combination of TMZ and radiation. TMZ was delivered by oral gavage daily for 5 consecutive days at a dose of 66 mg/kg/day, which provides similar serum concentrations in mice as compared to the standard clinical adjuvant dosing regimen of 200 mg/m2 for 5 days in humans 26-28. The time from tumor implantation to reaching a moribund state was recorded for each mouse, and median survivals following treatment with placebo, TMZ, RT or TMZ/RT are summarized in Table 1. To facilitate the interpretation of treatment response in each line, the survival ratio for each treatment was defined as the ratio of the median survival for mice receiving the treatment versus placebo (Table 2). Across all tumor lines, TMZ treatment alone was associated with a range in survival ratio from 1.1 to 6.9 (median 1.9). The range in survival ratio for RT alone was 1.0 to 4.6 (median 1.7) and the range in survival ratio for RT/TMZ was 1.2 to 7.6 (median 3.2). Thus, similar to clinical experience, the efficacy of RT, TMZ or RT/TMZ ranged widely with most tumors having an intermediate response to therapy.
Table 1.
Survival studies results following therapy with placebo/sham RT, TMZ, RT or RT/TMZ for 20 GBM xenograft lines
| placebo | TMZ | RT | RT/TMZ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GBM# |
Rx day |
n |
survival, days |
n |
survival, days |
n |
survival, days |
n |
survival, days |
| GBM5 | 22 | 8 | 55 | 10 | 321 | 10 | 139 | 9 | 349 |
| GBM6 | 37 | 8 | 41 | 6 | 57 | 7 | 42 | 4 | 98 |
| GBM8 | 44 | 8 | 58.5 | 8 | 123.5 | 8 | 107 | 8 | 189 |
| GBM10 | 36 | 7 | 41 | 7 | 55 | 9 | 66 | 6 | 76 |
| GBM12 | 11 | 10 | 15 | 10 | 53 | 10 | 37 | 10 | 85.5 |
| GBM14 | 24 | 10 | 33 | 10 | 186 | 10 | 55.5 | 9 | 188 |
| GBM15 | 29 | 10 | 54 | 10 | 252.5 | 9 | 250 | 10 | 252 |
| GBM16 | 23 | 8 | 47.5 | 8 | 325.5 | 8 | 64 | 8 | 343 |
| GBM22 | 38 | 8 | 43 | 8 | 154.5 | 8 | 43 | 8 | 325.5 |
| GBM26 | 65 | 8 | 82.5 | 8 | 99.5 | 7 | 130 | 7 | 130 |
| GBM28 | 30 | 8 | 33 | 7 | 56 | 7 | 68 | 8 | 82.5 |
| GBM34 | 46 | 8 | 85 | 8 | 366.5 | 8 | 156 | 7 | 391 |
| GBM36 | 44 | 8 | 125.5 | 8 | 228.5 | 8 | 194.5 | 8 | 307 |
| GBM38 | 45 | 9 | 55 | 10 | 80 | 9 | 57 | 9 | 75 |
| GBM39 | 28 | 10 | 31 | 10 | 120 | 10 | 50.5 | 10 | 202 |
| GBM43 | 22 | 5 | 24 | 8 | 43 | 7 | 41 | 5 | 55 |
| GBM44 | 31 | 9 | 35 | 7 | 59 | 9 | 104 | 10 | 113 |
| GBM46 | 30 | 10 | 45 | 10 | 51 | 9 | 104 | 10 | 114.5 |
| GBM58 | 51 | 10 | 91.5 | 10 | 102 | 9 | 100 | 10 | 111 |
| GBM59 | 39 | 9 | 46 | 9 | 90 | 9 | 74 | 10 | 254 |
Rx day - number of days following tumor implantation that the 5-day therapy course was completed.
n - number of animals in each treatment group.
Survival - indicates the median survival in days for the indicated treatment.
Table 2.
Survival benefit following TMZ, RT or TMZ/RT
| Survival Ratio (p-value) | ||||
|---|---|---|---|---|
| GBM# | MS-PCR | TMZ | RT | TMZ/RT |
| GBM5 | M | 5.84 (<0.0001) | 2.53 (0.0003) | 6.35 (<0.0001) |
| GBM6 | U | 1.39 (0.0005) | 1.02 (0.23) | 2.39 (0.04) |
| GBM8 | M | 2.11 (<0.0001) | 1.83 (0.001) | 3.23 (0.07) |
| GBM10 | U | 1.34 (0.10) | 1.61 (0.003) | 1.85 (0.0003) |
| GBM12 | M | 3.53 (<0.0001) | 2.47 (<0.0001) | 5.70 (<0.0001) |
| GBM14 | M | 5.64 (<0.0001) | 1.68 (<0.0001) | 5.70 (0.0003) |
| GBM15 | M | 4.68 (0.0001) | 4.63 (<0.0001) | 4.67 (0.0001) |
| GBM16 | M | 6.85 (0.02) | 1.35 (0.47) | 7.22 (0.10) |
| GBM22 | M | 3.59 (0.0009) | 1.00 (0.29) | 7.57 (0.0009) |
| GBM26 | U | 1.21 (0.003) | 1.58 (0.0001) | 1.58 (0.0001) |
| GBM28 | U | 1.70 (0.003) | 2.06 (0.0001) | 2.50 (<0.0001) |
| GBM34 | U | 4.31 (<0.0001) | 1.84 (0.01) | 4.60 (0.0001) |
| GBM36 | M | 1.82 (0.14) | 1.55 (0.05) | 2.45 (0.02) |
| GBM38 | U | 1.45 (0.0001) | 1.04 (0.21) | 1.36 (0.003) |
| GBM39 | M | 3.87 (<0.0001) | 1.63 (<0.0001) | 6.52 (<0.0001) |
| GBM43 | U | 1.79 (0.003) | 1.71 (0.04) | 2.29 (0.002) |
| GBM44 | U | 1.69 (0.05) | 2.97 (0.008) | 3.23 (<0.0001) |
| GBM46 | M | 1.13 (0.22) | 2.31 (0.0007) | 2.54 (<0.0001) |
| GBM58 | U | 1.11 (0.25) | 1.09 (0.35) | 1.21 (0.14) |
| GBM59 | M | 1.96 (<0.0001) | 1.61 (0.0004) | 5.52 (<0.0001) |
MS-PCR - methylation specific PCR; M - methylated, U - unmethylated Survival ratio is the ratio of median survival for treatment vs. placebo.
p-value refers to the comparison of survival for the indicated treatment relative to placebo treatment.
Therapy response relative to MGMT methylation status
The influence of MGMT methylation status on survival benefit following each treatment was evaluated in the 20 lines. As anticipated, MGMT methylated lines had a greater survival ratio following TMZ treatment alone than the unmethylated lines (Figure 2A, median survival ratio 3.6 vs. 1.5, respectively; p=0.008), and a similar association was observed for treatment with TMZ/RT (Figure 2B, median survival ratio 5.7 vs. 2.3, respectively; p=0.001). However, similar to clinical experience, there were several MGMT methylated tumors for which there was a nominal benefit from TMZ mono-therapy (GBM8, 36, 46, 59). Interestingly, 1 of these tumors significantly benefited from combined TMZ/RT (GBM59). There was no associated survival benefit following RT monotherapy for MGMT methylated tumors (Figure 2C, median survival ratio 1.7) relative to unmethylated tumors (median survival ratio 1.6; p=0.47). Thus, MGMT methylation status was significantly associated with response to TMZ or TMZ/RT but not RT alone.
Figure 2.
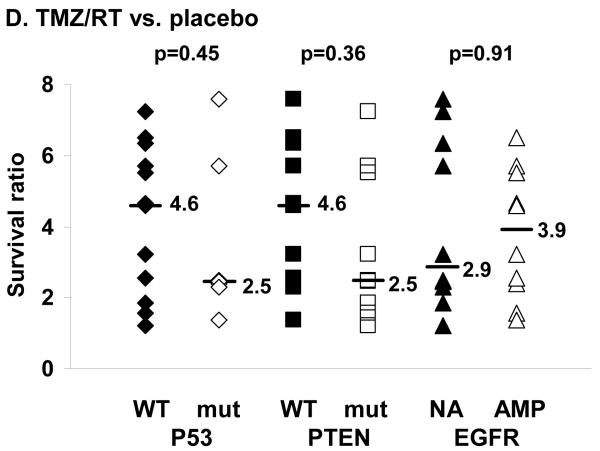
Survival benefit from therapy relative to MGMT, EGFR, p53 and PTEN status. Twenty GBM xenograft lines were evaluated for response to radiation therapy (RT) and temozolomide (TMZ) alone and in combination. For each xenograft line, mice with established intracranial tumors were randomized to treatment with placebo/sham RT treatment, RT (2 Gy twice daily × 5 days), TMZ (66 mg/kg/day × 5 days), or concomitant TMZ and RT (TMZ/RT). Mice were followed until reaching a moribund state, and the median survival for each treatment was calculated. To describe the efficacy of treatment relative to placebo, the survival ratio for any treatment was defined as the ratio of median survival for treated mice vs. median survival for placebo mice for each xenograft tested. In this way, a survival ratio of 1.0 would indicate no additional survival benefit for treatment compared to placebo, and a ratio of 2.0 would indicate a doubling in survival with treatment as compared to placebo. The survival ratio is plotted relative to MGMT methylation status (M - methylated; U - unmethylated) for A) TMZ, B) TMZ/RT, and C) RT. D) The survival ratio following TMZ/RT also is plotted relative to p53 status (WT -wild-type; mut - mutant), PTEN status (WT - wild-type, mut - mutant or homozygous deleted), and EGFR status (NA - non-amplified, AMP - amplified). The median survival ratio for a given molecular feature and the p-value from a two-sample rank sum test comparison between groups are shown for each figure.
The survival benefit associated with treatment also was evaluated in relationship to 3 common genetic lesions observed in GBM: p53 mutation, PTEN mutation or deletion, and EGFR amplification. The results of the genetic characterization for each tumor line are summarized in Supplemental Table 1. Of the 20 xenograft lines, 7 lines had p53 mutations, 7 lines were homozygous deleted for PTEN, 2 lines had mutations in the coding sequence for PTEN, 6 lines had amplification of wild-type EGFR, 3 lines had amplification of a vIII-mutant EGFR, and 1 line had amplification of a vII-mutant EGFR. In a simple pair-wise non-parametric comparison, there was no statistically significant correlation between genetic status defined by p53 mutation, PTEN mutation/deletion or EGFR amplification and survival ratio following TMZ/RT (Figure 2D), RT, or TMZ (data not shown). However, in a second analysis in which individual survival data for all mice treated on these studies were evaluated by a repeated measures ANOVA, there was a significant p53 status-treatment interaction (p=0.01), a marginally significant EGFR by treatment interaction (p=0.06) and no significant PTEN status by treatment interaction (p=0.24). Analyzing the p53 data separately by treatment status, mice with wild-type p53 tumors treated with TMZ had better survival than mice with mutant p53 tumors treated with TMZ (LSM survival 161 days versus 104 days, respectively; p=0.06); mice with wild-type p53 tumors treated with TMZ/RT also had better survival than mice with mutant p53 tumors treated with TMZ/RT (LSM survival 202 days versus 143 days, respectively; p=0.06). There was no difference when comparing mice with wild-type p53 versus mutant p53 tumors treated with RT (LSM survival 120 days versus 76 days, respectively; p=0.15). Collectively, these data suggest that wild-type p53 status may be associated with an increased sensitivity to TMZ-based therapies in the xenograft model.
Radiosensitizing effects of temozolomide
The combination of RT and TMZ was significantly more effective than either therapy alone in only a subset of tumors. To quantitate the extent of benefit for combined therapy relative to single agent therapy, the difference in median survival for TMZ/RT versus the more effective monotherapy was normalized to placebo survival (survival difference) for each tumor line using the data from Table 1. For the group as a whole, there was a broad range in this survival difference from -0.09 (GBM38) to 4.0 (GBM22). In relationship to MGMT methylation status, the median survival difference for methylated tumors was greater than for the unmethylated tumors (Figure 3; median survival difference 0.63 vs. 0.26, respectively; p=0.08). For the unmethylated tumors, none of the tumors had a survival difference associated with RT/TMZ that was greater than 1.0, while of the 11 MGMT methylated xenograft lines, 5 tumors had a survival difference greater than 1.0 (GBM8 - 1.1, GBM12 - 2.2, GBM39 - 2.7, GBM59 - 3.6, GBM22 - 4.0). Conversely, a subset of MGMT methylated tumors and most MGMT unmethylated tumors derived no additional survival benefit from combined therapy as compared to the most efficacious monotherapy (survival difference near 0). Thus, a significant additional benefit for concomitant TMZ/RT was observed only in a subset of MGMT methylated tumors.
Figure 3.
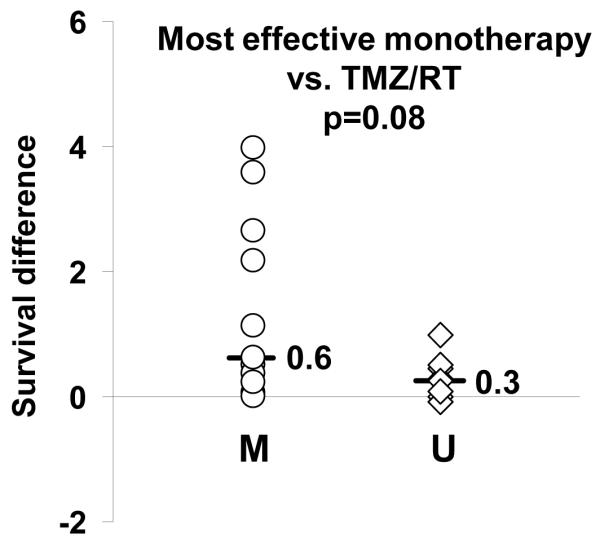
Additional survival benefit from concomitant therapy. The specific benefit of combined TMZ/RT compared to monotherapy was quantified by calculating a ‘survival difference’: (difference in the median survival for TMZ/RT versus most efficacious monotherapy) divided by placebo survival. Survival difference for each line is plotted relative to MGMT methylation status (M - methylated; U - unmethylated). The median survival difference for each group is shown, and the p-value for comparison between groups is shown.
Sequential versus concomitant therapy
The potential for synergistic interactions between RT and TMZ was evaluated by comparing the relative efficacy of sequential versus concurrent therapy. Using the same model system described above, mice with established intracranial GBM12 xenografts were randomized to therapy with placebo, TMZ alone (week 1, 66 mg/kg/day × 5d), RT alone (week 1, 2 Gy bid × 5 d), RT followed by TMZ on sequential weeks (RT week 1→TMZ week 2), TMZ followed by RT (TMZ week 1→RT week2), or RT concurrent with TMZ (TMZ week 1 +RT week 1). As seen in Figure 4, either RT alone or TMZ alone (survival ratio of 1.8 and 2.3, respectively) were significantly less effective than any of the combined therapy arms (p<0.001 for all comparisons). Of the combination arms, RT followed by TMZ (survival ratio 4.0) was the least effective regimen as compared to monotherapy with either RT or TMZ. In comparison, TMZ followed by RT and TMZ concurrent with RT both were significantly more effective at prolonging survival (survival ratio of 9.6 for TMZ→RT, p=0.001; survival ratio 12.9 for TMZ +RT, p<0.0001). There was no significant difference in efficacy for these latter 2 combination regimens (p=0.47). Thus, optimal efficacy with combined therapy required TMZ to be administered prior to or concurrent with RT in the GBM12 xenograft line.
Figure 4.
Efficacy of concomitant vs. sequential therapy with TMZ and RT. Mice with established GBM12 intracranial xenografts were randomized to treatment with placebo/sham RT, TMZ (66 mg/kg/day, days 1-5), RT (2 Gy twice daily, days 1-5), concurrent TMZ & RT × 1 week (both TMZ and RT, days 1-5), TMZ then RT (TMZ on days 1-5 followed by RT on days 8-12), and RT then TMZ (RT days 1-5 followed by TMZ days 8-12). The survival for each treatment is plotted. The survival benefit (ratio of median survival for treatment indicated vs. placebo) is shown in the legend. The p-values shown compare concurrent TMZ/RT to TMZ then RT and compare TMZ then RT to RT then TMZ.
DISCUSSION
Therapy with radiation and TMZ provides clinical benefit in a significant subset of patients with newly diagnosed GBM 1. This standard treatment regimen includes TMZ delivered daily for 6 weeks during radiotherapy followed by 6 to 12 months of adjuvant TMZ therapy. However, previous in vitro studies have demonstrated limited to no radiosensitizing effects at clinically relevant radiation doses of 2 to 3 Gy 7-11. Thus, the importance of concurrent therapy with TMZ combined with RT is unclear. In the current study, concomitant therapy with radiation and TMZ was associated with a survival benefit in a subset of tumors, and the significantly greater survival benefit observed with sequential therapy when TMZ was given prior to RT provides definitive evidence that TMZ has significant radiosensitizing effects in at least a subset of GBM tumors.
Previous laboratory studies evaluating radiation combined with TMZ have relied on limited numbers of established GBM tumor cell lines that are maintained by in vitro cell culture 7-11. In contrast, the current study evaluated the combination of radiation and TMZ in a panel of 20 GBM xenograft lines established directly from patient tumor samples and maintained by serial subcutaneous tumor passage. This method of tumor propagation maintains key molecular and morphologic phenotypes of the original patient tumors 29, 30, and of specific relevance to testing TMZ-based regimens, the Mayo xenograft model faithfully maintains the MGMT methylation status of the primary patient tumor samples from which they were derived (Figure 1). The TMZ dosing regimen used for these studies (66 mg/kg/day × 5 days) was selected to model the standard adjuvant clinical dosing regimen of 200 mg/m2/day × 5 days: previous studies have demonstrated similar plasma drug concentrations for the respective human and animal dosing regimens 26-28. Similar to clinical observations 2, TMZ responsiveness in the Mayo xenograft model was significantly associated with MGMT promoter methylation (Figure 2A). Also similar to clinical results, the MGMT methylation marker was not completely accurate for predicting TMZ responsiveness: GBM34 lacks MGMT promoter hypermethylation but was significantly sensitive to TMZ, while GBM8, 46, and 59 are MGMT promoter hypermethylated but are relatively resistant to TMZ. These results support the idea that the Mayo GBM Xenograft Panel is a highly clinically relevant model for evaluating TMZ-based regimens.
The combination of radiation and TMZ was evaluated in all xenograft lines to understand the potential contribution of the radiosensitizing effects of TMZ. By analyzing the difference in survival ratio for treatment with TMZ/RT versus the most effective monotherapy for each tumor line, we defined the additional survival benefit that can be obtained with combined therapy (Figure 3). In this analysis, concomitant therapy provided only limited additional survival benefit in the majority of tumors, and a marked survival benefit was observed only in about half of the MGMT methylated tumors. These data are consistent with previous in vitro studies suggesting that absence of MGMT activity is important for the radiosensitizing effects of TMZ 9, 11. MGMT specifically repairs O6-methylguanine lesions induced by TMZ, and in the absence of MGMT, O6MG lesions can persist for many days 33, 34. Consistent with the idea that persistent O6MG lesions may be important for the radiosensitizing effects of TMZ, treatment with TMZ for a week prior to RT was as effective as treating concurrently with TMZ and RT, and both treatments were significantly more effective than treating with RT and then TMZ (Figure 4). Importantly, our data extend the observations of the previous studies by demonstrating that while suppression of MGMT activity may be important for the radiosensitizing effects of TMZ, the lack of robust sensitizing effects in half of the MGMT methylated tumors suggest that additional molecular features may influence the radiosensitizing effects of TMZ.
Collectively, the results presented provide greater clarity regarding the importance of concomitant TMZ and RT therapy in the treatment of GBM. While the landmark EORTC 22891 randomized trial clearly defined the regimen of concomitant TMZ/RT followed by adjuvant TMZ as providing superior survival compared to RT alone, this study did not address the specific contribution of concomitant TMZ to patient survival. Given the equivocal results from previous in vitro studies evaluating the radiosensitizing effects of TMZ 7-11, the importance of concomitant TMZ with RT was unclear. In the current animal study, the relative efficacy of both TMZ and RT monotherapy was compared in each tumor line to the efficacy for combined therapy, and through this analysis, 5 MGMT methylated tumor lines (GBM 8, 12, 22, 39, 59) were identified as significantly benefiting from concomitant therapy. Ongoing studies in the laboratory are focused on understanding the mechanism of the selective radiosensitizing effects observed in these tumor lines in comparison to those that did not benefit. Until these mechanistic studies enable the identification of more robust predictors of response, the treatment of GBM patients with concomitant RT/TMZ should remain the standard of care. Within the context of novel clinical trial development, the results support the concept of testing novel radiosensitizing agents in combination with RT alone in tumors lacking MGMT methylation, while combining radiosensitizers with the therapeutic doublet of RT/TMZ in patients with MGMT methylated tumors.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the Mayo Foundation, by the National Cancer Institute (CA108961 and CA127716) and the Brain Tumor Funders Consortium.
Footnotes
Conflict of Interest Statement: none of the authors have a conflict of interest to disclose related to this manuscript.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 3.Paz MF, Yaya-Tur R, Rojas-Marcos I, et al. CpG island hypermethylation of the DNA repair enzyme methyltransferase predicts response to temozolomide in primary gliomas. Clin Cancer Res. 2004;10:4933–4938. doi: 10.1158/1078-0432.CCR-04-0392. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 5.McGinn CJ, Lawrence TS. Recent advances in the use of radiosensitizing nucleosides. Semin Radiat Oncol. 2001;11:270–280. doi: 10.1053/srao.2001.26002. [DOI] [PubMed] [Google Scholar]
- 6.Byfield JE. 5-Fluorouracil radiation sensitization--a brief review. Invest New Drugs. 1989;7:111–116. doi: 10.1007/BF00178197. [DOI] [PubMed] [Google Scholar]
- 7.Wedge SR, Porteous JK, Glaser MG, et al. In vitro evaluation of temozolomide combined with X-irradiation. Anti-Cancer Drugs. 1997;8:92–97. doi: 10.1097/00001813-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 8.van Rijn J, Heimans JJ, van den Berg J, et al. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–784. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-Mediated Radiation Enhancement in Glioblastoma: A Report on Underlying Mechanisms. Clin Cancer Res. 2006;12:4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 10.Combs SE, Schulz-Ertner D, Roth W, et al. In vitro responsiveness of glioma cell lines to multimodality treatment with radiotherapy, temozolomide, and epidermal growth factor receptor inhibition with cetuximab. Int J Radiat Oncol Biol Phys. 2007;68:873–882. doi: 10.1016/j.ijrobp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.van Nifterik KA, van den Berg J, Stalpers LJA, et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69:1246–1253. doi: 10.1016/j.ijrobp.2007.07.2366. [DOI] [PubMed] [Google Scholar]
- 12.Kitange GJ, Carlson BL, Schroeder MA, et al. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neurooncol. 2008:15228517-15222008–15228090. doi: 10.1215/15228517-2008-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinca EB, Lu KV, Sarkaria JN, et al. p53 Small-Molecule Inhibitor Enhances Temozolomide Cytotoxic Activity against Intracranial Glioblastoma Xenografts. Cancer Res. 2008;68:10034–10039. doi: 10.1158/0008-5472.CAN-08-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. see comment. [DOI] [PubMed] [Google Scholar]
- 16.Sarkaria JN, Carlson BL, Schroeder MA, et al. Use of an Orthotopic Xenograft Model for Assessing the Effect of Epidermal Growth Factor Receptor Amplification on Glioblastoma Radiation Response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 17.Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Clarke MJ, Carlson BL, et al. PTEN Loss Does Not Predict for Response to RAD001 (Everolimus) in a Glioblastoma Orthotopic Xenograft Test Panel. Clin Cancer Res. 2008;14:3993–4001. doi: 10.1158/1078-0432.CCR-07-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteller M, Hamilton SR, Burger PC, et al. Inactivation of the DNA Repair Gene O6-Methylguanine-DNA Methyltransferase by Promoter Hypermethylation is a Common Event in Primary Human Neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- 20.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-Repair Gene MGMT and the Clinical Response of Gliomas to Alkylating Agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 21.James CD, Galanis E, Frederick L, et al. Tumor suppressor gene alterations in malignant gliomas: histopathological associations and prognostic evaluation. International Journal of Oncology. 1999;15:547–553. doi: 10.3892/ijo.15.3.547. [DOI] [PubMed] [Google Scholar]
- 22.Giannini C, Hebrink D, Scheithauer BW, et al. Analysis of p53 mutation and expression in pleomorphic xanthoastrocytoma. Neurogenetics. 2001;3:159–162. doi: 10.1007/s100480100116. [DOI] [PubMed] [Google Scholar]
- 23.Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, James CD, Frederick L, et al. PTEN/MMAC1 mutations and EGFR amplification in glioblastomas. Cancer Research. 1997;57:5254–5257. [PubMed] [Google Scholar]
- 25.Hegi ME, Diserens A-C, Godard S, et al. Clinical Trial Substantiates the Predictive Value of O-6-Methylguanine-DNA Methyltransferase Promoter Methylation in Glioblastoma Patients Treated with Temozolomide. Clin Cancer Res. 2004;10:1871–1874. doi: 10.1158/1078-0432.ccr-03-0384. [DOI] [PubMed] [Google Scholar]
- 26.Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846–5852. [PubMed] [Google Scholar]
- 27.Baker SD, Wirth M, Statkevich P, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5:309–317. [PubMed] [Google Scholar]
- 28.Reid JM, Stevens DC, Rubin J, et al. Pharmacokinetics of 3-methyl-(triazen-1-yl)imidazole-4-carboximide following administration of temozolomide to patients with advanced cancer. Clin Cancer Res. 1997;3:2393–2398. [PubMed] [Google Scholar]
- 29.Giannini C, Sarkaria J, Saito A, et al. Patient Tumor EGFR and PDGFRA Gene Amplifications Retained in an Invasive Intracranial Xenograft Model of GBM. Neuro-Oncology. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandita A, Aldape KD, Zadeh G, et al. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 31.Pelloski CE, Rivera AL, De La Cruz Guerrero C, et al. MGMT Promoter Methylation is an Independent Prognostic Factor in the Absence of Alkylating Chemotherapy in Glioblastoma. International Journal of Radiation Oncology*Biology*Physics. 2008;72:S9–S9. [Google Scholar]
- 32.Sarkaria JN, Kitange GJ, James CD, et al. Mechanisms of Chemoresistance to Alkylating Agents in Malignant Glioma. Clin Cancer Res. 2008;14:2900–2908. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson PE, Cooper DP, Meyer TA, et al. Formation and persistence of O(6)-methylguanine in the mouse colon following treatment with 1,2-dimethylhydrazine as measured by an O(6)-alkylguanine-DNA alkyltransferase inactivation assay. Toxicol Lett. 2000;115:205–212. doi: 10.1016/s0378-4274(00)00193-4. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor PJ, Manning FC, Gordon AT, et al. DNA repair: kinetics and thresholds. Toxicol Pathol. 2000;28:375–381. doi: 10.1177/019262330002800304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.